Modeling and Molecular Dynamics Studies of Flavone―DENV E-3 Protein―SWCNT Interaction at the Flavonoid Binding Sites
Abstract
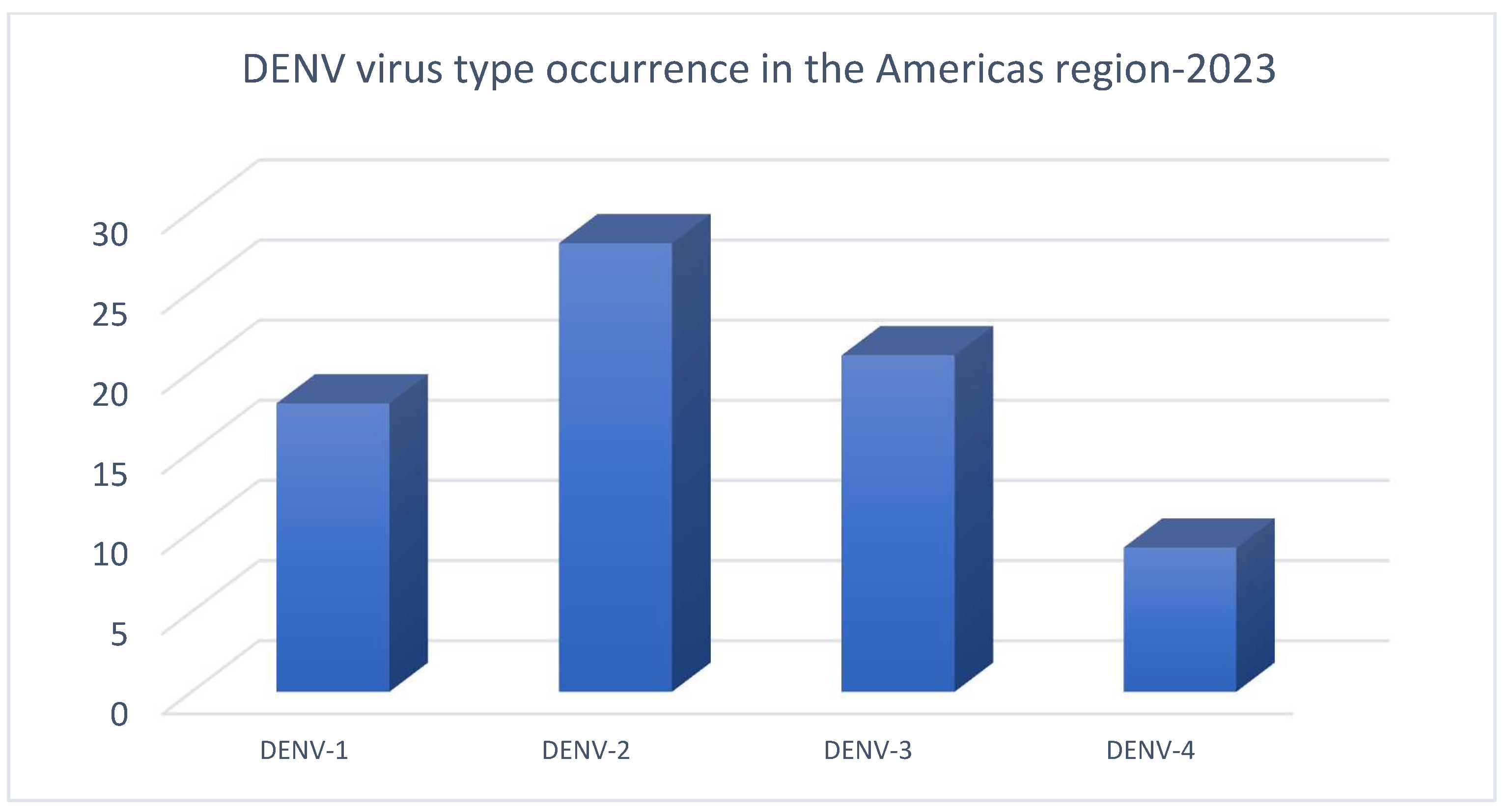
1. Introduction
2. Materials and Methods
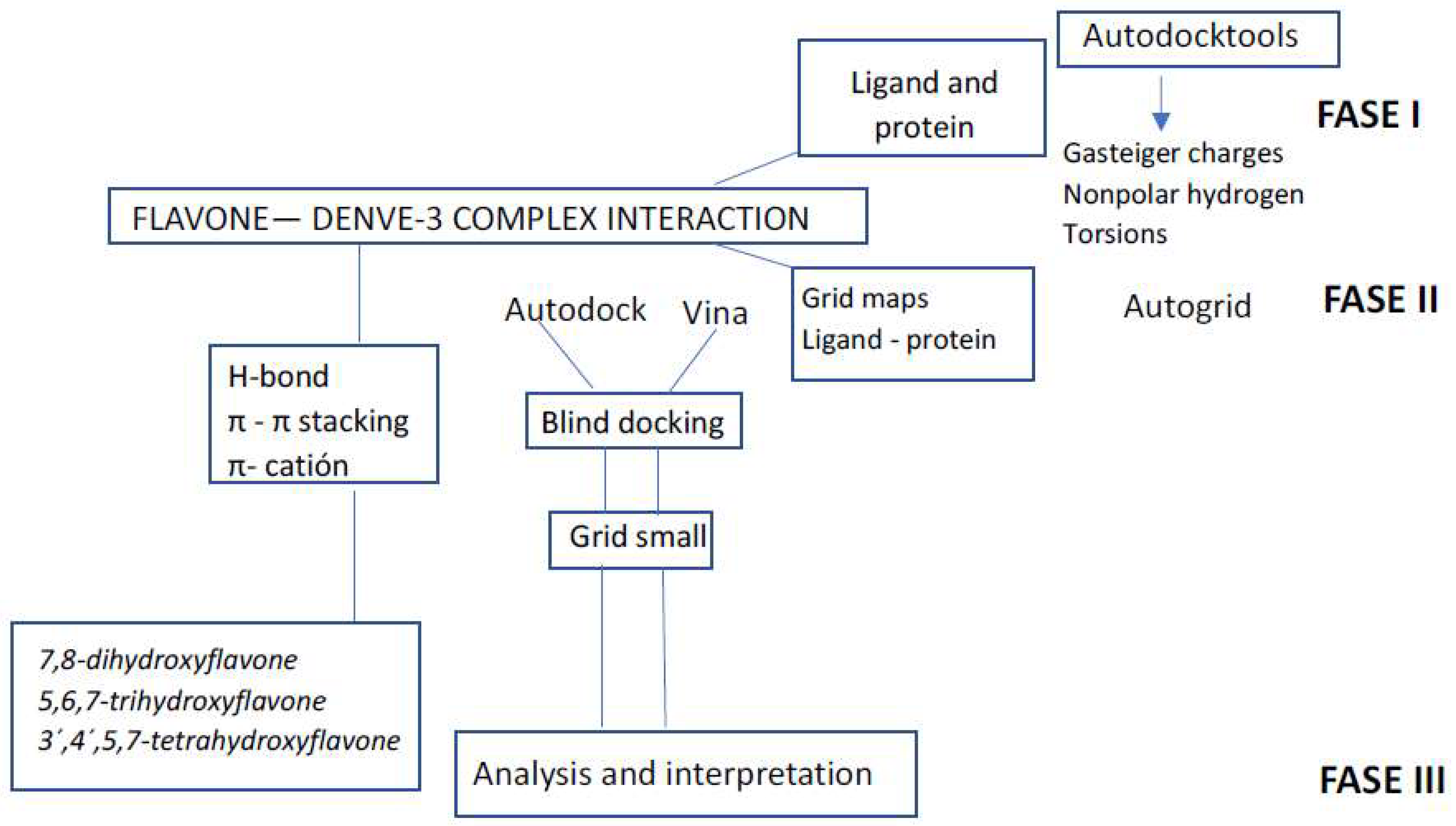
2.1. Docking Protocol
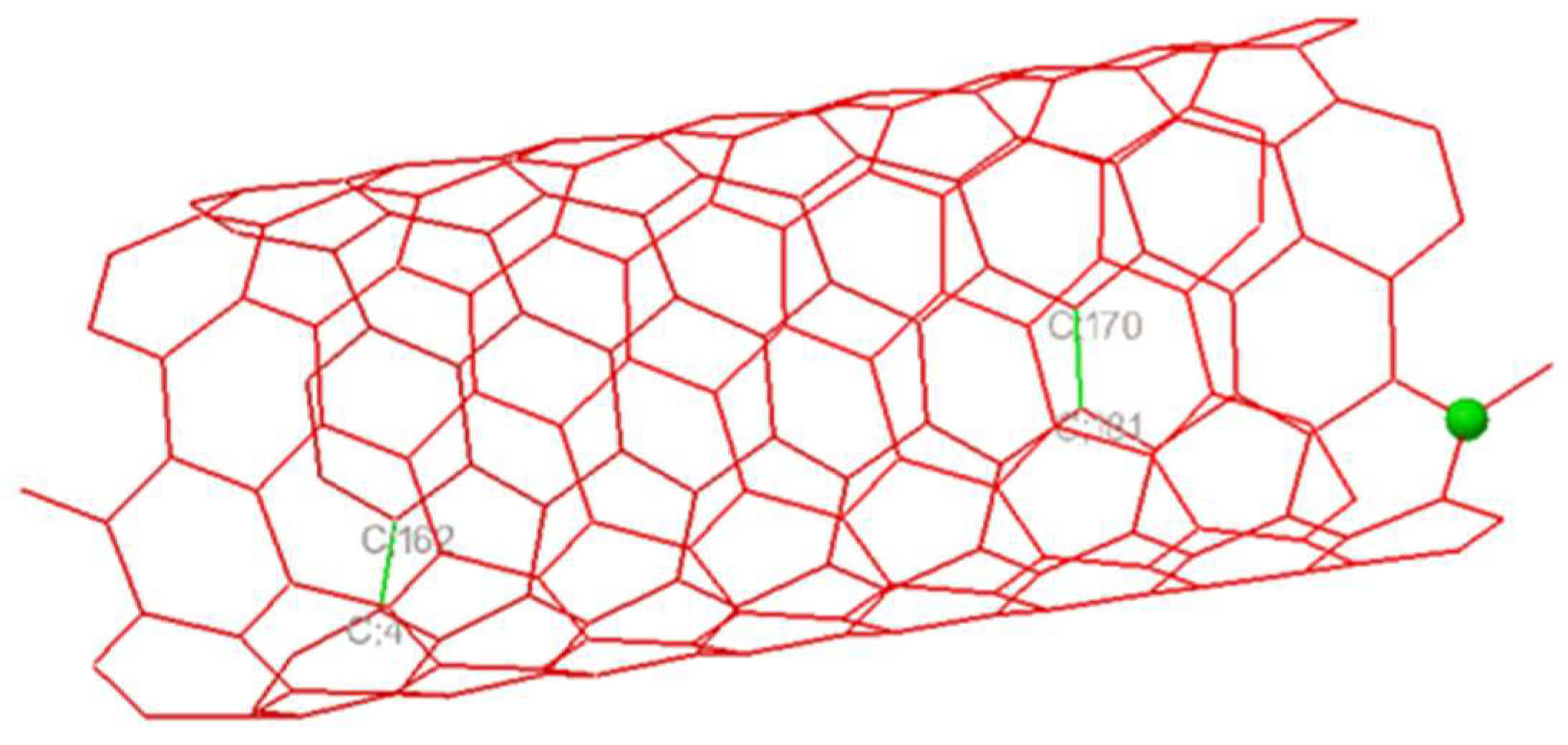
2.2. Nanotube Modeling
2.3. Molecular Dynamics
3. Results and Discussion
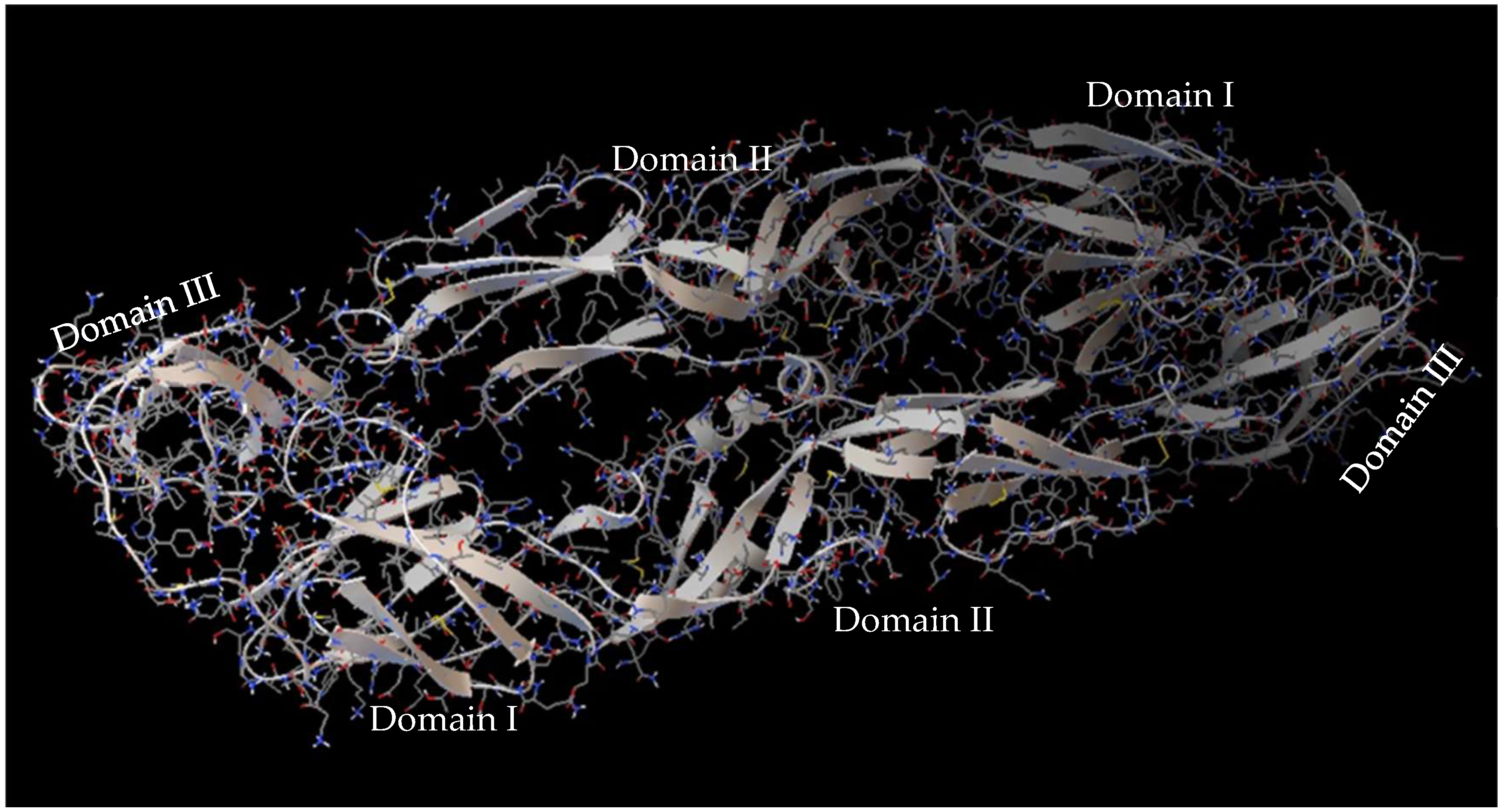
3.1. Interaction of DENV E-3–Flavone at Binding Site of Flavonoids
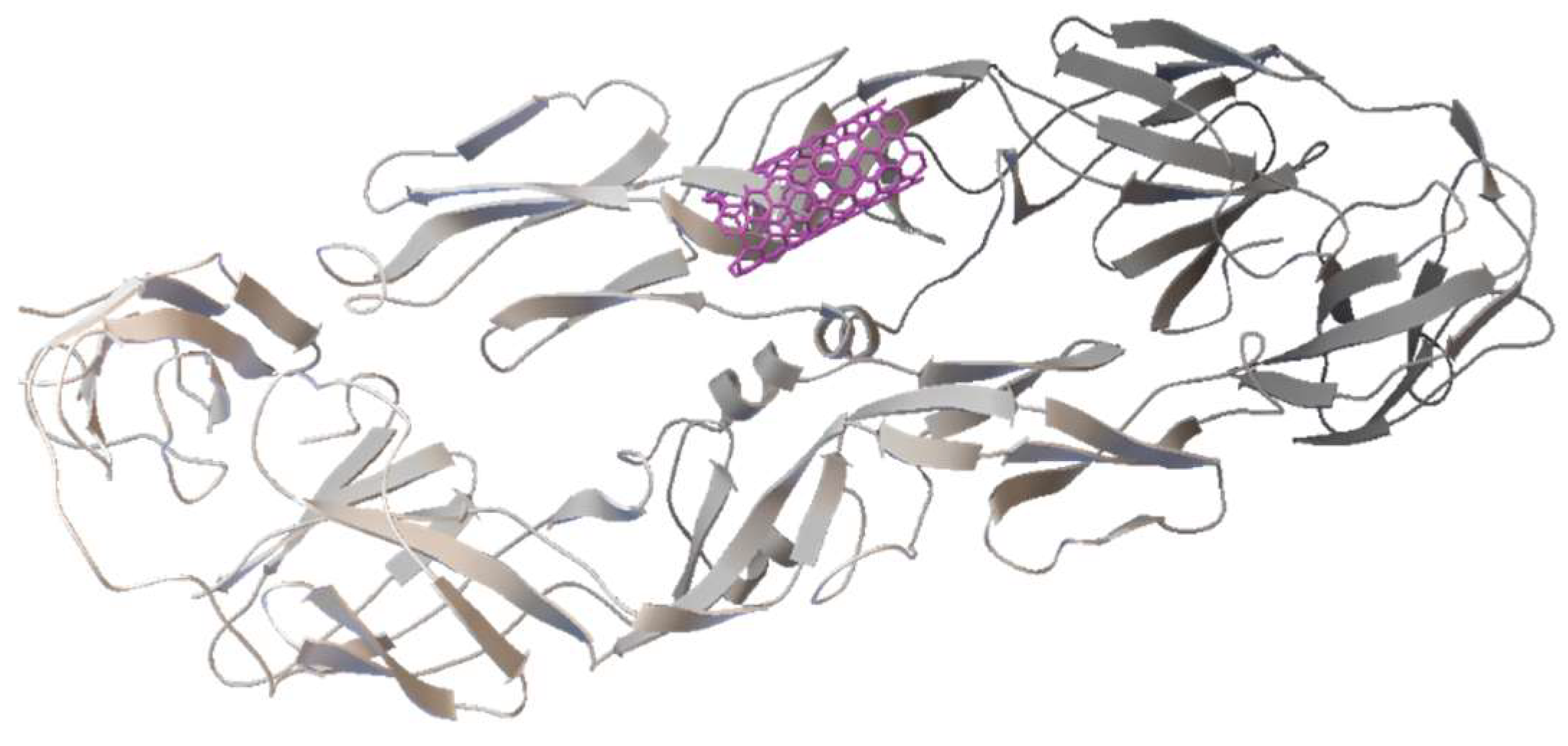
3.2. Flavone—DENV E-3—SWCNT Complex Interaction
3.2.1. Tropoflavin–DENV E-3—SWCNT
3.2.2. Baicalein–DENV E-3—SWCNT
3.2.3. Luteolin–DENV E-3―SWCNT
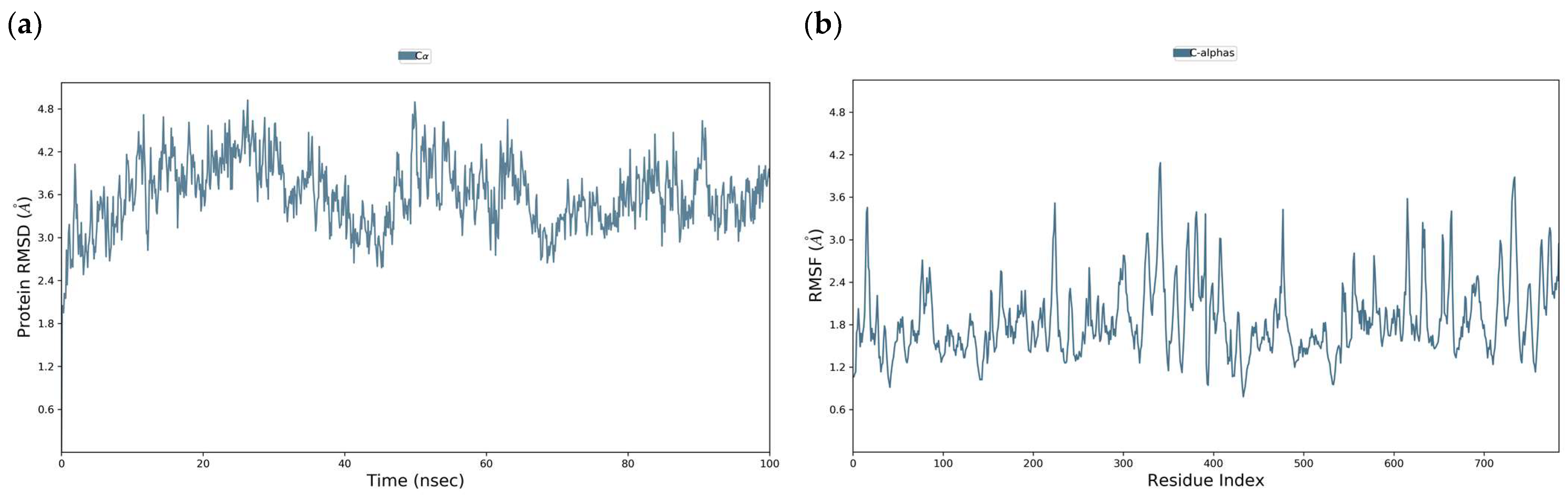
3.3. Molecular Dynamics of DENV E-3–Flavone Complexes at Flavonoid Binding Sites
3.3.1. Tropoflavin–DENV E-3 Complex
3.3.2. Baicalein–DENV E-3 Complex
3.3.3. Luteolin–DENV E-3 Complex
4. Limitations and Perspectives
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhiman, M.; Sharma, L.; Dadhich, A.; Dhawan, P.; Sharma, M.M. Traditional Knowledge to Contemporary Medication in the Treatment of Infectious Disease Dengue: A Review. Front. Pharmacol. 2022, 13, 750494. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.M.G.; Nogueira, R.M.R.; Schatzmayr, H.G.; Zanotto, P.M.; Gonzalo Bello, G. Phylogeography and evolutionary history of dengue virus type. Infect. Genet. Evol. 2009, 9, 716–725. [Google Scholar] [PubMed]
- World Health Organization. Disease Outbreak News; Dengue—Global Situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (accessed on 21 December 2023).
