Biosecurity Risk Factors and Predictive Index for Hepatitis E Virus Serological Status in Belgian Pig Farms: Conventional and Free-Range Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Serological Data
2.2. Biosecurity Questionnaire
2.3. Statistical Analysis
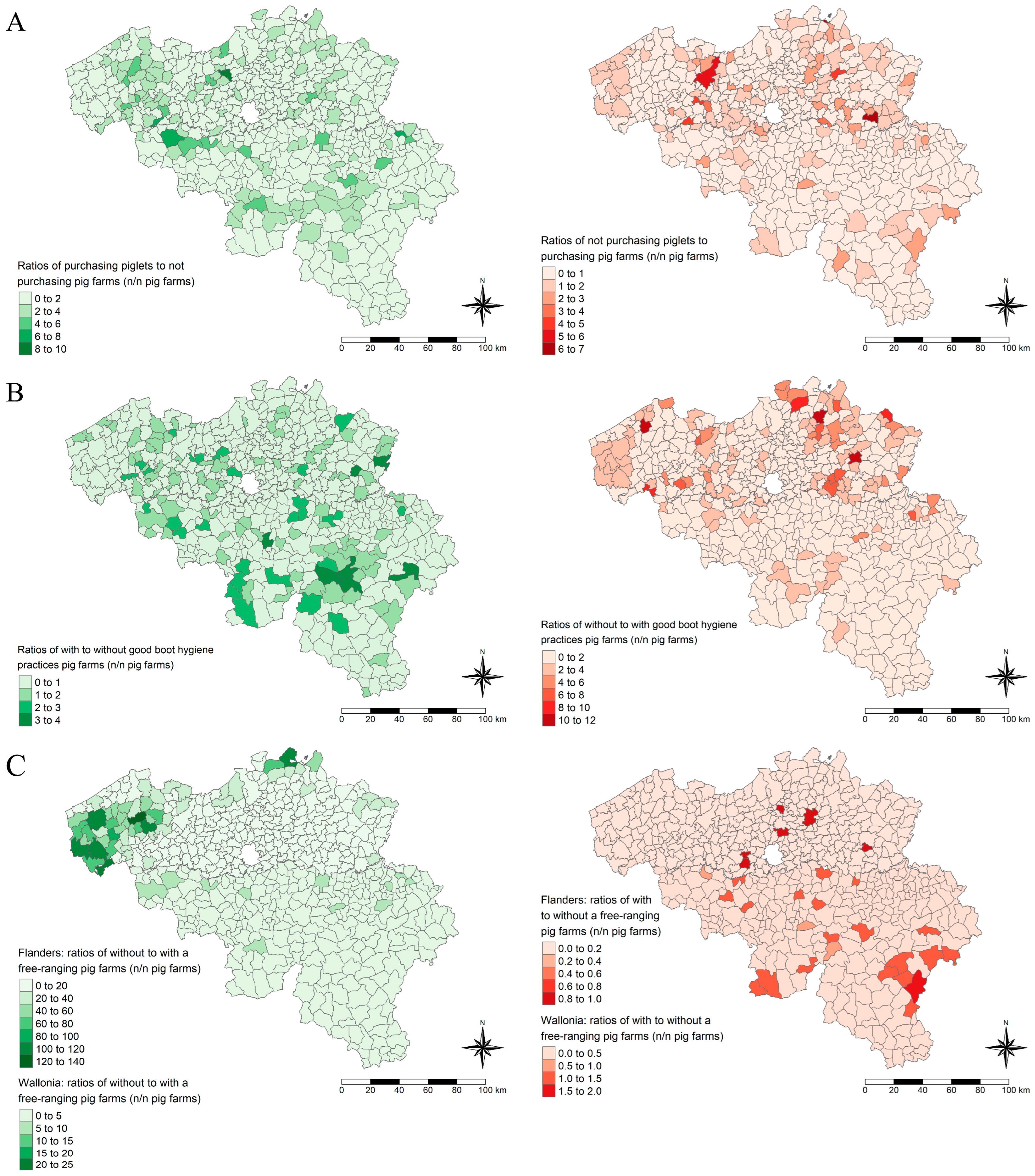
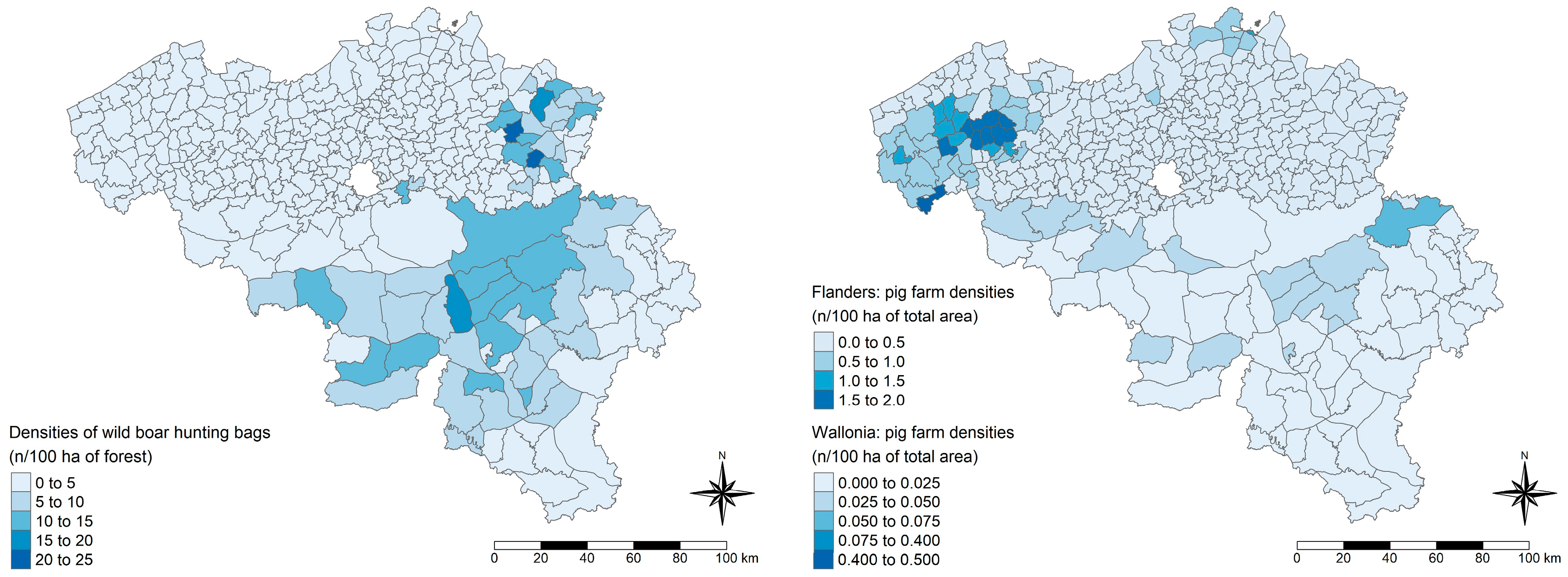
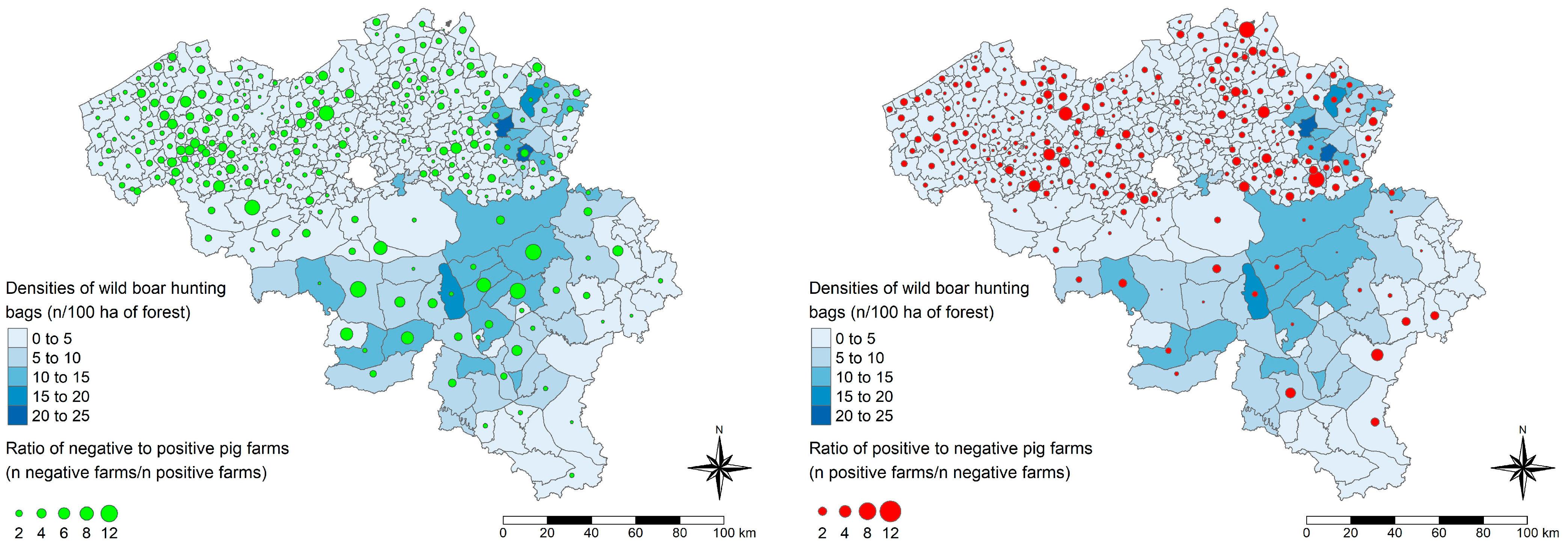
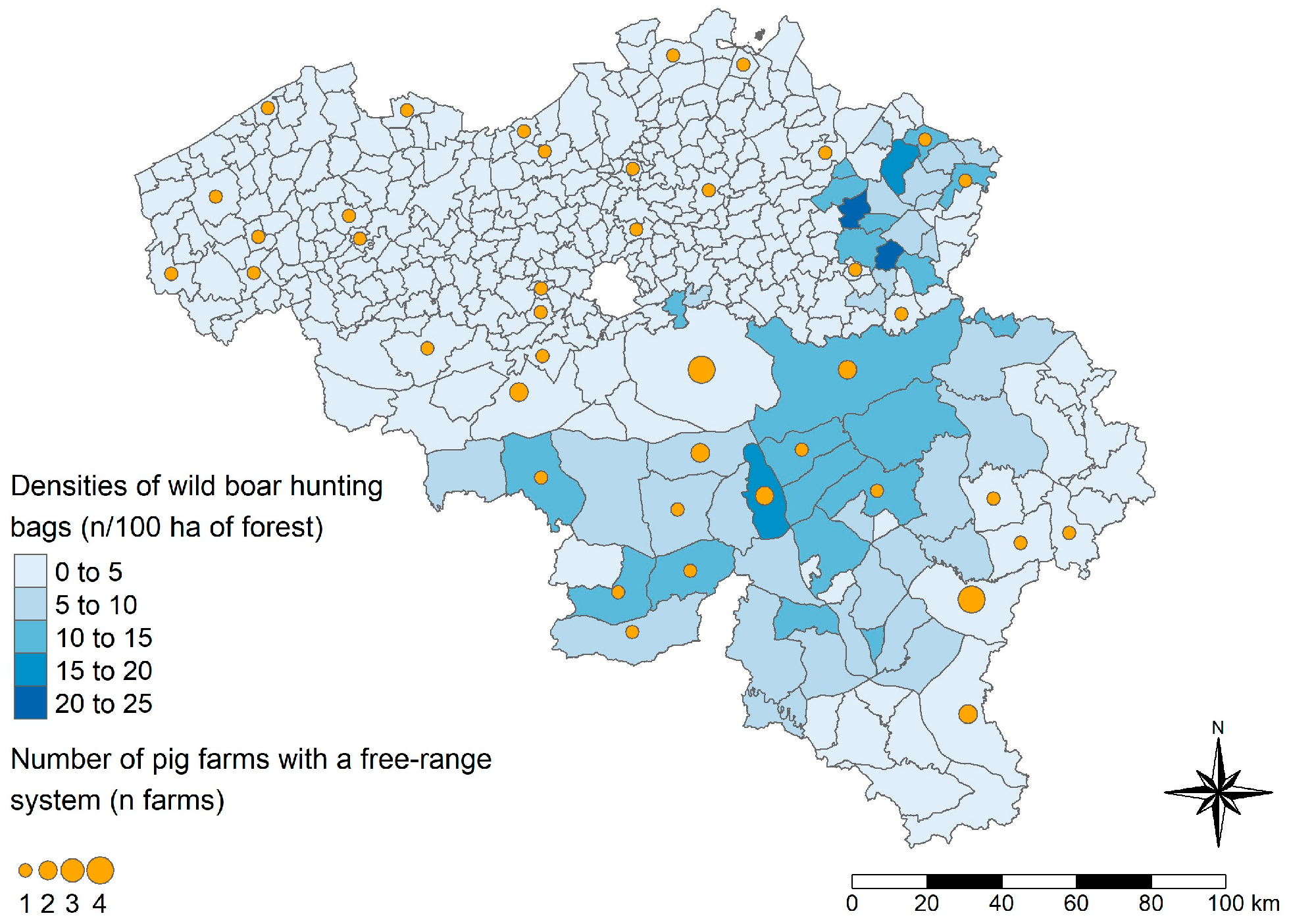
2.4. Spatial Visualization
3. Results
3.1. Bio-Exclusion: Piglet Purchase Protects Against Increased HEV Herd Seroprevalence
3.2. Bio-Compartmentalization: Free-Range Systems and Low Boot Hygiene Are Risk Factors for Increased HEV Herd Seroprevalence
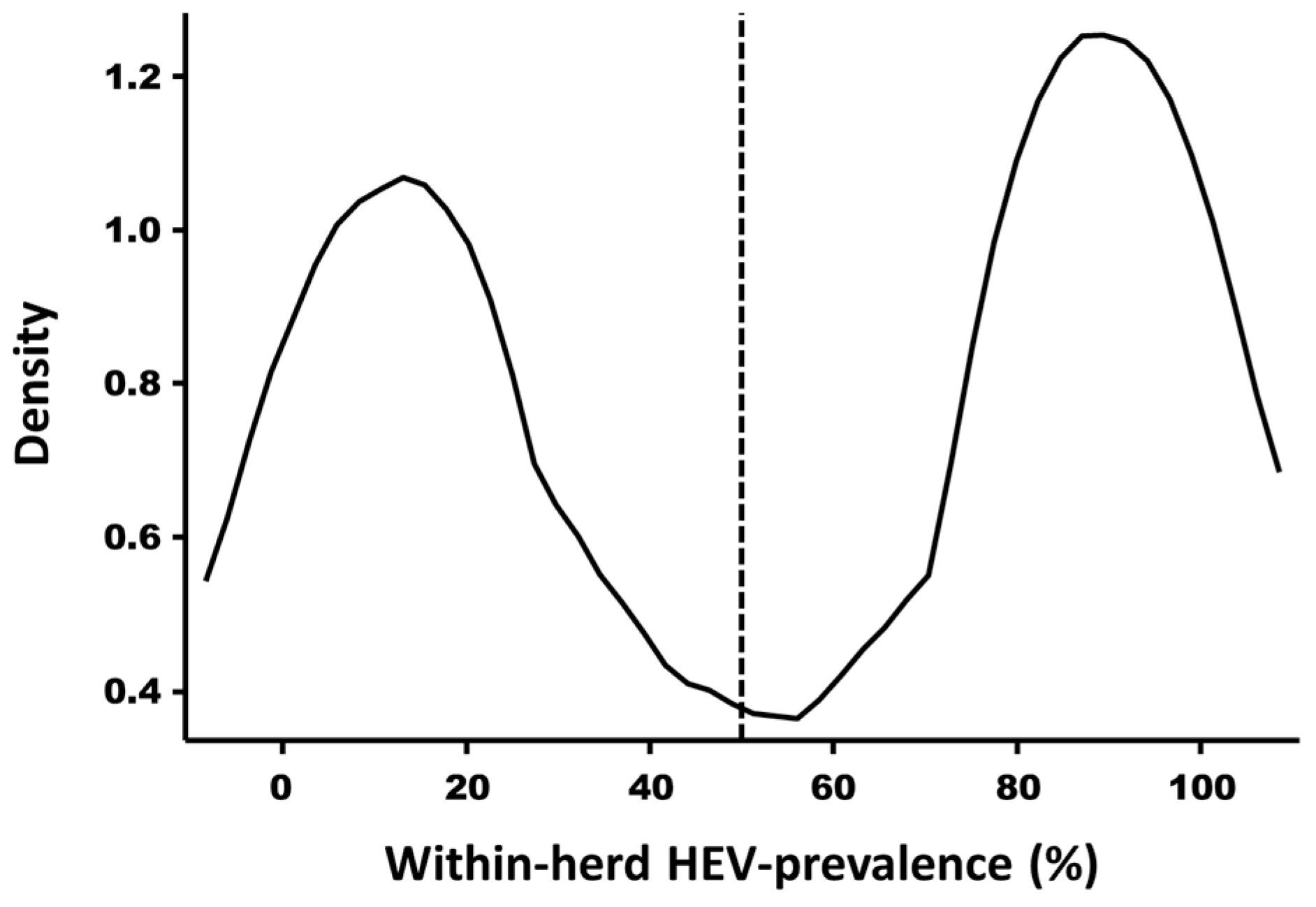
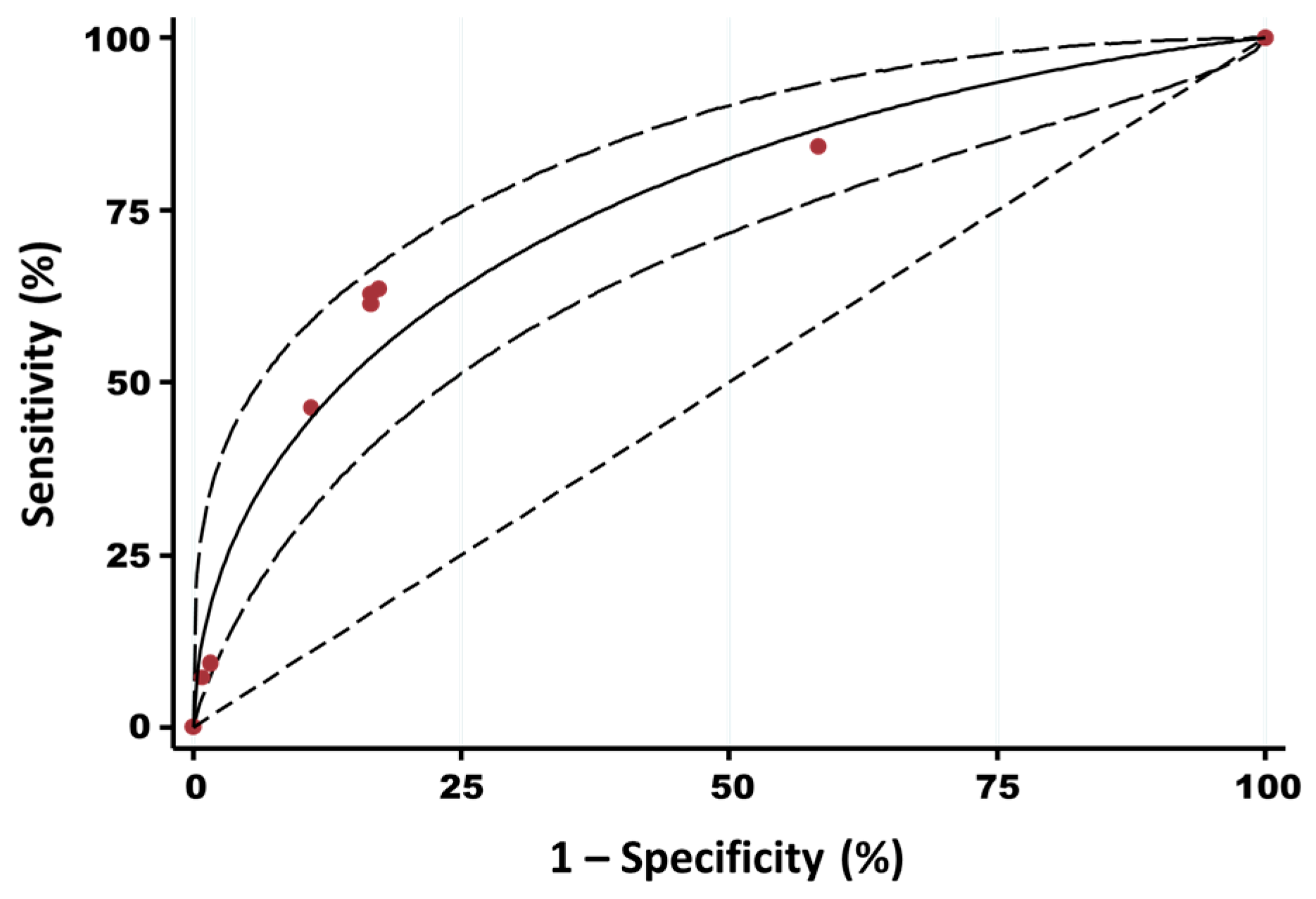
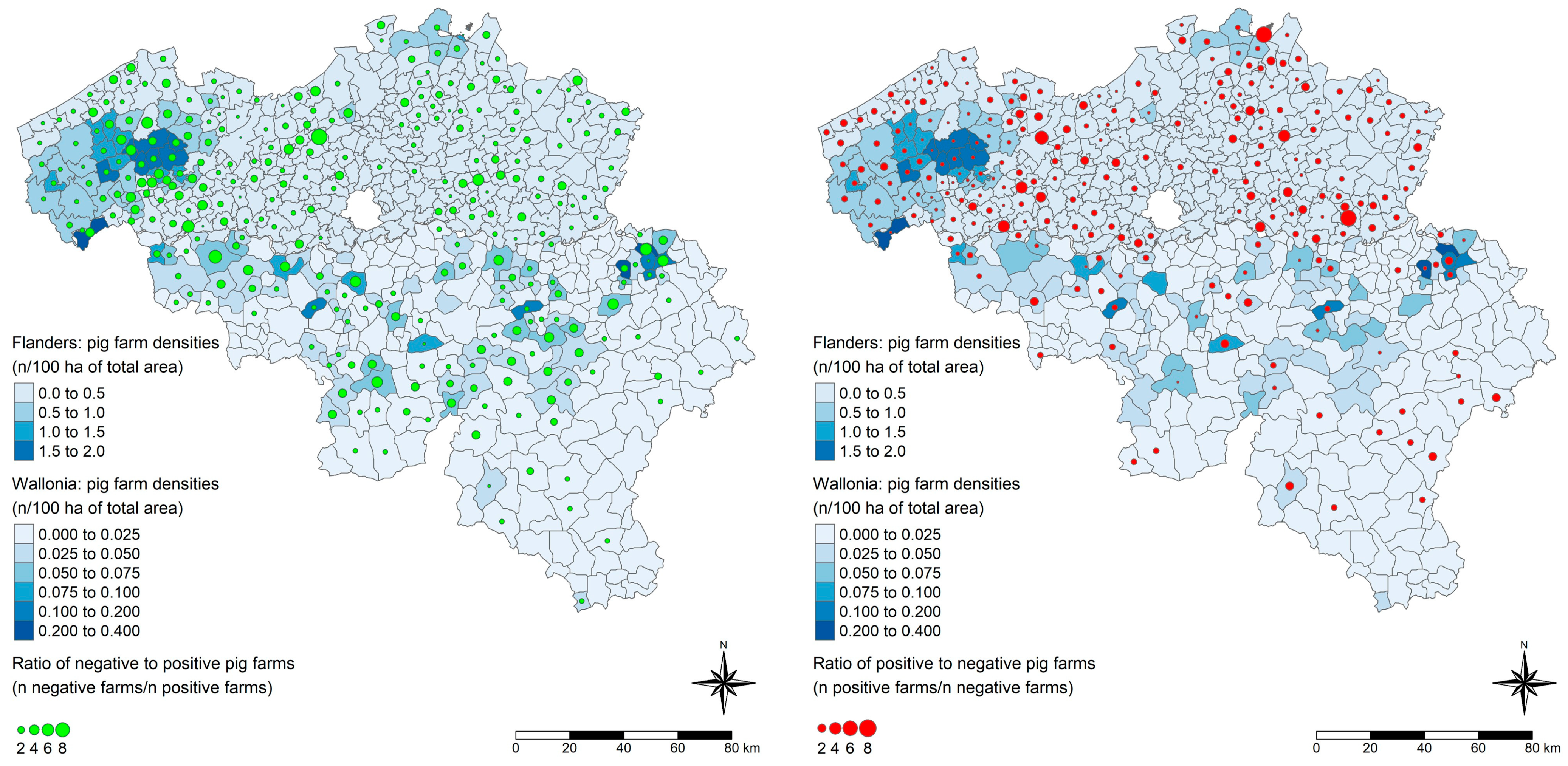
3.3. Prediction of the HEV Serological Status of Belgian Pig Farms and Spatial Visualization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HEV | Hepatitis E virus |
| WHO | World Health Organization |
| AFSCA/FAVV | Agence Fédérale pour la Sécurité de la Chaîne Alimentaire/Federaal Agentschap voor de Veiligheid van de Voedselketen |
| DGZ | Dierengezondheidszorg Vlaanderen |
| ARSIA | Animal Health Care Wallonia (Association Régionale de Santé et d’Identification Animales |
| SANITEL | Belgian Identification and Registration System |
| ELISA | Enzyme-linked immunosorbent assay |
| Se | Sensitivity |
| Sp | Specificity |
| OR | Odds ratio |
| BIC | Bayesian information criterion |
