Development of IgY-Based Passive Immunization Against Tilapia Lake Virus: Development and In Vitro Neutralization Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of a Recombinant TiLV-S4 Antigen
2.2. Chicken Immunization and Egg Collection
2.3. Total IgY Extraction
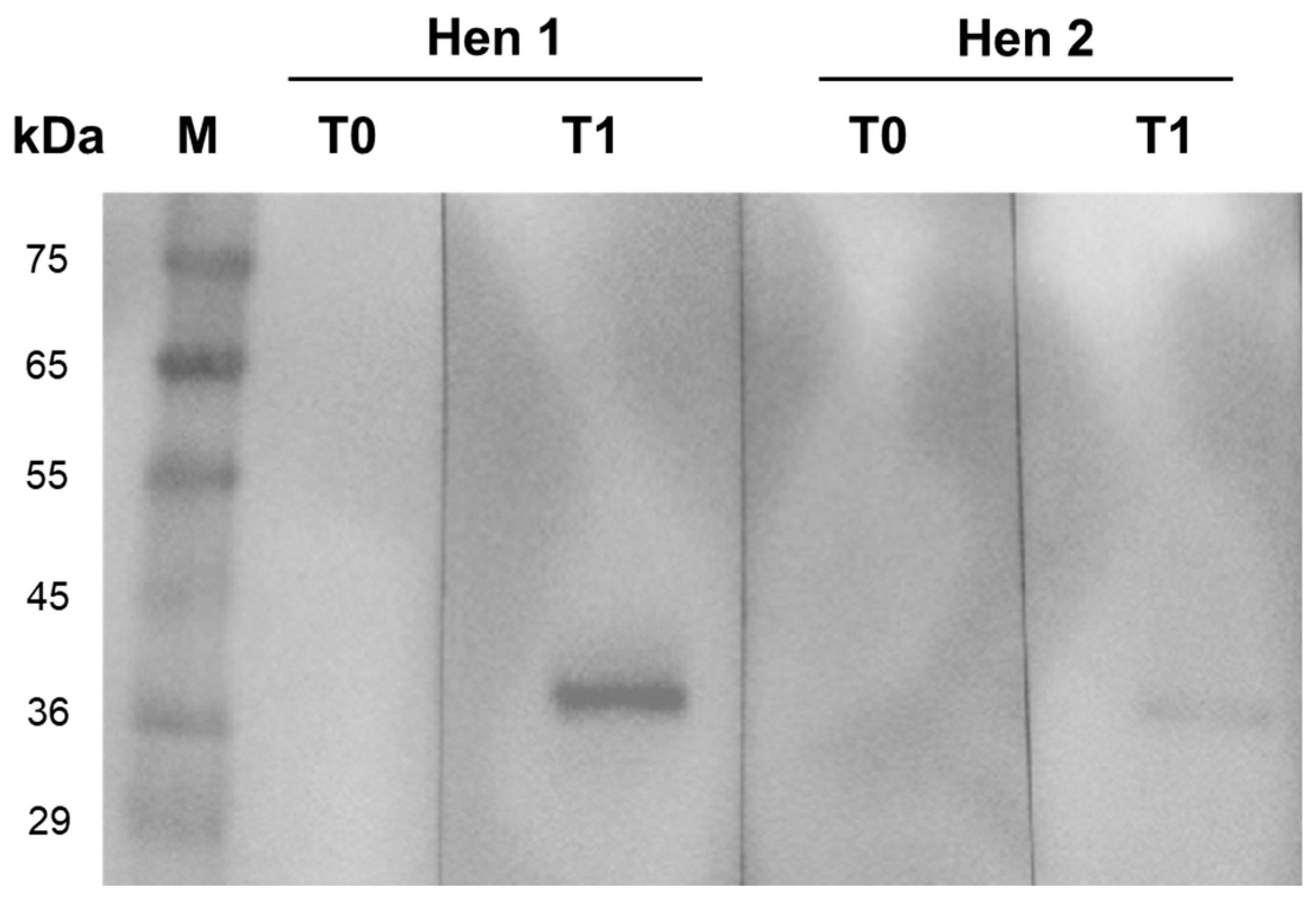
2.4. IgY Characterization by SDS-PAGE and Western Blot Analysis
2.5. In Vitro TiLV Neutralization Using Anti-TiLV-S4 IgY Antibodies
2.6. Virus Titration
2.7. Immunofluorescence Assay to Detect TiLV in the RHTiB Cell Line
2.8. Statistical Analysis
3. Results
3.1. Immunization and Preparation of Chicken IgY
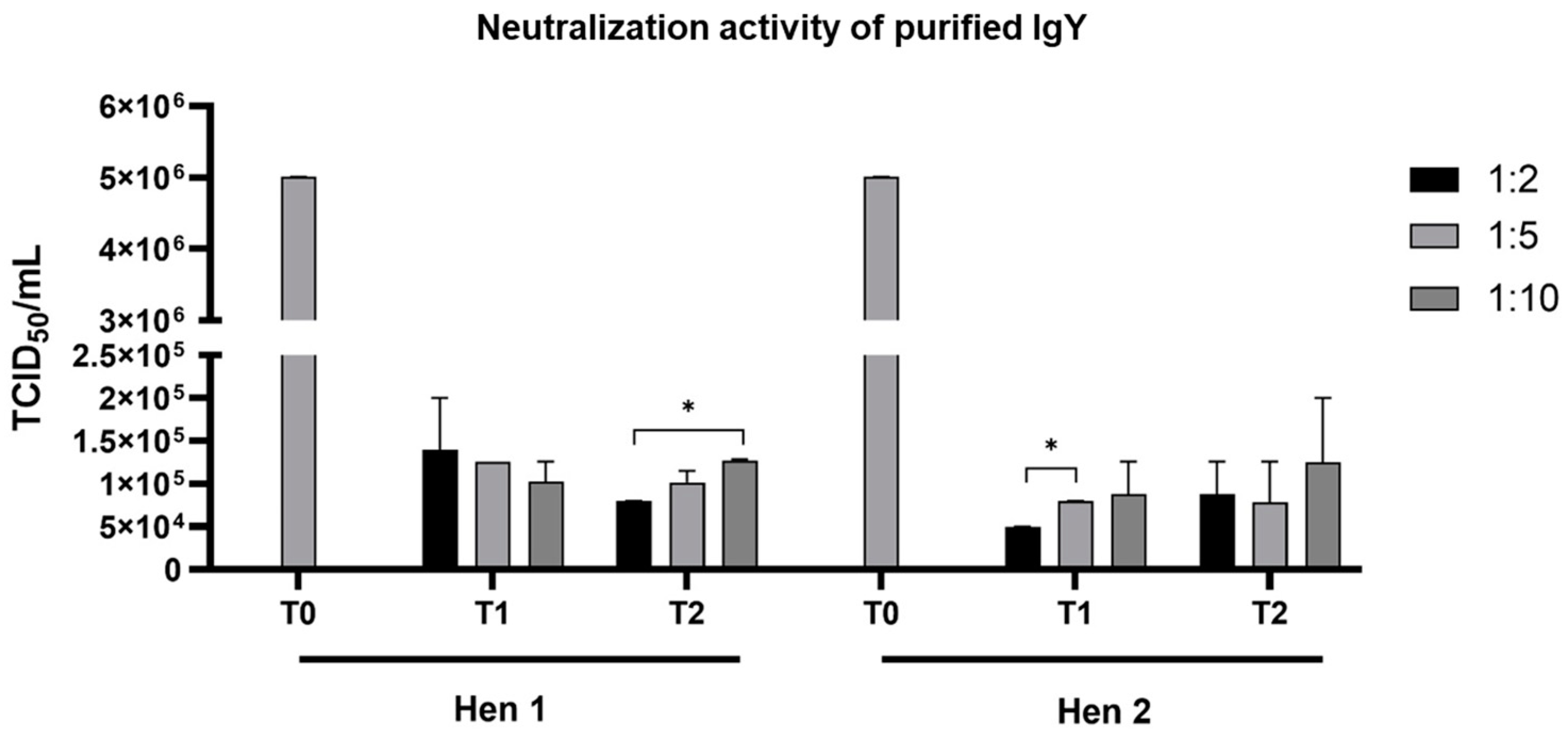
3.2. Neutralization of IgY Against TiLV
3.3. Immunofluorescence Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surachetpong, W.; Roy, S.R.K.; Nicholson, P. Tilapia lake virus: The story so far. J. Fish Dis. 2020, 43, 1115–1132. [Google Scholar] [CrossRef]
- Kembou-Ringert, J.E.; Steinhagen, D.; Readman, J.; Daly, J.M.; Adamek, M. Tilapia Lake Virus Vaccine Development: A Review on the Recent Advances. Vaccines 2023, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, E.; Mishra, N.; Briese, T.; Zody, M.C.; Tsofack, J.E.K.; Zamostiano, R.; Berkowitz, A.; Ng, J.; Nitido, A.; Corvelo, A.; et al. Characterization of a Novel Orthomyxo-like Virus Causing Mass Die-Offs of Tilapia. mBio 2016, 7, e00431-16. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Krupovic, M.; Surachetpong, W.; Wolf, Y.I.; Kuhn, J.H. ICTV Virus Taxonomy Profile: Amnoonviridae 2023. J. Gen. Virol. 2023, 104, 001903. [Google Scholar] [CrossRef] [PubMed]
- Clyde, C.W.; Tan, J.P.; Yeap, S.K.; Yong, C.Y. Current updates on viral infections affecting tilapia. In Aquaculture and Fisheries; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Liamnimitr, P.; Thammatorn, W.; U-thoomporn, S.; Tattiyapong, P.; Surachetpong, W. Non-lethal sampling for Tilapia Lake Virus detection by RT-qPCR and cell culture. Aquaculture 2018, 486, 75–80. [Google Scholar] [CrossRef]
- Kembou-Ringert, J.E.; Hotio, F.N.; Steinhagen, D.; Thompson, K.D.; Surachetpong, W.; Rakus, K.; Daly, J.M.; Goonawardane, N.; Adamek, M. Knowns and unknowns of TiLV-associated neuronal disease. Virulence 2024, 15, 2329568. [Google Scholar] [CrossRef]
- Yamkasem, J.; Tattiyapong, P.; Kamlangdee, A.; Surachetpong, W. Evidence of potential vertical transmission of tilapia lake virus. J. Fish Dis. 2019, 42, 1293–1300. [Google Scholar] [CrossRef]