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70-year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar]
- Weaver, S.C.; Vasilakis, N. Molecular evolution of dengue viruses: Contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect. Genet. Evol. 2009, 9, 523–540. [Google Scholar]
- Naveca, F.G.; Santiago, G.A.; Maito, R.M.; Meneses, C.A.R.; Nascimento, V.A.; Souza, V.C.; do Nascimento, F.O.; Silva, D.; Mejía, M.; Gonçalves, L.; et al. Reemergence of Dengue Virus Serotype 3, Brazil. Emerg. Infect. Dis. 2023, 29, 1482–1484. [Google Scholar]
- Adelino, T.; Lima, M.; Guimarães, N.R.; Xavier, J.; Fonseca, V.; Tomé, L.M.R.; Pereira, M.A.; Machado, V.F.; Alcantara, L.C.J.; Iani, F.C.d.M.; et al. Resurgence of Dengue Virus Serotype 3 in Minas Gerais, Brazil: A Case Report. Pathogens 2024, 13, 202. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, R.; Shen, H.; Wang, M.; Yin, Z.; Cheng, A. Structures and Functions of the Envelope Glycoprotein in Flavivirus Infections. Viruses 2017, 9, 338. [Google Scholar] [CrossRef]
- Hassandarvish, P.; Rothan, H.A.; Rezaei, S.; Yusof, R.; Abubakara, S.; Zandi, K. In silico study on baicalein and baicalin as inhibitors of dengue virus replication. RSC Adv. 2016, 6, 31235. [Google Scholar]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A Minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef]
- Stiasny, K.; Heinz, F.X. Flavivirus membrane fusion. J. Gen. Virol. 2006, 87, 2755–2766. [Google Scholar] [CrossRef]
- Kularatne, S.A.M. Dengue Fever. BMJ 2015, 351, h4661. [Google Scholar] [PubMed]
- Thomas, S.J. Is new dengue vaccine efficacy data a relief or cause for concern? npj Vaccines 2023, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Balde, D.; Talla, C.; Camara, D.; Barry, M.A.; Sagne, S.N.; Gueye, K.; Dia, C.A.K.M.; Sambe, B.S.; Fall, G.; et al. Molecular Evolution of Dengue Virus 3 in Senegal between 2009 and 2022: Dispersal Patterns and Implications for Prevention and Therapeutic Countermeasures. Vaccines 2023, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- García-Ariza, L.L.; González-Rivillas, N.; Díaz-Aguirre, C.J.; Rocha-Roa, C.; Padilla-Sanabria, L.; Castaño-Osorio, J.C. Antiviral Activity of an Indole-Type Compound Derived from Natural Products, Identified by Virtual Screening by Interaction on Dengue Virus NS5 Protein. Viruses 2023, 15, 1563. [Google Scholar] [CrossRef]
- Altamish, M.; Khan, M.; Baig, M.S.; Pathak, B.; Rani, V.; Akhtar, J.; Khan, A.A.; Ahmad, S.; Krishnan, A. Therapeutic Potential of Medicinal Plants against Dengue Infection: A Mechanistic Viewpoint. ACS Omega 2022, 7, 24048–24065. [Google Scholar]
- Ancuceanu, R.; Dinu, M.; Cristina Dinu-Pirvu, C.; Valentina Anuta, V.; Negulescu, V. Pharmacokinetics of B-Ring Unsubstituted Flavones. Pharmaceutics 2019, 11, 370. [Google Scholar] [CrossRef]
- Espíndola, C. Some Ways for the Synthesis of Chalcones—New Ways for the Synthesis of Flavon-3-ols. Mini-Rev. Org. Chem. 2020, 17, 647–673. [Google Scholar] [CrossRef]
- Espíndola, C. Some Nanocarrier’s Properties and Chemical Interaction Mechanisms with Flavones. Molecules 2023, 28, 2864. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, L.; Ma, S.; Liu, Y. Anti-Enterovirus 71 Agents of Natural Products. Molecules 2015, 20, 16320–16333. [Google Scholar] [CrossRef]
