Protection Against Rabies Induced by the Non-Replicative Viral Vectors MVA and Ad5 Expressing Rabies Glycoprotein
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viral Stocks
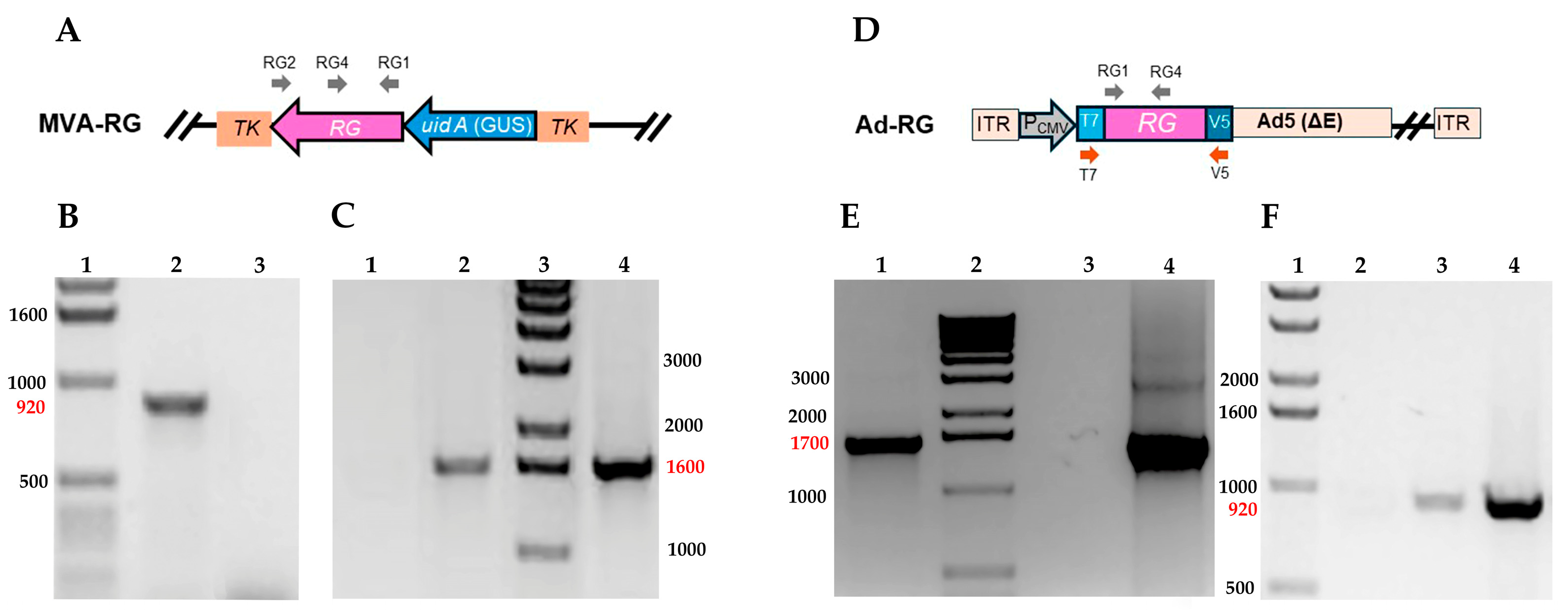
2.2. Construction of Recombinant MVA-RG
2.3. Generation of Recombinant Adenoviruses
2.4. Animals
2.5. Evaluation of Recombinant Viral Vector Efficacy in Homologous and Heterologous Immunization Schemes
2.6. Intracerebral RABV Challenge
3. Results
3.1. Construction and Molecular Characterization of Recombinant MVA and Ad5 Expressing Rabies Glycoprotein (RG)
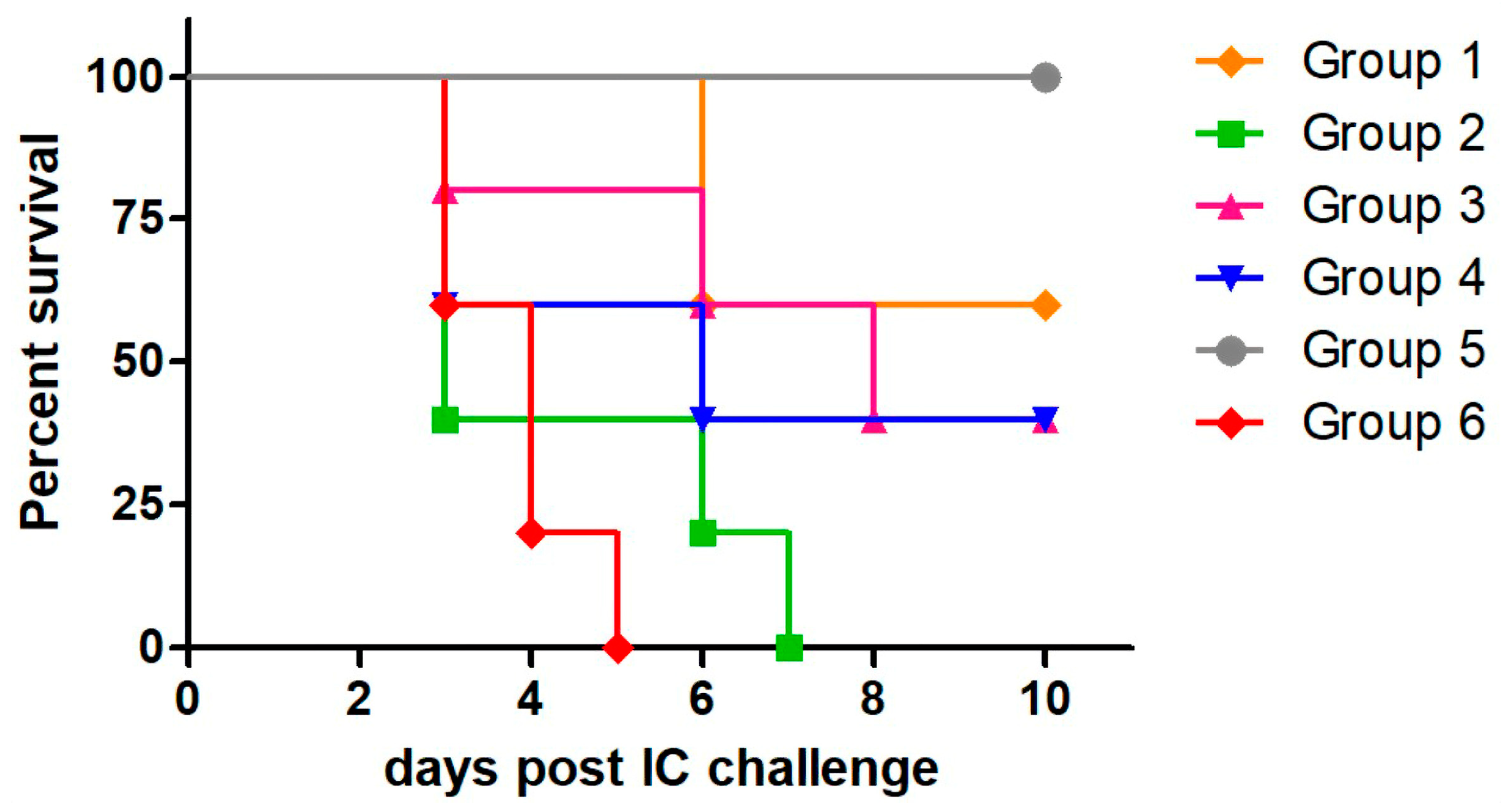
3.2. Protection Induced by MVA-RG and Ad-RG Against Intracerebral RABV Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wunner, W.H.; Conzelmann, K.-K. Rabies Virus. In Rabies, 4th ed.; Fooks, A.R., Jackson, A.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 43–81. ISBN 9780128187050. [Google Scholar]
- Kim, H.H.; Yang, D.K.; Nah, J.J.; Song, J.Y.; Cho, I.S. Comparison of the Protective Efficacy between Single and Combination of Recombinant Adenoviruses Expressing Complete and Truncated Glycoprotein, and Nucleoprotein of the Pathogenic Street Rabies Virus in Mice. Virol. J. 2017, 14, 122. [Google Scholar] [CrossRef]
- Ertl, H.C.J. Next Generation of Rabies Vaccines. In Rabies; Fooks, A.R., Jackson, A.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 509–526. ISBN 9780128187050. [Google Scholar]
- Rupprecht, C.E.; Buchanan, T.; Cliquet, F.; King, R.; Müller, T.; Yakobson, B.; Yang, D.K. A Global Perspective on Oral Vaccination of Wildlife against Rabies. J. Wildl. Dis. 2024, 60, 241–284. [Google Scholar] [CrossRef]
- Del Médico Zajac, M.; Garanzini, D.; Pérez, O.; Calamante, G. Recombinant Veterinary Vaccines Against Rabies: State of Art and Perspectives. In Emerging and Reemerging Viral Pathogens; Ennaji, M.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 225–242. ISBN 9780128149669. [Google Scholar]
- Wiktor, T.J.; Macfarlan, R.I.; Reagan, K.J.; Dietzschold, B.; Curtis, P.J.; Wunner, W.H.; Kieny, M.P.; Lathe, R.; Lecocq, J.P.; Mackett, M. Protection from Rabies by a Vaccinia Virus Recombinant Containing the Rabies Virus Glycoprotein Gene. Proc. Natl. Acad. Sci. USA 1984, 81, 7194–7198. [Google Scholar] [CrossRef]