| PI | Predictive index |
| ROC | Receiver operating characteristic |
| CO | Cut-off |
| SPW | Public Service of Wallonia |
| INBO | Instituut voor Natuur- en Bosonderzoek |
Appendix A
Appendix B
| Variable | Category |
|---|---|
| Type | Closed f-to-f herd |
| Mixed f-to-f herd | |
| Slaughter pig herd | |
| Other | |
| Size | 101–200 |
| 201–500 | |
| 501–1000 | |
| 1001–2000 | |
| 2001–4000 | |
| >4000 | |
| Internal biosecurity score | |
| External biosecurity score | |
| General biosecurity score | |
| Do the pigs have access to free range? | Yes |
| No | |
| Besides pigs, are there any other livestock animals on your farm? | Yes |
| No | |
| Are there any cattle on the farm? | Yes |
| No | |
| Do you purchase breeding pigs (sows/gilts/boars)? | Yes |
| No | |
| Are piglets purchased? | Yes |
| No | |
| When purchasing semen, is there proof that the health status and health management of the originating AI center/company are equal to or superior to those of your own farm? | Yes |
| No | |
| Not known | |
| No purchase of semen | |
| Is the transport vehicle bringing pigs to the slaughterhouse or other farms empty upon arrival at the farm, and are the animals loaded from an isolated loading dock or directly from the barn/central corridor? | Central corridor and empty vehicle |
| Central corridor and not empty vehicle | |
| Loading dock or no transport | |
| Is the manure/slurry removed through the dirty area of the farm? | Yes |
| No | |