- Wang, M.; Tao, L.; Xu, H. Chinese herbal medicines as a source of molecules with antienterovirus 71 activity. Chin Med. 2016, 11, 2. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.X.; OuYong, B.M.; Hassandarvish, P.; Poh, C.L.; Ramanathan, B. Antiviral activity of silymarin and baicalein against dengue virus. Sci. Rep. 2021, 11, 21221. [Google Scholar]
- Peng, M.; Watanabe, S.; Chan, K.W.K.; He, Q.; Zhao, Y.; Zhang, Z.; Lai, X.; Luo, D.; Vasudevan, S.G.; Li, G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antiviral Res. 2017, 143, 176–185. [Google Scholar] [PubMed]
- Boniface, P.K.; Ferreira, E.I. Flavonoids as efficient scaffolds: Recent trends for malaria, leishmaniasis, Chagas disease, and dengue. Phytother. Res. 2019, 33, 2473–2517. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Jusoh, S.A. Molecular Docking and Molecular Dynamics Simulation Studies to Predict Flavonoid Binding on the Surface of DENV2 E Protein. Interdiscip. Sci. Comput. Life Sci. 2017, 9, 499–511. [Google Scholar] [CrossRef]
- Espíndola, C. Analysis of molecular interactions between flavones and dengue DENV E—3 protein by In silico approach. Scirea J. Chem. 2023, 8, 27–53. [Google Scholar] [CrossRef]
- Shirasu, K.; Kitayama, S.; Liu, F.; Yamamoto, G.; Hashida, T. Molecular dynamics simulations and theoretical model for engineering tensile properties of single-and multi-walled carbon nanotubes. Nanomaterials 2021, 11, 795. [Google Scholar] [CrossRef]
- Skariyachan, S.; Gopal, D.; Deshpande, D.; Joshi, A.; Uttarkarf, A.; Niranjan, V. Carbon fullerene and nanotube are probable binders to multiple targets of SARS-CoV-2: Insights from computational modeling and molecular dynamic simulation studies. Infect. Genet. Evol. 2021, 96, 105155. [Google Scholar]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar]
- Batra, S.; Sharma, S.; Mehra, N.K. Carbon Nanotubes for Drug Delivery Applications. In Handbook of Carbon Nanotubes; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulha, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001. [Google Scholar]
- Espíndola, C.; Correa, A.J.; López-López, M.; López-Cornejo, P.; Bernal, E.; Lebrón, J.A.; Ostos, F.J.; Benhnia, M.R.-E.-I.; Moyá, M.L. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics 2022, 14, 2806. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, C.; Gómez-Méndez, A.; Moyá, M.L.; López-Vallejo, F.; Bernal, E.; Lebrón, J.A.; Martín, V.I.; Ostos, F.J.; López-Cornejo, P.; López-López, M. The Role of Pristine Carbon Nanotubes as Nanocarriers of 7,8-Dihydroxyflavone. J. Delivery Sci. Technol. 2024, 100, 106068. [Google Scholar]
- Versiani, A.F.; Astigarraga, R.G.; Rocha, E.S.; Barboza, A.P.; Kroon, E.G.; Rachid, M.A.; Souza, D.G.; Ladeira, L.O.; Barbosa-Stancioli, E.F.; Jorio, A.; et al. Multi-walled carbon nanotubes functionalized with recombinant Dengue virus 3 envelope proteins induce significant and specific immune responses in mice. J. Nanobiotechnol. 2017, 15, 26. [Google Scholar]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Olsen, L.R.; Zhang, G.L.; Keskin, D.B.; Reinherz, E.L.; Brusic, V. Conservation analysis of dengue virus t-cell epitope-based vaccine candidates using peptide block entropy. Front. Immunol. 