- Dong, H.T.; Senapin, S.; Gangnonngiw, W.; Nguyen, V.V.; Rodkhum, C.; Debnath, P.P.; Delamare-Deboutteville, J.; Mohan, C.V. Experimental infection reveals transmission of tilapia lake virus (TiLV) from tilapia broodstock to their reproductive organs and fertilized eggs. Aquaculture 2020, 515, 734541. [Google Scholar] [CrossRef]
- Taengphu, S.; Kayansamruaj, P.; Kawato, Y.; Delamare-Deboutteville, J.; Mohan, C.V.; Dong, H.T.; Senapin, S. Concentration and quantification of Tilapia tilapinevirus from water using a simple iron flocculation coupled with probe-based RT-qPCR. PeerJ 2022, 10, e13157. [Google Scholar] [CrossRef]
- Prasartset, T.; Surachetpong, W. Simultaneous detection of three important viruses affecting tilapia using a multiplex PCR assay. J. Fish Dis. 2023, 46, 459–464. [Google Scholar] [CrossRef]
- Thammatorn, W.; Rawiwan, P.; Surachetpong, W. Minimal risk of tilapia lake virus transmission via frozen tilapia fillets. J. Fish Dis. 2019, 42, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Aich, N.; Paul, A.; Choudhury, T.G.; Saha, H. Tilapia Lake Virus (TiLV) disease: Current status of understanding. Aquac. Fish. 2022, 7, 7–17. [Google Scholar] [CrossRef]
- Jaemwimol, P.; Sirikanchana, K.; Tattiyapong, P.; Mongkolsuk, S.; Surachetpong, W. Virucidal effects of common disinfectants against tilapia lake virus. J. Fish Dis. 2019, 42, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Barría, A.; Trinh, T.Q.; Mahmuddin, M.; Benzie, J.A.H.; Chadag, V.M.; Houston, R.D. Genetic parameters for resistance to Tilapia Lake Virus (TiLV) in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 522, 735126. [Google Scholar] [CrossRef]
- Lertwanakarn, T.; Trongwongsa, P.; Yingsakmongkol, S.; Khemthong, M.; Tattiyapong, P.; Surachetpong, W. Antiviral Activity of Ribavirin against Tilapia tilapinevirus in Fish Cells. Pathogens 2021, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Tattiyapong, P.; Kitiyodom, S.; Yata, T.; Jantharadej, K.; Adamek, M.; Surachetpong, W. Chitosan nanoparticle immersion vaccine offers protection against tilapia lake virus in laboratory and field studies. Fish Shellfish Immunol. 2022, 131, 972–979. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.-Z.; Gao, L.-H.; Miao, B.; Zheng, J.-S.; Pu, D.-C.; Zhang, Q.-Q.; Zeng, W.-W.; Wang, D.-S.; Su, S.-Q.; et al. Identification and pathogenetic study of tilapia lake virus (TiLV) isolated from naturally diseased tilapia. Aquaculture 2023, 565, 739166. [Google Scholar] [CrossRef]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev. Aquac. 2023, 15, 41–56. [Google Scholar] [CrossRef]
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S. Therapeutic antibodies for infectious diseases. Bull. World Health Organ. 2017, 95, 235–237. [Google Scholar] [CrossRef]