- Yarosh, O.K.; Wandeler, A.I.; Graham, F.L.; Campbell, J.B.; Prevec, L. Human Adenovirus Type 5 Vectors Expressing Rabies Glycoprotein. Vaccine 1996, 14, 1257–1264. [Google Scholar] [CrossRef]
- Altenburg, A.F.; Kreijtz, J.H.C.M.; de Vries, R.D.; Song, F.; Fux, R.; Rimmelzwaan, G.F.; Sutter, G.; Volz, A. Modified Vaccinia Virus Ankara (MVA) as Production Platform for Vaccines against Influenza and Other Viral Respiratory Diseases. Viruses 2014, 6, 2735–2761. [Google Scholar] [CrossRef]
- Volz, A.; Sutter, G. Modified vaccinia virus Ankara: History, value in basic research, and current perspectives for vaccine development. Adv. Virus Res. 2017, 97, 187–243. [Google Scholar]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; Di Pilato, M.; Esteban, M. The Evolution of Poxvirus Vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef]
- Fougeroux, C.; Holst, P.J. Future Prospects for the Development of Cost-Effective Adenovirus Vaccines. Int. J. Mol. Sci. 2017, 18, 686. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; An, Y.; Chen, Z. Construction and Application of Adenoviral Vectors. Mol. Ther. Nucleic Acids 2023, 34, 102027. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef]
- Ferrer, M.F.; Del Médico Zajac, M.P.; Zanetti, F.A.; Valera, A.R.; Zabal, O.; Calamante, G. Recombinant MVA Expressing Secreted Glycoprotein D of BoHV-1 Induces Systemic and Mucosal Immunity in Animal Models. Viral Immunol. 2011, 24, 331–339. [Google Scholar] [CrossRef]
- Zanetti, F.A.; Rudak, L.; Micucci, M.; Conte Grand, D.; Luque, A.; Russo, S.; Taboga, O.; Perez, O.; Calamante, G. Obtención y Evaluación Preliminar de Un Virus Canarypox Recombinante Como Candidato a Vacuna Antirrábica. Rev. Argent. Microbiol. 2012, 44, 75–84. [Google Scholar]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Weyer, J.; Rupprecht, C.E.; Mansc, J.; Viljoen, G.J.; Nel, L.H. Generation and Evaluation of a Recombinant Modified Vaccinia Virus Ankara Vaccine for Rabies. Vaccine 2007, 25, 4213–4222. [Google Scholar] [CrossRef]