| Are there specific suction pipes on the farm for removing slurry from the pit (permanent slurry removal pipes on the farm)? | Yes |
| No | |
| Do the rendering company or the feed supplier handle carcass removal or silo filling without entering the farm buildings? | Yes |
| No | |
| Does the farmer use a feed supplier that meets specific hygiene requirements (e.g., Salmonella-free, heat treatment)? | Yes |
| No or not known | |
| Is a bacteriological analysis of the drinking water conducted annually at the source and the storage tank? | Yes |
| No | |
| Is a bacteriological analysis of the drinking water conducted annually at the main outlets (where the animals drink)? | Yes |
| No | |
| Are specific measures taken for the supply of equipment (e.g., cleaning and disinfection, quarantine period in a designated area)? | Yes |
| No | |
| Is a period of no contact with pigs (more than 12 h) required for all visitors before they are allowed to enter the barns? | Yes |
| No | |
| Is there a hygiene lock available, and is it always used when visitors access the livestock buildings? | Yes |
| No | |
| Are all livestock buildings accessible to visitors only through the hygiene lock? | Yes |
| No | |
| No hygiene lock | |
| Is vermin (rats, mice, etc.) considered a problem on the farm? | Yes |
| No | |
| Are the surroundings of the farm (nearby buildings) paved and kept clean (e.g., waste removal, control of weeds) to prevent infestations by rodents/wild animals? | Yes |
| No | |