- Zhang, M.; Geng, H.; Tariq Javed, M.; Xu, L.; Li, X.; Wang, L.; Li, S.; Xu, Y. Passive protection of Japanese pufferfish (Takifugu rubripes) against Vibrio harveyi infection using chicken egg yolk immunoglobulins (IgY). Aquaculture 2021, 532, 736009. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Q.; Lin, J. Egg Yolk Antibody for Passive Immunization: Status, Challenges, and Prospects. J. Agric. Food Chem. 2023, 71, 5053–5061. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Mine, Y. Egg Yolk Antibodies for Passive Immunity. Annu. Rev. Food Sci. Technol. 2012, 3, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef] [PubMed]
- Hanly, W.C.; Artwohl, J.E.; Bennett, B.T. Review of Polyclonal Antibody Production Procedures in Mammals and Poultry. ILAR J. 1995, 37, 93–118. [Google Scholar] [CrossRef]
- El-Kafrawy, S.A.; Abbas, A.T.; Oelkrug, C.; Tahoon, M.; Ezzat, S.; Zumla, A.; Azhar, E.I. IgY antibodies: The promising potential to overcome antibiotic resistance. Front. Immunol. 2023, 14, 1065353. [Google Scholar] [CrossRef]
- Härtle, S.; Magor, K.E.; Göbel, T.W.; Davison, F.; Kaspers, B. Chapter 6—Structure and Evolution of Avian Immunoglobulins. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 103–120. [Google Scholar]
- Pereira, E.P.V.; van Tilburg, M.F.; Florean, E.O.P.T.; Guedes, M.I.F. Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef]
- Schade, R.; Calzado, E.G.; Sarmiento, R.; Chacana, P.A.; Porankiewicz-Asplund, J.; Terzolo, H.R. Chicken Egg Yolk Antibodies (IgY-technology): A Review of Progress in Production and Use in Research and Human and Veterinary Medicine. Altern. Lab. Anim. 2005, 33, 129–154. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Jin, L.; Zhen, Y.; Lu, Y.; Li, S.; You, J.; Wang, L. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: A review. Biotechnol. Adv. 2011, 29, 860–868. [Google Scholar] [CrossRef]
- Gutierrez, M.A.; Miyazaki, T.; Hatta, H.; Kim, M. Protective properties of egg yolk IgY containing anti-Edwardsiella tarda antibody against paracolo disease in the Japanese eel, Anguilla japonica Temminck & Schlegel. J. Fish Dis. 1993, 16, 113–122. [Google Scholar] [CrossRef]
- Norqvist, A.; Hagström, A.; Wolf-Watz, H. Protection of rainbow trout against vibriosis and furunculosis by the use of attenuated strains of Vibrio anguillarum. Appl. Environ. Microbiol. 1989, 55, 1400–1405. [Google Scholar] [CrossRef]