- Jackson, L.A.; Frey, S.E.; El Sahly, H.M.; Mulligan, M.J.; Winokur, P.L.; Kotloff, K.L.; Campbell, J.D.; Atmar, R.L.; Graham, I.; Anderson, E.J.; et al. Safety and Immunogenicity of a Modified Vaccinia Ankara Vaccine Using Three Immunization Schedules and Two Modes of Delivery: A Randomized Clinical Non-Inferiority Trial. Vaccine 2017, 35, 1675–1682. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Nitayaphan, S.; Chariyalertsak, S.; Kaewkungwal, J.; Dawson, P.; Dhitavat, J.; Phonrat, B.; Akapirat, S.; Karasavvas, N.; Wieczorek, L.; et al. Late Boosting of the RV144 Regimen with AIDSVAX B/E and ALVAC-HIV in HIV-Uninfected Thai Volunteers: A Double-Blind, Randomised Controlled Trial. Lancet HIV 2020, 7, e238–e248. [Google Scholar] [CrossRef]
- Palgen, J.L.; Tchitchek, N.; Rodriguez-Pozo, A.; Jouhault, Q.; Abdelhouahab, H.; Dereuddre-Bosquet, N.; Contreras, V.; Martinon, F.; Cosma, A.; Lévy, Y.; et al. Innate and Secondary Humoral Responses Are Improved by Increasing the Time between MVA Vaccine Immunizations. NPJ Vaccines 2020, 5, 24. [Google Scholar] [CrossRef]
- Kalodimou, G.; Jany, S.; Freudenstein, A.; Schwarz, J.H.; Limpinsel, L.; Rohde, C.; Kupke, A.; Becker, S.; Volz, A.; Tscherne, A.; et al. Short- and Long-Interval Prime-Boost Vaccination with the Candidate Vaccines MVA-SARS-2-ST and MVA-SARS-2-S Induces Comparable Humoral and Cell-Mediated Immunity in Mice. Viruses 2023, 15, 1180. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Padron-Regalado, E.; Thompson, C.P.; Kupke, A.; Wells, D.; Sloan, M.A.; Grehan, K.; Temperton, N.; Lambe, T.; Warimwe, G.; et al. ChAdOx1 and MVA Based Vaccine Candidates against MERS-CoV Elicit Neutralising Antibodies and Cellular Immune Responses in Mice. Vaccine 2017, 35, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Wan, M.; Shi, W.; Liu, J.; Zhao, Z.; Xu, Y.; Wei, W.; Sun, B.; Gao, F.; Cai, L.; et al. Performance of Homologous and Heterologous Prime-Boost Immunization Regimens of Recombinant Adenovirus and Modified Vaccinia Virus Ankara Expressing an Ag85B-TB10.4 Fusion Protein against Mycobacterium Tuberculosis. J. Microbiol. Biotechnol. 2018, 28, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Dema, B.; Rodriguez-Garcia, R.; López-Camacho, C.; Leoratti, F.M.S.; Lall, A.; Remarque, E.J.; Kocken, C.H.M.; Reyes-Sandoval, A. Evaluation of Chimpanzee Adenovirus and MVA Expressing TRAP and CSP from Plasmodium Cynomolgi to Prevent Malaria Relapse in Nonhuman Primates. Vaccines 2020, 8, 363. [Google Scholar] [CrossRef]