| Do pets (dogs or cats) have access to the livestock buildings? | Yes |
| No | |
| Can birds enter the livestock buildings? | Yes |
| No | |
| Are there grilles placed at the air inlets? | Yes |
| No | |
| Is the farm located in an area with low or high pig density? | High |
| Low | |
| Are there any other pig farms within a 500 m radius of your farm? | Yes |
| No | |
| Is slurry from other farms spread on neighboring land directly adjacent to the livestock buildings (within a 500 m radius)? | Yes |
| No | |
| Are vehicles transporting animals from other farms frequently (at least once a day) passing on the road near the farm? | Yes |
| No | |
| Have wild boars been spotted in the vicinity of the farm (within a 10 km radius)? | Yes |
| No | |
| Are footbaths present at the entrance of the farm, and are they used? | Yes |
| No | |
| Are the buildings constructed to prevent wild boars from intruding or coming into contact with the pigs? | Yes |
| No |
| Variable | Category |
|---|---|
| Size | 101–200 |
| 201–500 | |
| 501–1000 | |
| 1001–2000 | |
| 2001–4000 | |
| >4000 | |
| Internal biosecurity score | |
| External biosecurity score | |
| General biosecurity score | |
| Do the pigs have access to free range? | Yes |
| No | |
| Are there any cattle on the farm? | Yes |
| No | |
| Are the animals loaded from an isolated loading dock or directly from the barn/central corridor? | Central corridor or barn |
| Loading dock | |
| Is the manure/slurry removed through the dirty area of the farm? | Yes |