- Lee, S.B.; Mine, Y.; Stevenson, R.M.W. Effects of Hen Egg Yolk Immunoglobulin in Passive Protection of Rainbow Trout against Yersinia ruckeri. J. Agric. Food Chem. 2000, 48, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, H.; Chao, J.; Jian, S.; Wu, X.; Lu, J.; Wang, J.; Chen, C.; Liu, Y. Polyvalent passive vaccine candidates from egg yolk antibodies (IgY) of important outer membrane proteins (PF1380 and ExbB) of Pseudomonas fluorescens in fish. Fish Shellfish Immunol. 2023, 143, 109211. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, H.; Cui, P.; Chen, J.; Chao, J.; Wu, X.; Lu, J.; Zhang, X.; Xu, G.; Liu, Y. Differential polyvalent passive immune protection of egg yolk antibodies (IgY) against live and inactivated Vibrio fluvialis in fish. Fish Shellfish Immunol. 2024, 151, 109751. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; He, H.; Sato, A.; Hatta, H.; Nakao, M.; Somamoto, T. Ulcer disease prophylaxis in koi carp by bath immersion with chicken egg yolk containing anti-Aeromonas salmonicida IgY. Res. Vet. Sci. 2015, 99, 82–86. [Google Scholar] [CrossRef]
- Zhenxing, L.; Ke, H.; Yanping, M.; Le, H.T.H.; Feng, G.-q.; Jiangyao, M.; Zhiling, L.; Li, Y. Oral Passive Immunization of Carp Cyprinus carpio with Anti-CyHV-3 Chicken Egg Yolk Immunoglobulin (IgY). Fish Pathol. 2014, 49, 113–120. [Google Scholar]
- Yi, L.; Qin, Z.; Lin, H.; Zhou, Y.; Li, J.; Xu, Z.; Babu, V.S.; Lin, L. Features of chicken egg yolk immunoglobulin (IgY) against the infection of red-spotted grouper nervous necrosis virus. Fish Shellfish Immunol. 2018, 80, 534–539. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Yan, L.; Liu, W.; Shi, H.; Lu, Y.; Liu, X. Protective effects of egg yolk immunoglobulins (IgY) on juvenile groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) with red-spotted grouper nervous necrosis virus infection. Aquaculture 2021, 545, 737218. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Sirikanchana, K.; Surachetpong, W. Development and validation of a reverse transcription quantitative polymerase chain reaction for tilapia lake virus detection in clinical samples and experimentally challenged fish. J. Fish Dis. 2018, 41, 255–261. [Google Scholar] [CrossRef]
- Sanyalukruechai, C.; Watthanasakphuban, N.; Khemthong, M.; Surachetpong, W.; Rattanaporn, K. Expression and purification of recombinant tilapia lake virus segment 4 protein and its in-vitro biological activity for potential use in vaccine development. Sci. Rep. 2024, 14, 31529. [Google Scholar] [CrossRef]
- Polson, A.; von Wechmar, M.B.; van Regenmortel, M.H.V. Isolation of Viral IgY Antibodies from Yolks of Immunized Hens. Immunol. Commun. 1980, 9, 475–493. [Google Scholar] [CrossRef]