- Bruña-Romero, O.; Gonzá Lez-Aseguinolaza, G.; Hafalla, J.C.R.; Tsuji, M.; Nussenzweig, R.S. Complete, Long-Lasting Protection against Malaria of Mice Primed and Boosted with Two Distinct Viral Vectors Expressing the Same Plasmodial Antigen. Proc. Natl. Acad. Sci. USA 2001, 98, 11491–11496. [Google Scholar] [CrossRef]
- Coughlan, L.; Sridhar, S.; Payne, R.; Edmans, M.; Milicic, A.; Venkatraman, N.; Lugonja, B.; Clifton, L.; Qi, C.; Folegatti, P.M.; et al. Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. EBioMedicine 2018, 29, 146–154. [Google Scholar] [CrossRef]
- Paris, R.M.; Kim, J.H.; Robb, M.L.; Michael, N.L. Prime-Boost Immunization with Poxvirus or Adenovirus Vectors as a Strategy to Develop a Protective Vaccine for HIV-1. Expert Rev. Vaccines 2010, 9, 1055–1069. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Flaxman, A.; Jenkin, D.; Makinson, R.; Kingham-Page, L.; Bellamy, D.; Lopez, F.R.; Sheridan, J.; Poulton, I.; Aboagye, J.; et al. Safety and Immunogenicity of Adenovirus and Poxvirus Vectored Vaccines Against a Mycobacterium Avium Complex Subspecies. Vaccines 2021, 9, 262. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, W.; Yan, L.; Sun, P.; Xu, T.; Zhu, Y.; Liu, L.; Tian, L.; He, H.; Wei, Y.; et al. Recombinant Human Adenovirus Type 5 Co-Expressing RABV G and SFTSV Gn Induces Protective Immunity Against Rabies Virus and Severe Fever With Thrombocytopenia Syndrome Virus in Mice. Front. Microbiol. 2020, 11, 1473. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, Z.; Xue, X.; Zheng, W.; Xu, T.; Liu, L.; Tian, L.; Wang, X.; He, H.; Zheng, X. A Bivalent Human Adenovirus Type 5 Vaccine Expressing the Rabies Virus Glycoprotein and Canine Distemper Virus Hemagglutinin Protein Confers Protective Immunity in Mice and Foxes. Front. Microbiol. 2020, 11, 1070. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, N.; Vemula, S.V.; Couëtil, L.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Impact of Preexisting Adenovirus Vector Immunity on Immunogenicity and Protection Conferred with an Adenovirus-Based H5N1 Influenza Vaccine. PLoS ONE 2012, 7, e33428. [Google Scholar] [CrossRef]