2011, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Lima, E.N.; Octaviano, A.J.M.; Piqueira, J.R.C.; Diaz, R.S.; Justo, J.F. Coronavirus and Carbon Nanotubes: Seeking Immunological Relationships to Discover Immunotherapeutic Possibilities. Int. J. Nanomed. 2022, 17, 751–781. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Lazim, R.; Suh, D.; Choi, S. Advances in Molecular Dynamics Simulations and Enhanced Sampling Methods for the Study of Protein Systems. Int. J. Mol. Sci. 2020, 21, 6339. [Google Scholar] [CrossRef]
- Skyner, R.E.; McDonagh, J.L.; Groom, C.R.; van Mourik, T.; Mitchell, J.B. A review of methods for the calculation of solution free energies and the modelling of systems in solution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C.; Kroemer, H. Thermal Physics, 2nd ed.; W.H. Freeman and Company: San Francisco, CA, USA, 1980; p. 31. [Google Scholar]
- Gibbs, J.W. Elementary Principles in Statistical Mechanics; Charles Scribner’s Sons: New York, NY, USA, 1902. [Google Scholar]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A Gen. Phys. 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- D. E. Shaw Research. Schrödinger Release 2019–3: Desmond Molecular Dynamics System; Tools, Maestro-Desmond Interoperability, Ed.; Schrödinger: New York, NY, USA, 2019. [Google Scholar]
- Das, S.; Bora, N.; Rohman, M.A.; Sharma, R.; Jha, A.N.; Roy, A.S. Molecular recognition of bio-active flavonoids quercetin and rutin by bovine hemoglobin: An overview of the binding mechanism, thermodynamics, and structural aspects through multi-spectroscopic and molecular dynamics simulation studies. Phys. Chem. Chem. Phys. 2018, 20, 21668–21684. [Google Scholar] [CrossRef] [PubMed]
- Guirakhoo, F.; Hunt, A.R.; Lewis, J.G.; Roehrig, J.T. Selection and partial characterization of dengue 2 virus mutants that induce fusion at elevated pH. Virology 1993, 194, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.L.; Lee, P.L.; Chen, H.W.; Chen, L.K.; Kao, C.L.; King, C.C. Analysis of the steps involved in dengue virus entry into host cells. Virology 1999, 257, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sanchez, E.; Altmeyer, R.; Amara, A.; Schwartz, O.; Fieschi, F.; Virelizier, J.L.; Arenzana-Seisdedos, F.; Despres, P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003, 4, 723–728. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 6986–6991. [Google Scholar] [CrossRef]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Variable Surface Epitopes in the Crystal Structure of Dengue Virus Type 3 Envelope Glycoprotein. J. Virol. 2005, 79, 1223–1231. [Google Scholar] [CrossRef]
- Horton, R.H.; Moran, L.A.; Scrimgeour, K.G.; Perry, M.D.; Rawn, J.D. Principles of Biochemistry, 4th ed.; Publishing as Prentice Hall, Copyright; Chapter Pearson Education, Inc.: London, UK, 2006; ISBN 0-13-145306-8. [Google Scholar]
- Mondotte, J.A.; Lozach, P.-Y.; Amara, A.; Gamarnik, A.V. Essential Role of Dengue Virus Envelope Protein N Glycosylation at Asparagine-67 during Viral Propagation. J. Virol. 2007, 81, 7136–7148. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.A.; Diamond, M.S.; Harris, E. Dendritic cells in dengue virus infection: Targets of virus replication and mediators of immunity. Front. Immunol. 2014, 5, 647. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.L.; Pierson, T.C.; Sanchez, M.D.; Ahmed, A.A.; Murtadha, M.M.; Doms, R.W. N-linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. J. Virol. 2005, 79, 13262–13274. [Google Scholar] [CrossRef]