| No | |
| Are there specific suction pipes on the farm for removing slurry from the pit (permanent slurry removal pipes on the farm)? | Yes |
| No | |
| Is the carcass storage site secured to prevent access by dogs, cats, rodents, or wild animals? | Yes |
| No | |
| Does the farmer wear disposable gloves when handling carcasses or wash and disinfect their hands afterward? | Never |
| Sometimes | |
| Always | |
| Is it required to wash and disinfect hands before entering the livestock buildings? | Yes |
| No | |
| Is vermin (rats, mice, etc.) considered a problem on the farm? | Yes |
| No | |
| Are the surroundings of the farm (near the buildings) paved and kept clean (e.g., waste removal, weed control) to prevent infestations by rodents/wild animals? | Yes |
| No | |
| Do pets (dogs or cats) have access to the livestock buildings? | Yes |
| No | |
| Can birds enter the livestock buildings? | Yes |
| No | |
| Are there grilles placed at the air inlets? | Yes |
| No | |
| Is the health status of the farm (e.g., serology, reasons for seizure at the slaughterhouse, etc.) evaluated regularly (i.e., at least once a year)? | Yes |
| No | |
| Are sick animals always handled after healthy animals? | Yes |
| No | |
| Are specific clothing and footwear for each compartment available and changed between compartments? | Never |
| Sometimes | |
| Always | |
| Are hands washed and/or disinfected between livestock compartments? | Never |
| Sometimes | |