- De Andrade Pereira, B.; Bouillet, L.E.M.; Dorigo, N.A.; Fraefel, C.; Bruna-Romero, O. Adenovirus Specific Pre-Immunity Induced by Natural Route of Infection Does Not Impair Transduction by Adenoviral Vaccine Vectors in Mice. PLoS ONE 2015, 10, e0145260. [Google Scholar] [CrossRef]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-Existing Immunity against Ad Vectors: Humoral, Cellular, and Innate Response, What’s Important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Xiang, Z.Q.; Greenberg, L.; Ertl, H.C.; Rupprecht, C.E. Protection of Non-Human Primates against Rabies with an Adenovirus Recombinant Vaccine. Virology 2014, 450-451, 243–249. [Google Scholar] [CrossRef]
- Napolitano, F.; Merone, R.; Abbate, A.; Ammendola, V.; Horncastle, E.; Lanzaro, F.; Esposito, M.; Contino, A.M.; Sbrocchi, R.; Sommella, A.; et al. A next Generation Vaccine against Human Rabies Based on a Single Dose of a Chimpanzee Adenovirus Vector Serotype c. PLoS Negl. Trop. Dis. 2020, 14, e0008459. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, D.; Ritchie, A.J.; Aboagye, J.; Fedosyuk, S.; Thorley, L.; Provstgaad-Morys, S.; Sanders, H.; Bellamy, D.; Makinson, R.; Xiang, Z.Q.; et al. Safety and Immunogenicity of a Simian-Adenovirus-Vectored Rabies Vaccine: An Open-Label, Non-Randomised, Dose-Escalation, First-in-Human, Single-Centre, Phase 1 Clinical Trial. Lancet Microbe 2022, 3, e663–e671. [Google Scholar] [CrossRef] [PubMed]
- Phadke, V.K.; Gromer, D.J.; Rebolledo, P.A.; Graciaa, D.S.; Wiley, Z.; Sherman, A.C.; Scherer, E.M.; Leary, M.; Girmay, T.; McCullough, M.P.; et al. Safety and Immunogenicity of a ChAd155-Vectored Rabies Vaccine Compared with Inactivated, Purified Chick Embryo Cell Rabies Vaccine in Healthy Adults. Vaccine 2024, 42, 126441. [Google Scholar] [CrossRef]

| Prime | Boost | |
|---|---|---|
| Group 1 | MVA-RG (4 × 107 PFU, IP) | MVA-RG (4 × 107 PFU, IP) |
| Group 2 | MVA-GFP (1 × 107 PFU, IP) | MVA-GFP (1 × 107 PFU, IP) |
| Group 3 | Ad-RG (1 × 108 PFU, IM) | MVA-RG (4 × 107 PFU, IP) |