- Putri, G.N.; Gudla, C.S.; Singh, M.; Huan, C.N.; Haji, I.F.F.; Oo, Y.; Yee, T.J.H.; Jie, W.J.F.; Hann, C.J.J.; Selvam, V.; et al. Expanding the anti-flaviviral arsenal: Discovery of a baicalein-derived Compound with potent activity against DENV and ZIKV. Antiviral Res. 2023, 220, 105739. [Google Scholar] [CrossRef]
- Fidel, D.A.; Macalino, S.J.Y.; Posadas, G., II; Carrillo, M.C.O. Structural and Functional Analysis of Dengue Virus Non-Structural Protein 5 (NS5) Using Molecular Dynamics. Crystals 2023, 13, 63. [Google Scholar] [CrossRef]
- Zong, K.; Li, W.; Xu, Y.; Zhao, X.; Cao, R.; Yan, H.; Li, X. Design, Synthesis, Evaluation and Molecular Dynamics Simulation of Dengue Virus NS5-RdRp Inhibitors. Pharmaceuticals 2023, 16, 1625. [Google Scholar] [CrossRef] [PubMed]
- Raj, U.; Varadwaj, P.K. Flavonoids as multi-target inhibitors for proteins associated with Ebola Virus: In Silico discovery using virtual screening and molecular docking studies. Pharmacogn. Mag. 2016, 8, 132–141. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Dwivedi, V.D.; Prasad Tripathi, I.P.; Bharadwaj, S.; Kaushik, A.C.; Sarad Kumar, M.S. Identification of new potent inhibitors of dengue virus NS3 protease from traditional Chinese medicine database. VirusDisease 2016, 27, 220–225. [Google Scholar] [CrossRef][Green Version]
- Racherla, R.G.; Katari, S.K.; Mohan, A.; Amineni, U.; Badur, M.; Chaudhury, A.; Nagaraja, M.; Kodavala, S.; Kante, M.; Kalawat, U. Molecular Characterization and Identification of Potential Inhibitors for ‘E’ Protein of Dengue Virus. Viruses 2022, 14, 940. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hasnat, S.; Akter, S.; Mim, M.M.; Tahcin, A.; Hoque, M.; Sutradhar, D.; Keya, M.A.A.; Sium, N.R.; Hossain, S.; et al. Computational identification of Vernonia cinerea-derived phytochemicals as potential inhibitors of nonstructural protein 1 (NSP1) in dengue virus serotype. Front. Pharmacol. 2024, 15, 1465827. [Google Scholar]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar]
- Roschek, B.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Alberte, Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar]
- Lu, P.; Zhang, T.; Ren, Y.; Rao, H.; Lei, J.; Zhao, G.; Wang, M.; Gong, D.; Cao, Z. A Literature Review on the Antiviral Mechanism of Luteolin. Nat. Prod. Commun. 2023, 18, 1934578X231171521. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. In vitro antiviral activity of Fisetin, Rutin and Naringenin against Dengue virus type. J. Med. Plants Res. 2011, 5, 5534–5539. [Google Scholar]

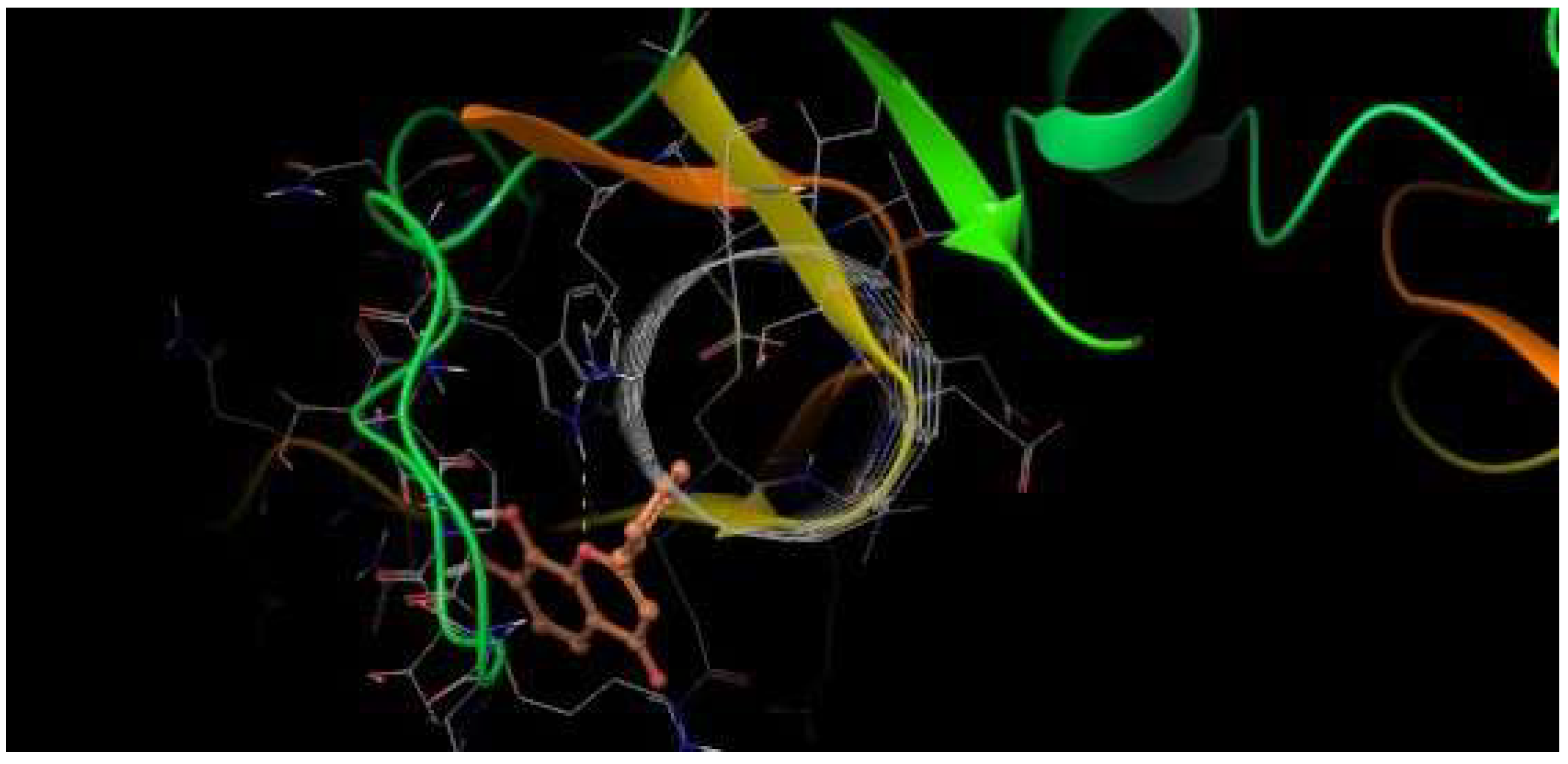
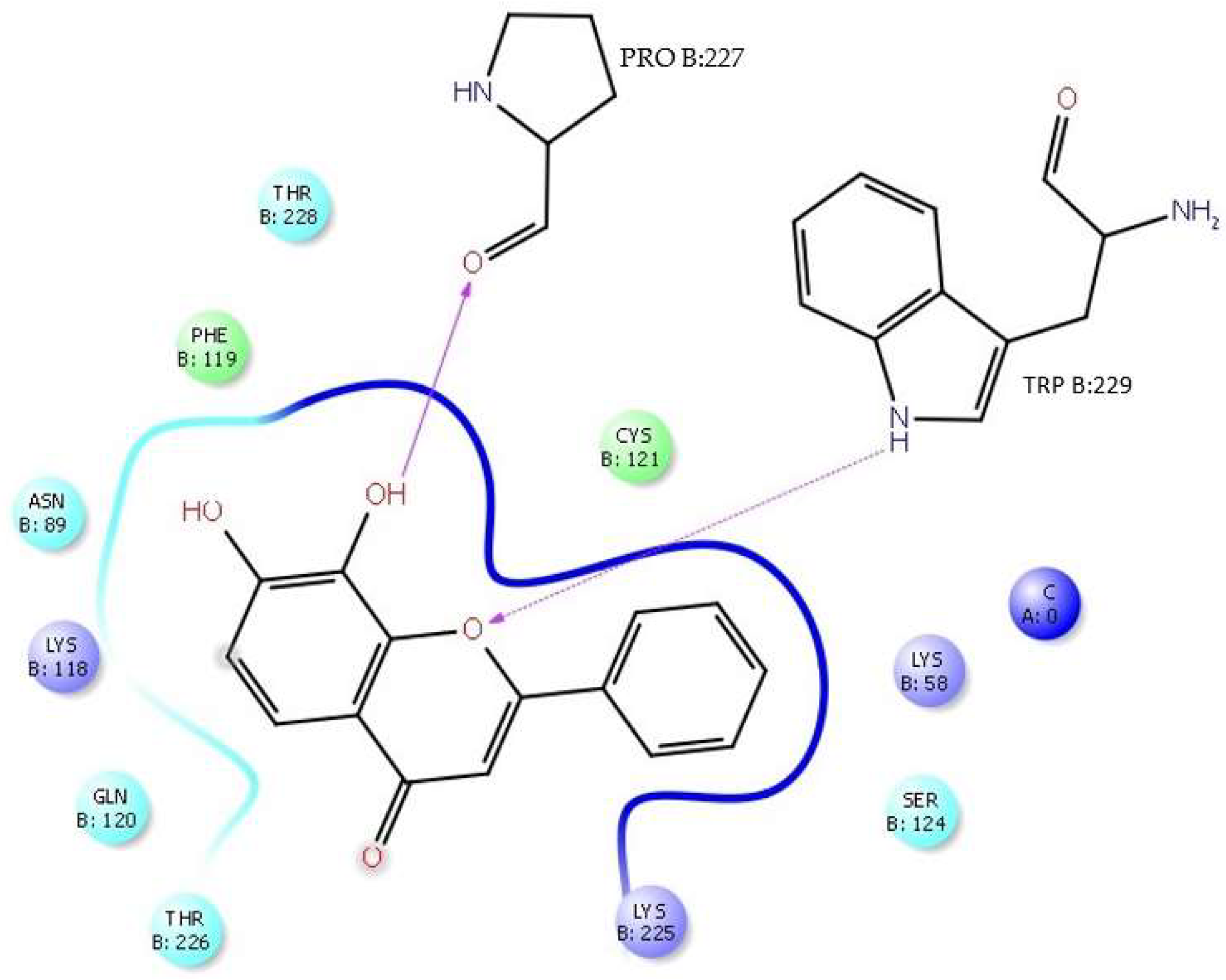
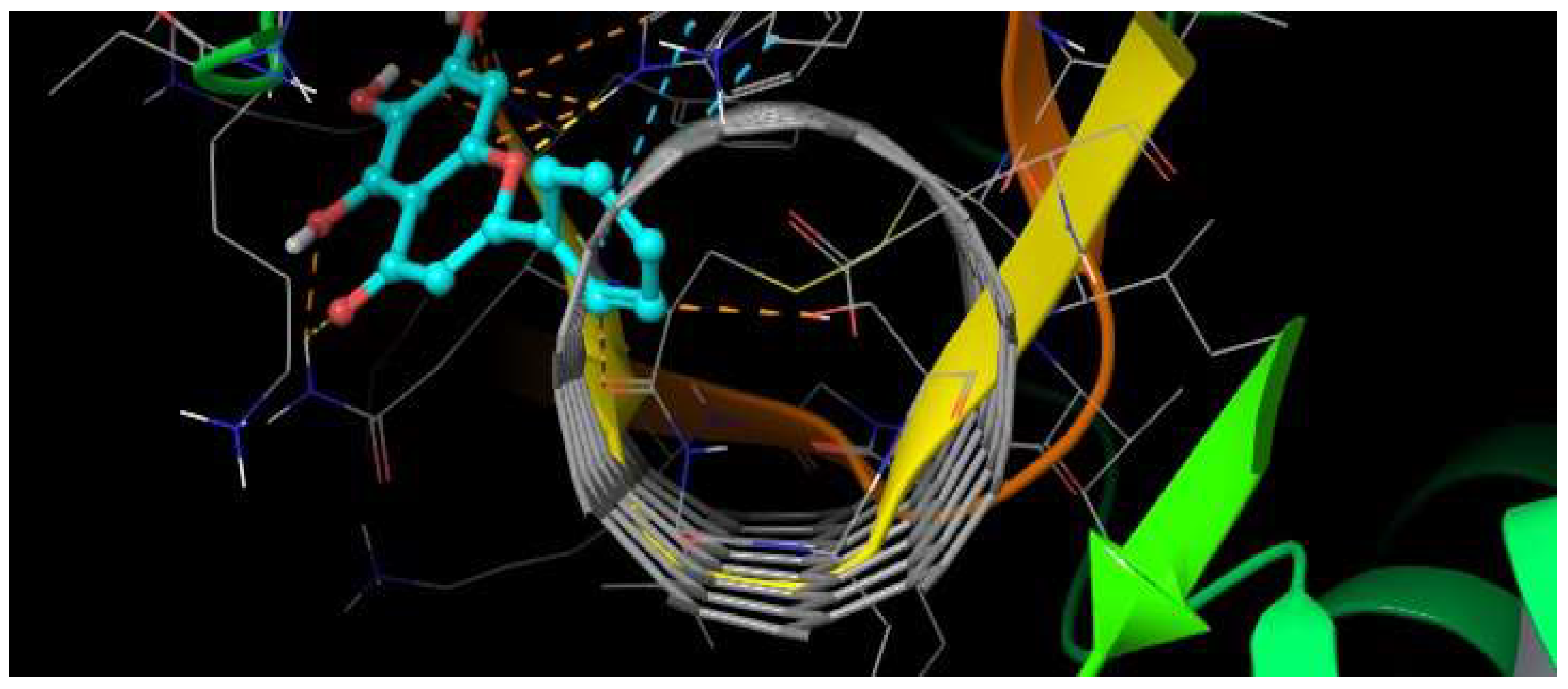
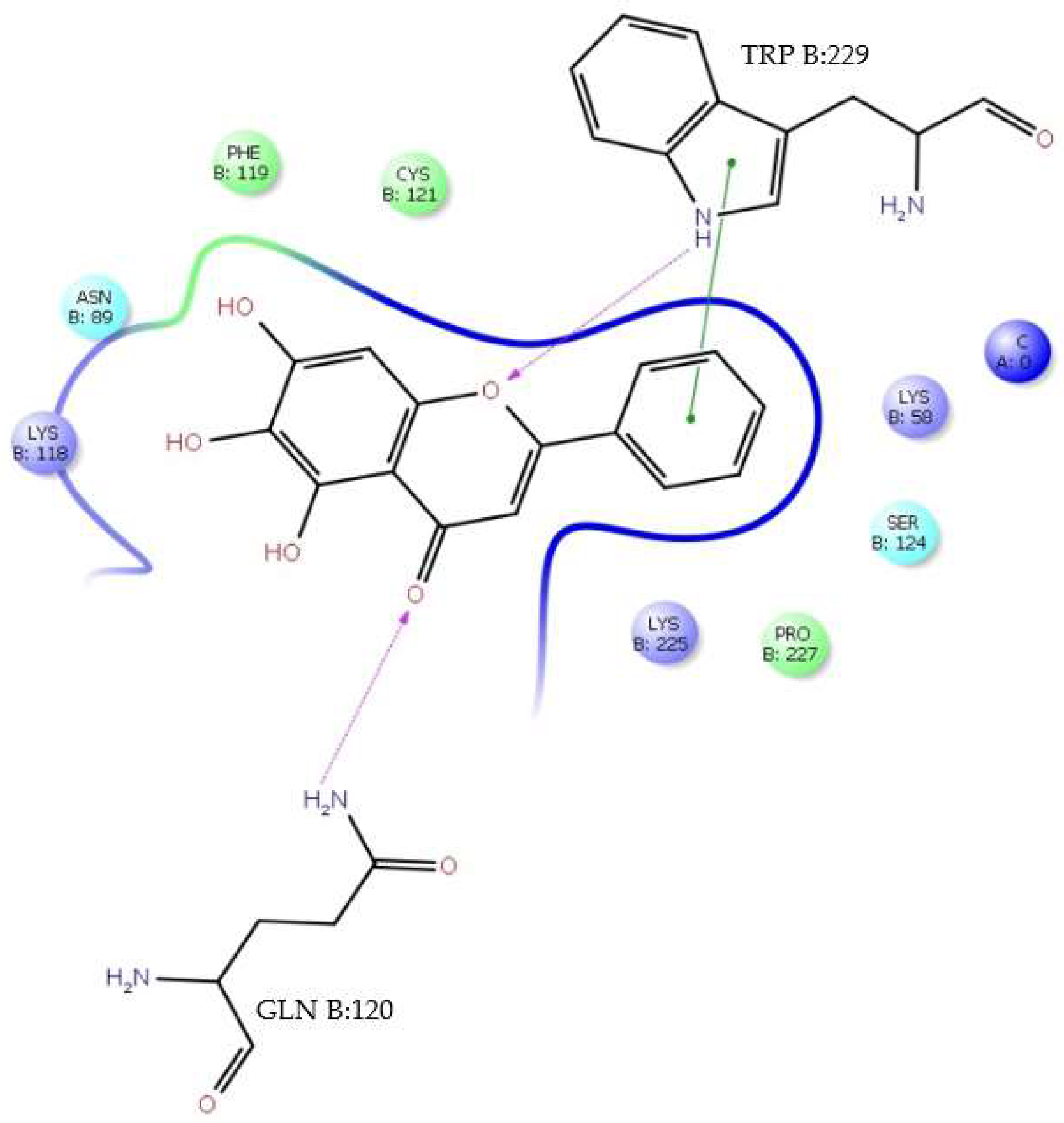

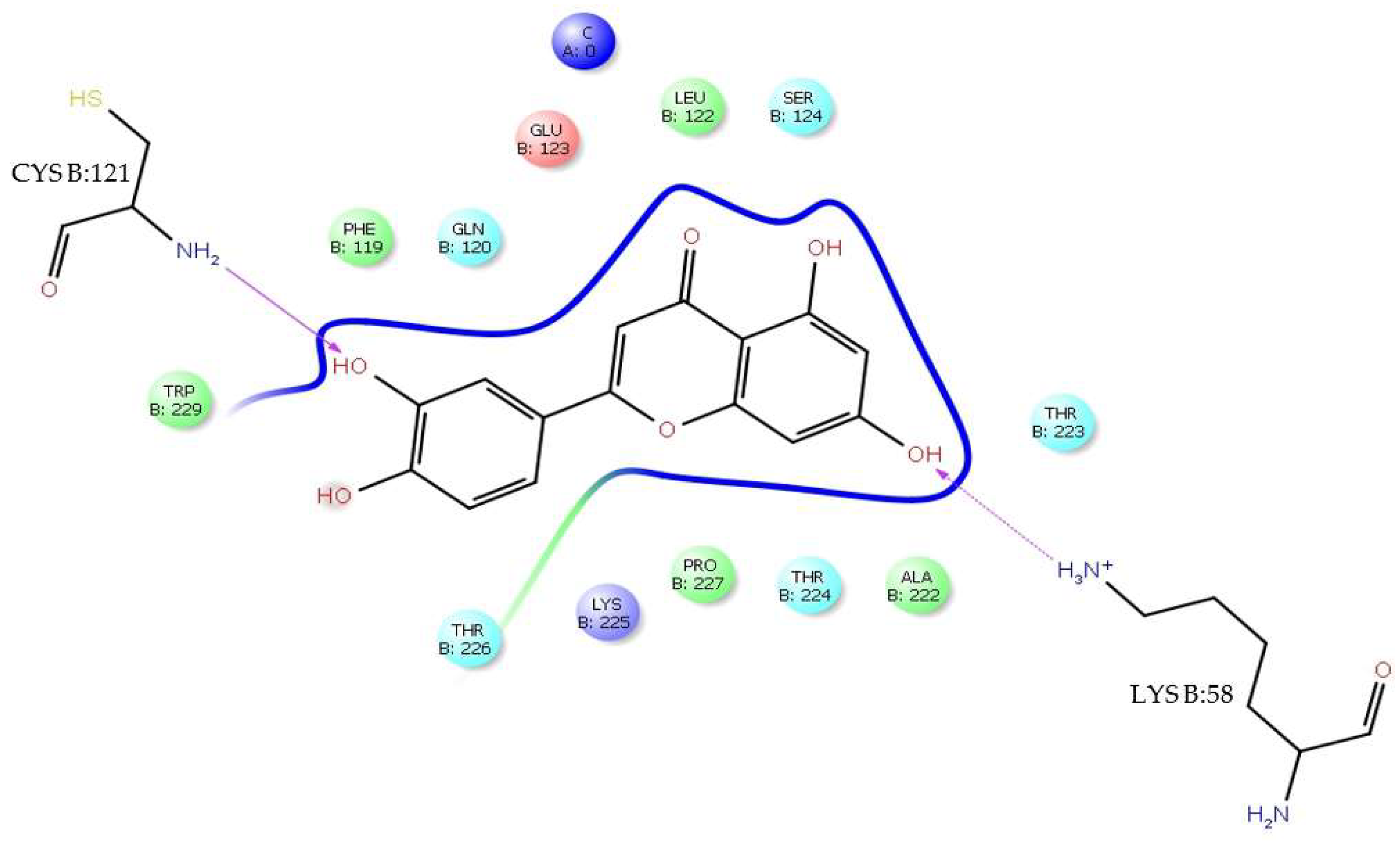
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.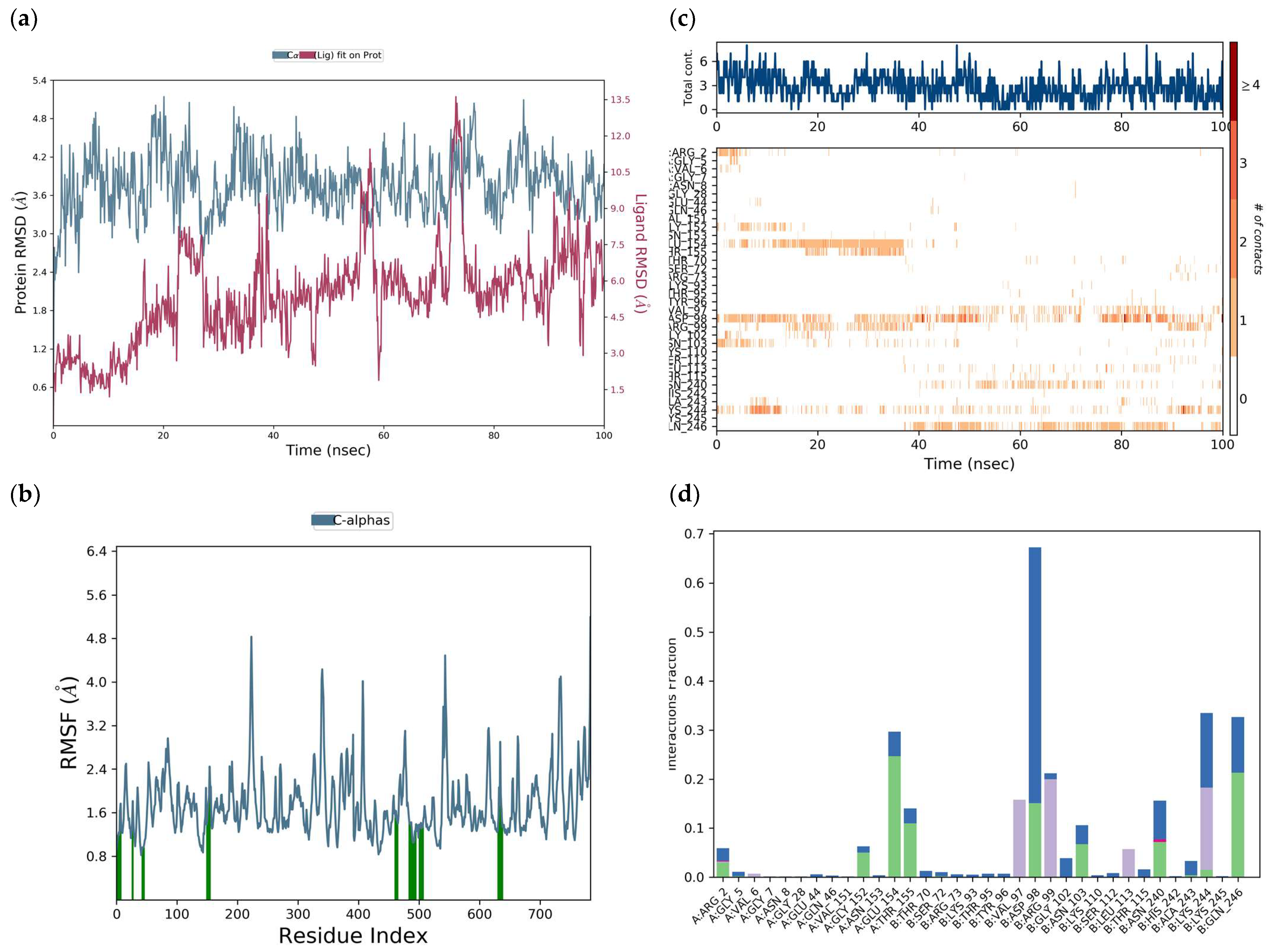
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.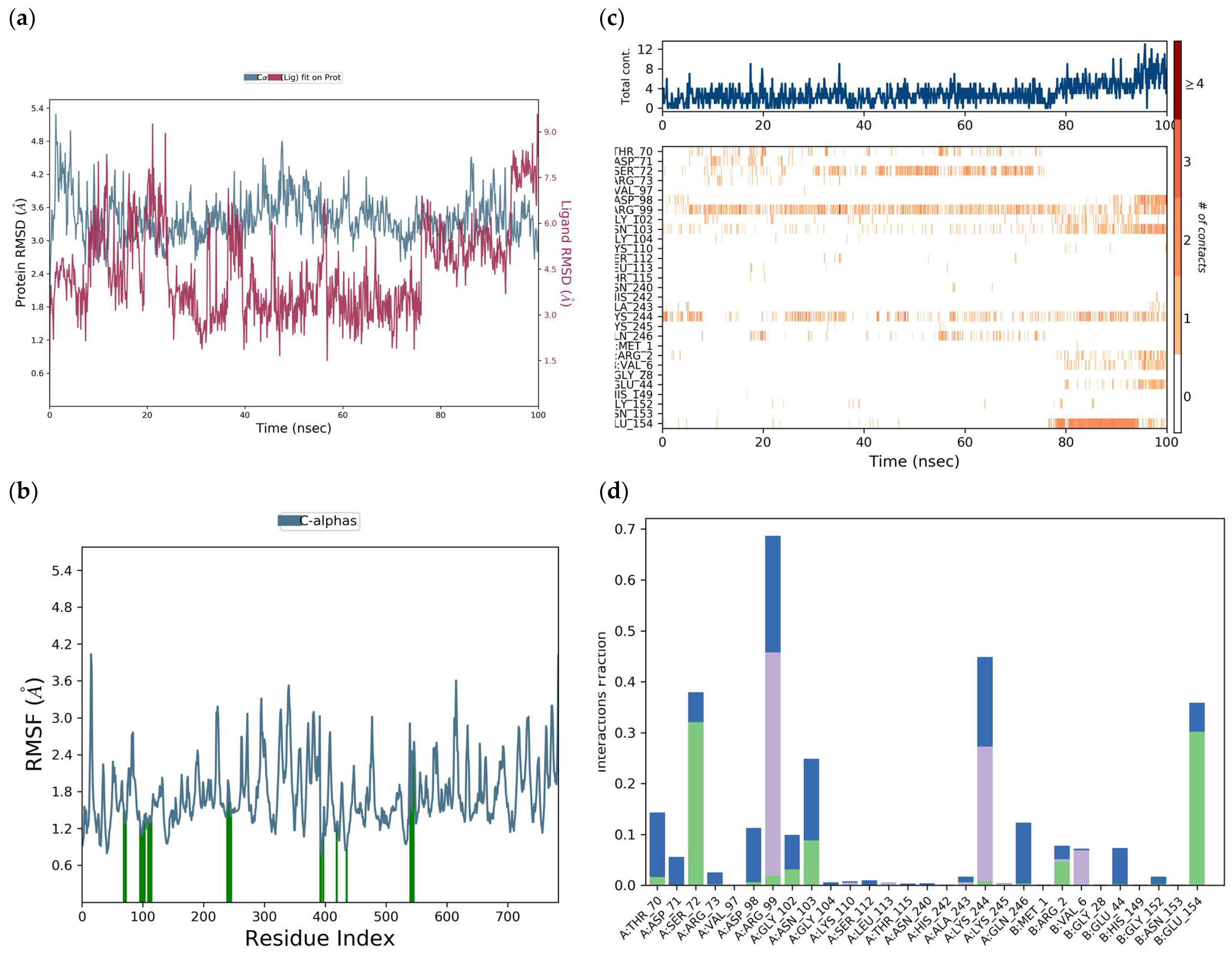
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.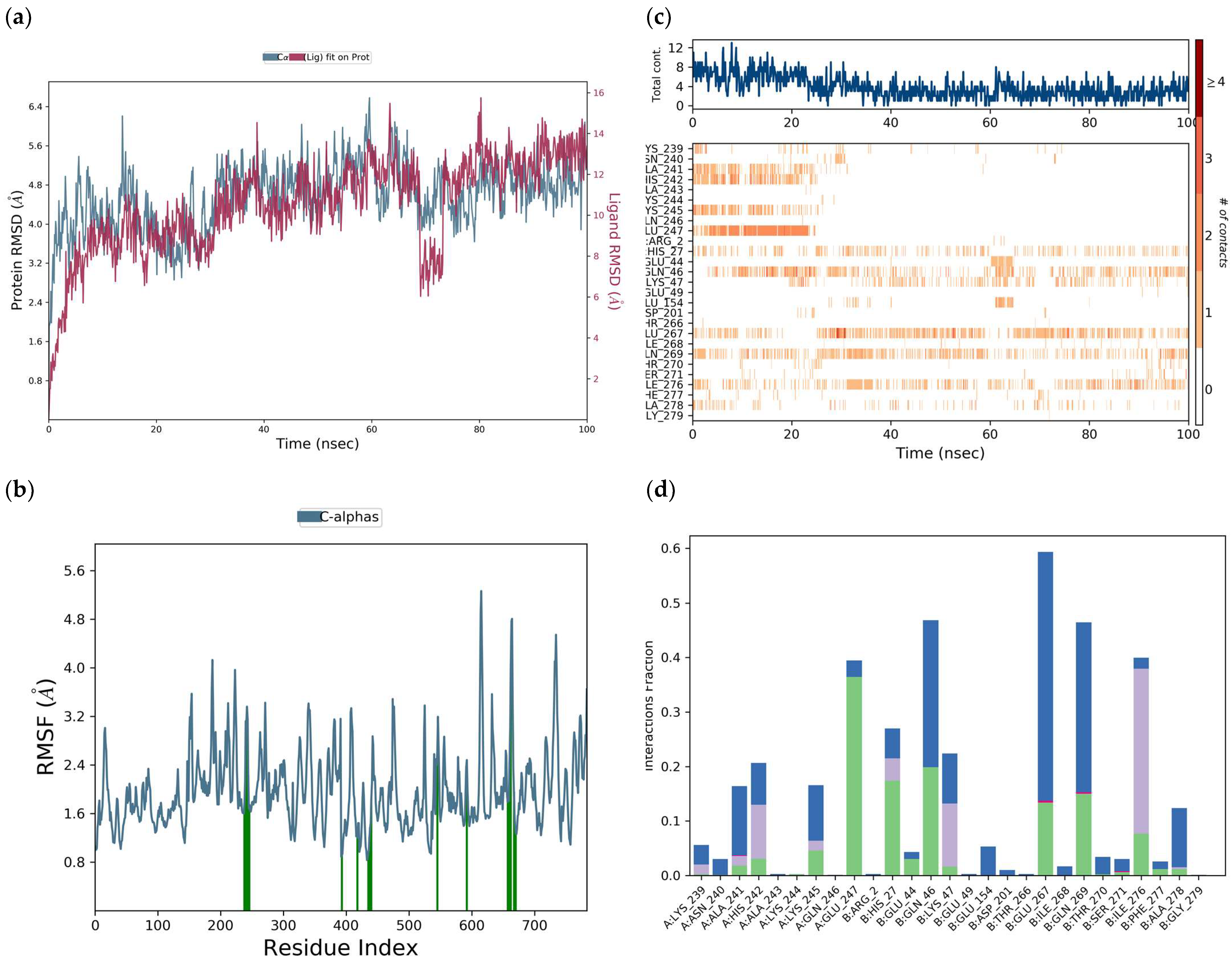
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.