| Always | |
| Are footbaths/boot washers placed between different compartments, or are boots changed between compartments? | Yes |
| No | |
| On the farm, is all work conducted from the youngest pigs to the oldest? | Yes |
| No | |
| Is the equipment required for a particular group of animals arranged according to the work order to avoid using the same equipment across different groups? | Yes |
| No | |
| Is there a cleaning and disinfection protocol for equipment (e.g., brooms, shovels) after use, and is this protocol followed? | Yes |
| No | |
| Is the equipment clearly identified or recognizable (e.g., by color coding) as being reserved for a specific area or age group? | Yes |
| No | |
| Are syringes (for injections) available and used specifically for each age group? | Yes |
| No | |
| Are needles (for injections) available and used specifically for each age group? | Yes |
| No | |
| Are the buildings/rooms cleaned and disinfected after each production cycle? | Yes |
| No | |
| Are the corridors cleaned and disinfected after moving animals? | Not always |
| Always | |
| Are footbaths present at the entrance of the farm, and are they used? | Yes |
| No | |
| Is the liquid in the footbath changed immediately when it becomes visibly contaminated? | Yes |
| No | |
| No footbath |
References
- Purdy, M.A.; Drexler, J.F.; Meng, X.J.; Norder, H.; Okamoto, H.; Van der Poel, W.H.M.; Reuter, G.; de Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 11 June 2024).