| Group 4 | Ad-RG (1 × 108 PFU, IM) | MVA-GFP (1 × 107 PFU, IP) |
| Group 5 | Commercial vaccine (0.25 mL, IP) | Commercial vaccine (0.25 mL, IP) |
| Group 6 | PBS (0.8 mL, IP) | PBS (0.8 mL, IP) |
| Group | Surviving Animals/Total Animals | Protection After Challenge (%) |
|---|---|---|
| 1 | 3/5 | 60 |
| 2 | 0/5 | 0 |
| 3 | 2/5 | 40 |
| 4 | 2/5 | 40 |
| 5 | 10/10 | 100 |
| 6 | 0/10 | 0 |
| Prime | Boost | |
|---|---|---|
| Group 1 | Ad-RG 1 × 106 PFU (IM) | Ad-RG 1 × 106 PFU (IM) |
| Group 2 | Ad-RG 1 × 107 PFU (IM) | Ad-RG 1 × 107 PFU (IM) |
| Group 3 | Ad-RG 1 × 106 PFU (IM) | Ad-RG 1 × 107 PFU (IM) |
| Group 4 | Ad-GFP 1 × 106 PFU (IM) | Ad-GFP 1 × 106 PFU (IM) |
| Group 5 | Commercial vaccine (0.25 mL, IP) | Commercial vaccine (0.25 mL, IP) |
| Group 6 | PBS (0.8 mL, IP) | PBS (0.8 mL, IP) |
| Group | Surviving Animals/Total Animals | Protection After Challenge (%) |
|---|---|---|
| 1 | 7/10 | 70 |
| 2 | 3/5 * | 60 |
| 3 | 10/10 | 100 |
| 4 | 0/5 | 0 |
| 5 | 10/10 | 100 |
| 6 | 10/10 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garanzini, D.P.; Micucci, M.A.; Torres Lopez, A.; Perez, O.; Calamante, G.; Del Medico Zajac, M.P. Protection Against Rabies Induced by the Non-Replicative Viral Vectors MVA and Ad5 Expressing Rabies Glycoprotein. Viruses 2025, 17, 476. https://doi.org/10.3390/v17040476
Garanzini DP, Micucci MA, Torres Lopez A, Perez O, Calamante G, Del Medico Zajac MP. Protection Against Rabies Induced by the Non-Replicative Viral Vectors MVA and Ad5 Expressing Rabies Glycoprotein. Viruses. 2025; 17(4):476. https://doi.org/10.3390/v17040476
Chicago/Turabian StyleGaranzini, Debora Patricia, Matias Ariel Micucci, Annalies Torres Lopez, Oscar Perez, Gabriela Calamante, and Maria Paula Del Medico Zajac. 2025. "Protection Against Rabies Induced by the Non-Replicative Viral Vectors MVA and Ad5 Expressing Rabies Glycoprotein" Viruses 17, no. 4: 476. https://doi.org/10.3390/v17040476
APA StyleGaranzini, D. P., Micucci, M. A., Torres Lopez, A., Perez, O., Calamante, G., & Del Medico Zajac, M. P. (2025). Protection Against Rabies Induced by the Non-Replicative Viral Vectors MVA and Ad5 Expressing Rabies Glycoprotein. Viruses, 17(4), 476. https://doi.org/10.3390/v17040476