- Pauly, D.; Chacana, P.; Gutierrez Calzado, E.J.; Brembs, B.; Schade, R. IgY Technology: Extraction of Chicken Antibodies from Egg Yolk by Polyethylene Glycol (PEG) Precipitation. J. Vis. Exp. 2011, 51, e3084. [Google Scholar] [CrossRef]
- Metheenukul, P.; Surachetpong, W.; Prasertsincharoen, N.; Arreesrisom, P.; Thengchaisri, N. Comparison of immunoglobulin Y antibody production in new and spent laying hens. Vet. World 2024, 17, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Dechavichitlead, W.; Waltzek, T.B.; Surachetpong, W. Tilapia develop protective immunity including a humoral response following exposure to tilapia lake virus. Fish Shellfish Immunol. 2020, 106, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Mohamad, A.; Khemthong, M.; Trongwongsa, P.; Lertwanakarn, T.; Setthawong, P.; Surachetpong, W. A New Cell Line from the Brain of Red Hybrid Tilapia (Oreochromis spp.) for Tilapia Lake Virus Propagation. Animals 2024, 14, 1522. [Google Scholar] [CrossRef]
- Klimentzou, P.; Paravatou-Petsotas, M.; Zikos, C.; Beck, A.; Skopeliti, M.; Czarnecki, J.; Tsitsilonis, O.; Voelter, W.; Livaniou, E.; Evangelatos, G.P. Development and immunochemical evaluation of antibodies Y for the poorly immunogenic polypeptide prothymosin alpha. Peptides 2006, 27, 183–193. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Yamkasem, J.; Surachetpong, W.; Lin, Y.-J.; You, S.-H.; Lu, T.-H.; Chen, C.-Y.; Wang, W.-M.; Liao, C.-M. Assessing the effect of probiotics on tilapia lake virus-infected tilapia: Transmission and immune response. J. Fish Dis. 2022, 45, 1117–1132. [Google Scholar] [CrossRef]
- Mohamad, A.; Yamkasem, J.; Paimeeka, S.; Khemthong, M.; Lertwanakarn, T.; Setthawong, P.; Nuez-Ortin, W.G.; Isern Subich, M.M.; Surachetpong, W. Efficacy of Feed Additives on Immune Modulation and Disease Resistance in Tilapia in Coinfection Model with Tilapia Lake Virus and Aeromonas hydrophila. Biology 2024, 13, 938. [Google Scholar] [CrossRef]
- Tabll, A.A.; Shahein, Y.E.; Omran, M.M.; Hussein, N.A.; El-Shershaby, A.; Petrovic, A.; Glasnovic, M.; Smolic, R.; Smolic, M. Monoclonal IgY antibodies: Advancements and limitations for immunodiagnosis and immunotherapy applications. Ther. Adv. Vaccines Immunother. 2024, 12, 25151355241264520. [Google Scholar] [CrossRef]
- Sugino, H.; Nitoda, T.; Juneja, L.R. General Chemical Composition of Hen Eggs. In Hen Eggs; CRC: Boca Raton, FL, USA, 2018; pp. 13–24. [Google Scholar]
- Zhang, L.; Lin, L.; Qin, Z. A review on the application of chicken immunoglobulin Y in aquaculture. Rev. Aquac. 2024, 16, 536–551. [Google Scholar] [CrossRef]