 H-bonds,
H-bonds,  hydrophobic,
hydrophobic,  Ionic,
Ionic,  Water bridge.
Water bridge.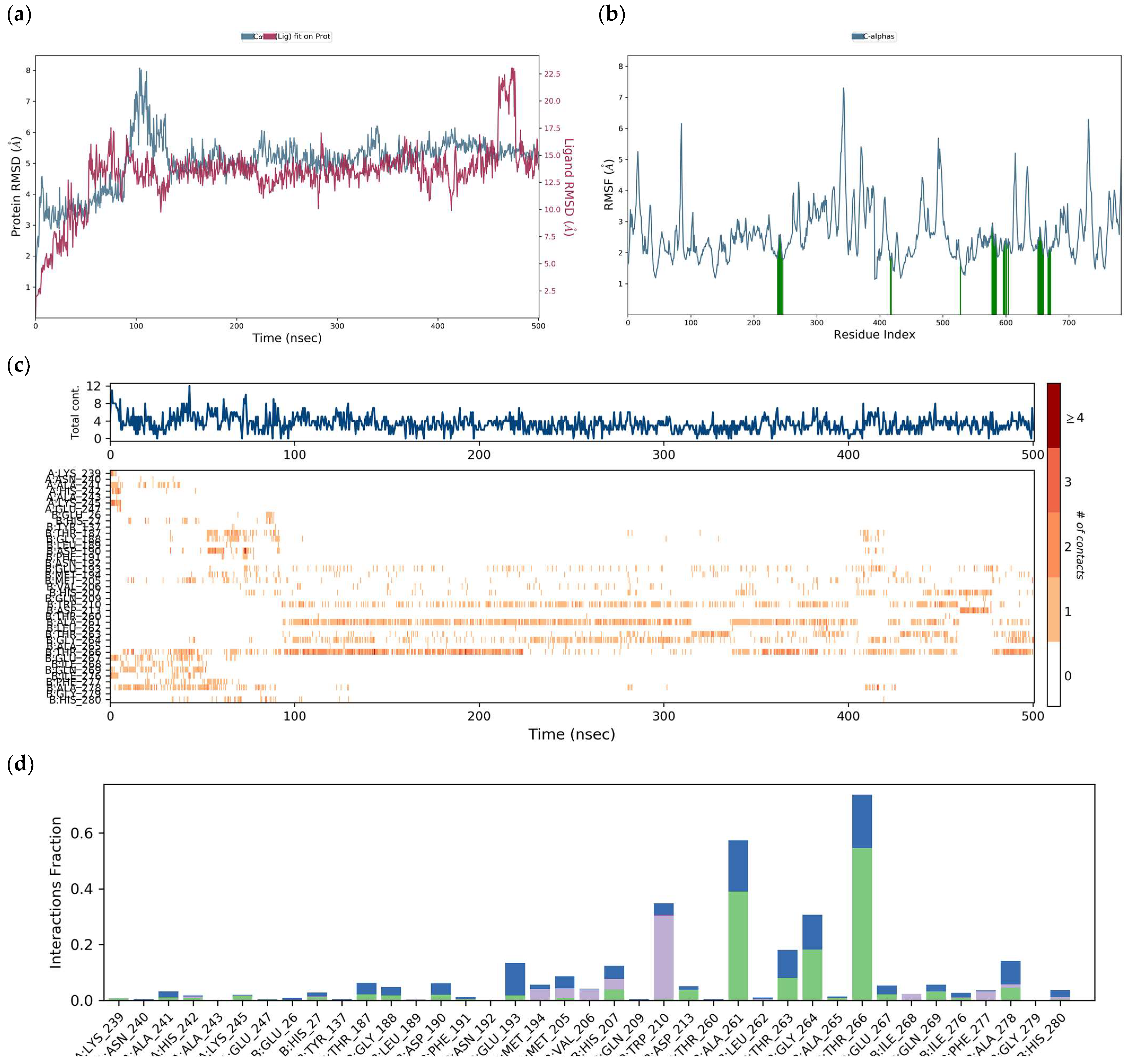
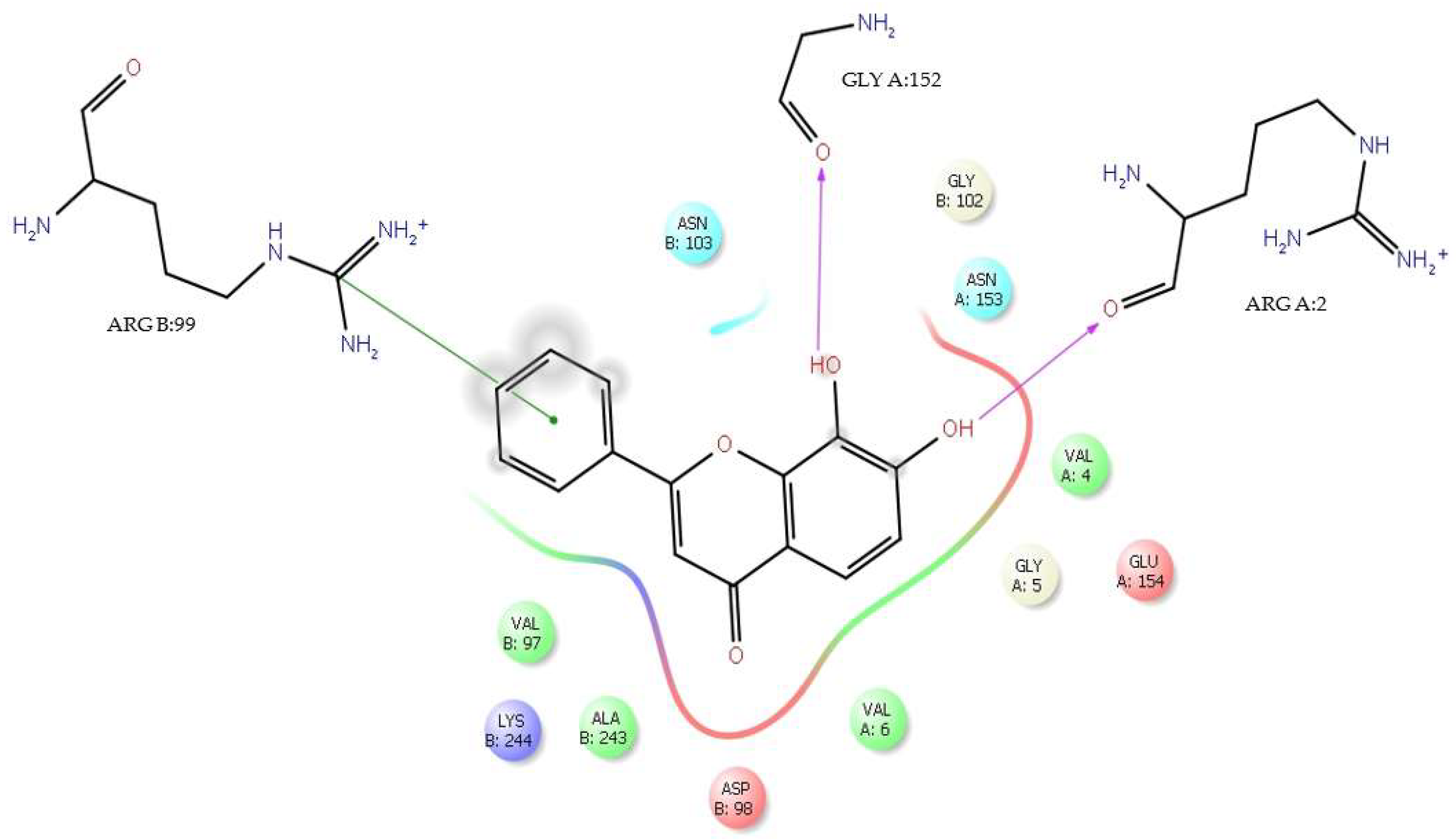
| Flavone | ΔG (Kcal/mol) | H-Bond Interaction with DENV E-3 | Residue/Flavone Interaction Functional Groups | Distance (Å) |
|---|---|---|---|---|
| Tropoflavin | −7.0 | GlyA:152 | C=O―8-OH | 2.37 |
| −4.49 | LysA:245 | NH3+―7-OH | 2.42 | |
| Baicalein | −3.3 | ArgA:99 | NH2―C=O | 2.45 |
| Luteolin | −5.19 | LysA:245 | C=O―3’-OH | 2.45 |
| Flavone | ΔG (kcal/mol) | π-Cation Interaction | Residue/Flavone Interaction Functional Groups | Distance (Å) |
|---|---|---|---|---|
| Baicalein | −6.4 | LysA:244 | NH3+—A ring | 3.72 |
| Interaction Type | Residue |
|---|---|
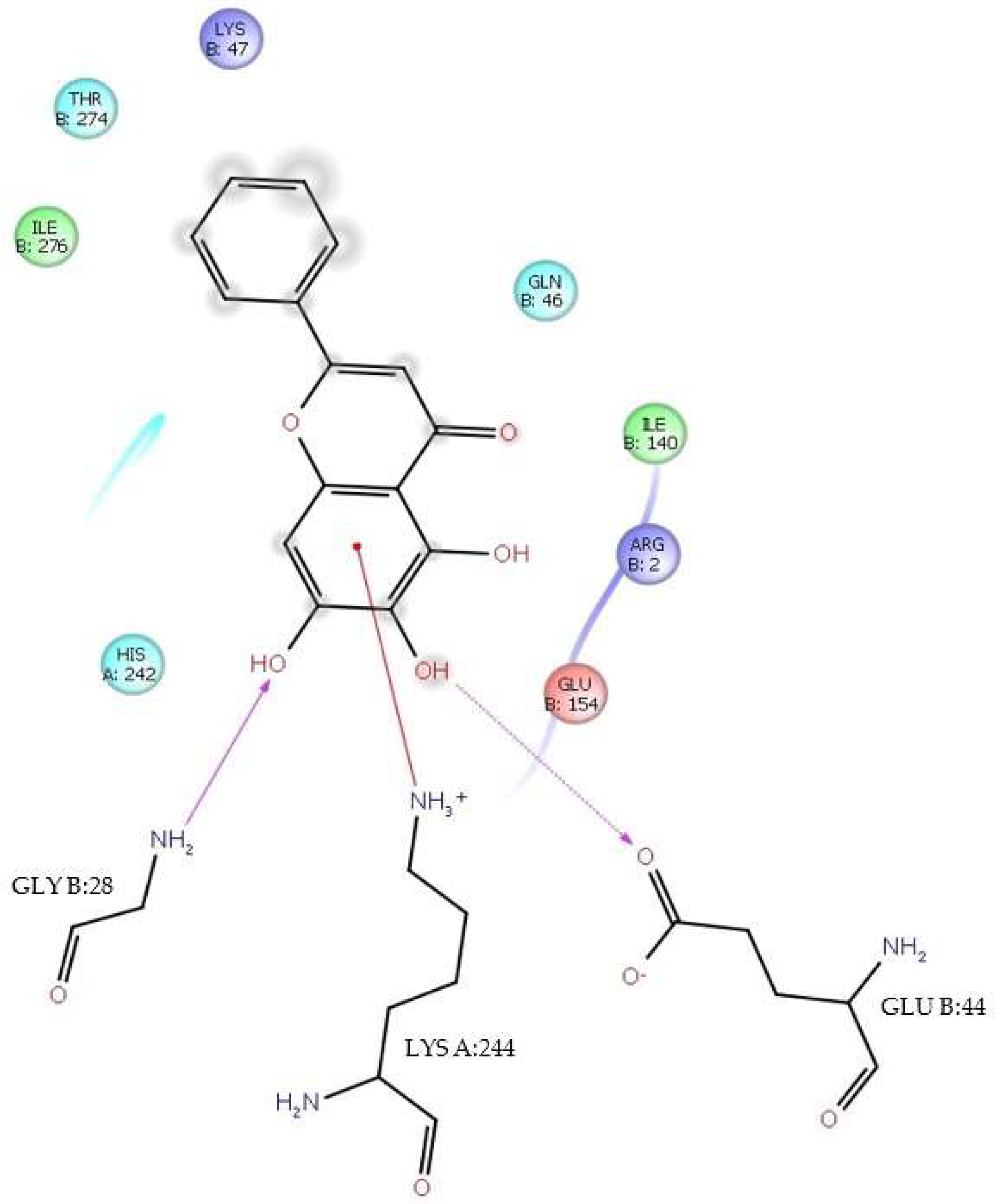
| H-bond | AsnB:103, GlyA:152, GluA:154, ArgA:2, AspB:98, AlaB:243, LysB:244 |
| Water bridge | AsnB:103, GlyB:102, GlyA:152, AsnA:153, GluA:154, ArgA:2, GlyA:5, AspB:98, AlaB:243, LysB:244 |
| Ionic | ArgA:2 |
| π-cation | ValA:6, LysB:244, ValB:97 |
| Interaction Type | Residue |
|---|---|
| H-bond | ArgA:99, GluB:154, LysA:244, AspA:98, GlyA:102, AsnA:103 |
| Water bridge | ArgA:99, GluB:154, GlnA:246, GlyB:152, AspA:98, GlyA:102, AsnA:103 |
| π-cation | ArgA:99, LysA:244, ValB:6 |