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Purdy, M.A. Consensus Proposals for Classification of the Family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Tan, B.H.; Chi-Yuan Teo, E.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Kim Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Mauroy, A.; Pavio, N.; Purdy, M.A.; Rose, N.; Thiry, E.; de Oliveira-Filho, E.F. Hepatitis E Virus and Related Viruses in Animals. Transbound. Emerg. Dis. 2017, 64, 37–52. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef]
- Singh, M.P.; Majumdar, M.; Goyal, K.; Lakshmi, P.V.M.; Bhatia, D.; Ratho, R.K. Investigation of Suspected Viral Hepatitis Outbreaks in North West India. Diagn. Microbiol. Infect. Dis. 2016, 84, 309–314. [Google Scholar] [CrossRef]
- Belei, O.; Ancusa, O.; Mara, A.; Olariu, L.; Amaricai, E.; Folescu, R.; Zamfir, C.L.; Gurgus, D.; Motoc, A.G.; Stânga, L.C.; et al. Current Paradigm of Hepatitis E Virus Among Pediatric and Adult Patients. Front. Pediatr. 2021, 9, 721918. [Google Scholar] [CrossRef]
- Kamar, N.; Legrand-Abravanel, F.; Izopet, J.; Rostaing, L. Hepatitis E Virus: What Transplant Physicians Should Know. Am. J. Transplant. 2012, 12, 2281–2287. [Google Scholar] [CrossRef]
- Sue, P.K.; Pisanic, N.; Heaney, C.D.; Forman, M.; Valsamakis, A.; Jackson, A.M.; Ticehurst, J.R.; Montgomery, R.A.; Schwarz, K.B.; Nelson, K.E.; et al. Hepatitis E Virus Infection Among Solid Organ Transplant Recipients at a North American Transplant Center. Open Forum Infect. Dis. 2016, 3, ofw006. [Google Scholar] [CrossRef]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E Virus Infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef]
- Ho, E.; Schenk, J.; Hutse, V.; Suin, V.; Litzroth, A.; Blaizot, S.; Herzog, S.A.; Verburgh, V.; Jacques, M.; Rahman, A.; et al. Stable HEV IgG Seroprevalence in Belgium between 2006 and 2014. J. Viral Hepat. 2020, 27, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-J.; Halbur, P.G.; Shapiro, M.S.; Govindarajan, S.; Bruna, J.D.; Mushahwar, I.K.; Purcell, R.H.; Emerson, S.U. Genetic and Experimental Evidence for Cross-Species Infection by Swine Hepatitis E Virus. J. Virol. 1998, 72, 9714–9721. [Google Scholar] [CrossRef] [PubMed]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative Pathogenesis of Infection of Pigs with Hepatitis E Viruses Recovered from a Pig and a Human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The Global Epidemiology of Hepatitis E Virus Infection: A Systematic Review and Meta-Analysis. Liver Int. 2020, 40, 1516–1528. [Google Scholar] [CrossRef]
- Leblanc, D.; Ward, P.; Gagné, M.J.; Poitras, E.; Müller, P.; Trottier, Y.L.; Simard, C.; Houde, A. Presence of Hepatitis E Virus in a Naturally Infected Swine Herd from Nursery to Slaughter. Int. J. Food Microbiol. 2007, 117, 160–166. [Google Scholar] [CrossRef]
- Casas, M.; Pujols, J.; Rosell, R.; de Deus, N.; Peralta, B.; Pina, S.; Casal, J.; Martín, M. Retrospective Serological Study on Hepatitis E Infection in Pigs from 1985 to 1997 in Spain. Vet. Microbiol. 2009, 135, 248–252. [Google Scholar] [CrossRef]
- Rose, N.; Lunazzi, A.; Dorenlor, V.; Merbah, T.; Eono, F.; Eloit, M.; Madec, F.; Pavio, N. High Prevalence of Hepatitis E Virus in French Domestic Pigs. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 419–427. [Google Scholar] [CrossRef]
- Martinelli, N.; Luppi, A.; Cordioli, P.; Lombardi, G.; Lavazza, A. Prevalence of Hepatitis E Virus Antibodies in Pigs in Northern Italy. Infect. Ecol. Epidemiol. 2011, 1, 7331. [Google Scholar] [CrossRef]
- Lange, H.; Overbo, J.; Borgen, K.; Dudman, S.; Hoddevik, G.; Urdahl, A.M.; Vold, L.; Sjurseth, S.K. Hepatitis E in Norway: Seroprevalence in Humans and Swine. Epidemiol. Infect. 2017, 145, 181–186. [Google Scholar] [CrossRef]
- Pavio, N.; Merbah, T.; Thébault, A. Frequent Hepatitis E Virus Contamination in Food Containing Raw Pork Liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef]
- Wielick, C.; Ludwig-Begall, L.; Faes, C.; Ribbens, S.; Saegerman, C.; Thiry, E. A Randomized Large-Scale Cross-Sectional Serological Survey of Hepatitis E Virus Infection in Belgian Pig Farms. Microorganisms 2023, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Agence Fédérale Pour la SÉCURITÉ de la Chaîne Alimentaire. Circulaire Contenant des Instructions Pour les Vétérinaires d’exploitation Concernant l’évaluation des Risques Pour les Exploitations Porcines. Available online: https://favv-afsca.be/sites/default/files/2023-11/20210531_circ_instructionveterinairesanalysederisque_FR_v1_.pdf (accessed on 4 March 2023).