- Madera-Contreras, A.M.; Solano-Texta, R.; Cisneros-Sarabia, A.; Bautista-Santos, I.; Vences-Velázquez, G.; Vences-Velázquez, A.; Cortés-Sarabia, K. Optimized method for the extraction of contaminant-free IgY antibodies from egg yolk using PEG 6000. MethodsX 2022, 9, 101874. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-e.; Wen, J.; Zhao, S.; Zhang, K.; Zhou, Y. Prophylaxis and therapy of pandemic H1N1 virus infection using egg yolk antibody. J. Virol. Methods 2014, 206, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Arasteh, N.; Aminirissehei, A.H.; Yousif, A.N.; Albright, L.J.; Durance, T.D. Passive immunization of rainbow trout (Oncorhynchus mykiss) with chicken egg yolk immunoglobulins (IgY). Aquaculture 2004, 231, 23–36. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, X.; Lin, J. Ex Vivo Evaluation of Egg Yolk IgY Degradation in Chicken Gastrointestinal Tract. Front. Immunol. 2021, 12, 746831. [Google Scholar] [CrossRef]
- Li, X.-Y.; Jin, L.-J.; McAllister, T.A.; Stanford, K.; Xu, J.-Y.; Lu, Y.-N.; Zhen, Y.-H.; Sun, Y.-X.; Xu, Y.-P. Chitosan−Alginate Microcapsules for Oral Delivery of Egg Yolk Immunoglobulin (IgY). J. Agric. Food Chem. 2007, 55, 2911–2917. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setthawong, P.; Yamkasem, J.; Khemthong, M.; Tattiyapong, P.; Metheenukul, P.; Prasertsincharoen, N.; Lertwanakarn, T.; Thengchaisri, N.; Surachetpong, W. Development of IgY-Based Passive Immunization Against Tilapia Lake Virus: Development and In Vitro Neutralization Assays. Viruses 2025, 17, 448. https://doi.org/10.3390/v17030448
Setthawong P, Yamkasem J, Khemthong M, Tattiyapong P, Metheenukul P, Prasertsincharoen N, Lertwanakarn T, Thengchaisri N, Surachetpong W. Development of IgY-Based Passive Immunization Against Tilapia Lake Virus: Development and In Vitro Neutralization Assays. Viruses. 2025; 17(3):448. https://doi.org/10.3390/v17030448
Chicago/Turabian StyleSetthawong, Piyathip, Jidapa Yamkasem, Matepiya Khemthong, Puntanat Tattiyapong, Pornphimon Metheenukul, Noppadol Prasertsincharoen, Tuchakorn Lertwanakarn, Naris Thengchaisri, and Win Surachetpong. 2025. "Development of IgY-Based Passive Immunization Against Tilapia Lake Virus: Development and In Vitro Neutralization Assays" Viruses 17, no. 3: 448. https://doi.org/10.3390/v17030448
APA StyleSetthawong, P., Yamkasem, J., Khemthong, M., Tattiyapong, P., Metheenukul, P., Prasertsincharoen, N., Lertwanakarn, T., Thengchaisri, N., & Surachetpong, W. (2025). Development of IgY-Based Passive Immunization Against Tilapia Lake Virus: Development and In Vitro Neutralization Assays. Viruses, 17(3), 448. https://doi.org/10.3390/v17030448