| Interaction Type | Residue |
|---|---|
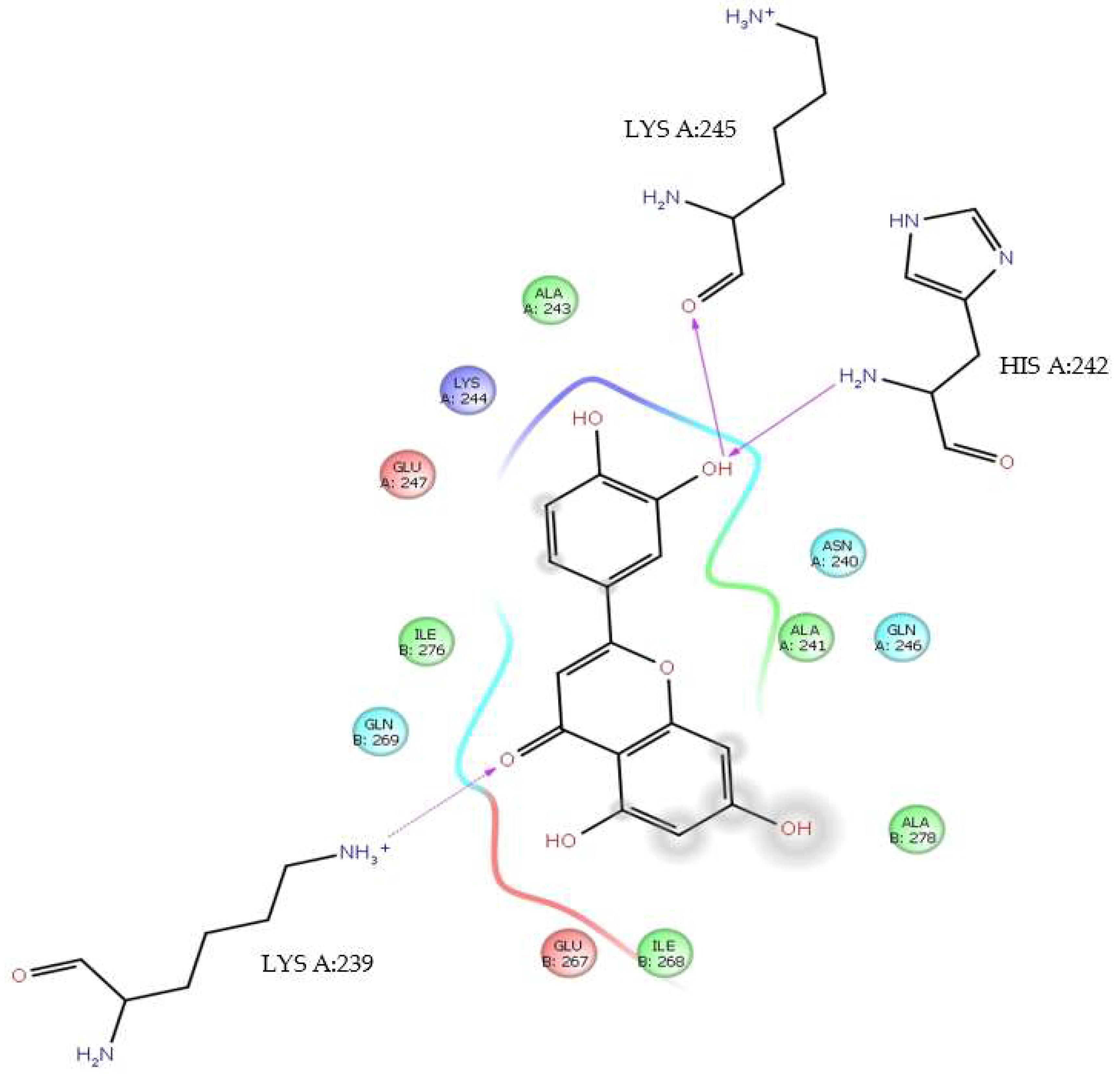
| H-bond | LysA:245, HisA:242, AlaA:241, GlnA:246, AlaB:278, GluB:267, GlnB:269, IleB:276, GluA:247 |
| Water bridge | LysA:245, HisA:242, AsnA:240, AlaA:241, GlnA:246, AlaB:278, GluB:267, IleB:268, LysA:239, GlnB:269, IleB:276, GluA:247 |
| Ionic | AlaA:241, GluB:267, GlnB:269 |
| π-cation | LysA:245, HisA:242, AlaA:241, AlaB:278, LysA:239, IleB:276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espíndola, C. Modeling and Molecular Dynamics Studies of Flavone―DENV E-3 Protein―SWCNT Interaction at the Flavonoid Binding Sites. Viruses 2025, 17, 525. https://doi.org/10.3390/v17040525
Espíndola C. Modeling and Molecular Dynamics Studies of Flavone―DENV E-3 Protein―SWCNT Interaction at the Flavonoid Binding Sites. Viruses. 2025; 17(4):525. https://doi.org/10.3390/v17040525
Chicago/Turabian StyleEspíndola, Cecilia. 2025. "Modeling and Molecular Dynamics Studies of Flavone―DENV E-3 Protein―SWCNT Interaction at the Flavonoid Binding Sites" Viruses 17, no. 4: 525. https://doi.org/10.3390/v17040525
APA StyleEspíndola, C. (2025). Modeling and Molecular Dynamics Studies of Flavone―DENV E-3 Protein―SWCNT Interaction at the Flavonoid Binding Sites. Viruses, 17(4), 525. https://doi.org/10.3390/v17040525