- Wavreille, J.; Rondia, P.; Decruyenaere, V.; Jamar, D.; Stilmant, D.; Gengler, N.; Mayeres, P.; Beckers, Y.; Sindic, M.; Bartiaux-Thill, N. La Diversification En Productions Animales. In 10ème Carrefour des Productions Animales: L’élevage: Hier, Aujourd’hui, Demain Quelles Attentes Pour Quels Enjeux? Gembloux, Belgium, 2005; pp. 53–57. Available online: https://orbi.uliege.be/bitstream/2268/122510/1/La%20diversification%20en%20productions%20animales.pdf (accessed on 14 March 2025).
- Meester, M.; Bouwknegt, M.; Hakze-van der Honing, R.; Vernooij, H.; Houben, M.; van Oort, S.; van der Poel, W.H.M.; Stegeman, A.; Tobias, T. Repeated Cross-Sectional Sampling of Pigs at Slaughter Indicates Varying Age of Hepatitis E Virus Infection within and between Pig Farms. Vet. Res. 2022, 53, 50. [Google Scholar] [CrossRef]
- Rutjes, S.A.; Bouwknegt, M.; Van Der Giessen, J.W.; De Roda Husman, A.M.; Reusken, C.B.E.M. Seroprevalence of Hepatitis e Virus in Pigs from Different Farming Systems in the Netherlands. J. Food Prot. 2014, 77, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Licoppe, A.; Fett, T.; Thomas, I.; Brochier, B.; Thiry, E.; Linden, A. Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound. Emerg. Dis. 2017, 64, 764–773. [Google Scholar] [CrossRef]
- 23 JUILLET 2013—Arrêté Ministériel Portant Exécution de l’arrêté Royal du 12 Octobre 2010 Relatif à la Lutte Contre la Maladie d’Aujeszky. Moniteur Belge. Available online: https://www.ejustice.just.fgov.be/mopdf/2013/07/30_1.pdf#Page19 (accessed on 22 November 2022).
- Biocheck. UGent. Available online: https://biocheckgent.com/en/questionnaires/pigs-indoor (accessed on 5 December 2024).
- Laanen, M.; Beek, J.; Ribbens, S.; Vangroenweghe, F.; Maes, D.; Dewulf, J. Bioveiligheid op Varkensbedrijven: Ontwikkeling van een Online Scoresysteem en de Resultaten van de Eerste 99 Deelnemende Bedrijven. Vlaams Diergeneeskd. Tijdschr. 2010, 79, 302–306. [Google Scholar] [CrossRef]
- Renault, V.; Humblet, M.F.; Pham, P.N.; Saegerman, C. Biosecurity at Cattle Farms: Strengths, Weaknesses, Opportunities and Threats. Pathogens 2021, 10, 1315. [Google Scholar] [CrossRef]
- Faes, C.; Aerts, M.; Litière, S.; Méroc, E.; Van Der Stede, Y.; Mintiens, K. Estimating Herd Prevalence on the Basis of Aggregate Testing of Animals. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 155–174. [Google Scholar] [CrossRef]
- Saegerman, C.; Gilbert, A.; Donneau, A.F.; Gangolf, M.; Diep, A.N.; Meex, C.; Bontems, S.; Hayette, M.P.; D’Orio, V.; Ghuysen, A. Clinical Decision Support Tool for Diagnosis of COVID-19 in Hospitals. PLoS ONE 2021, 16, e0247773. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On Determining the Most Appropriate Test Cut-off Value: The Case of Tests with Continuous Results. Biochem. Med. 2016, 26, 297. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Perkins, N.J.; Liu, A.; Bondell, H. Optimal Cut-Point and Its Corresponding Youden Index to Discriminate Individuals Using Pooled Blood Samples. Epidemiology 2005, 16, 73–81. [Google Scholar] [CrossRef]
- Youden, W. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science (1979) 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Alarcón, L.V.; Alberto, A.A.; Mateu, E. Biosecurity in Pig Farms: A Review. Porc. Health Manag. 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Q.; Mou, J.; Gong, G.; Yang, Z.; Cui, L.; Zhu, J.; Ju, G.; Hua, X. Hepatitis E Virus Infection among Domestic Animals in Eastern China. Zoonoses Public Health 2008, 55, 291–298. [Google Scholar] [CrossRef]
- Seminati, C.; Mateu, E.; Peralta, B.; de Deus, N.; Martin, M. Distribution of Hepatitis E Virus Infection and Its Prevalence in Pigs on Commercial Farms in Spain. Vet. J. 2008, 175, 130–132. [Google Scholar] [CrossRef]
- Vitral, C.L.; Pinto, M.A.; Lewis-Ximenez, L.L.; Khudyakov, Y.E.; Santos, D.R.; dos Gaspar, A.M.C. Serological Evidence of Hepatitis E Virus Infection in Different Animal Species from the Southeast of Brazil. Mem. Inst. Oswaldo Cruz 2005, 100, 117–122. [Google Scholar] [CrossRef]
- Walachowski, S.; Dorenlor, V.; Lefevre, J.; Lunazzi, A.; Eono, F.; Merbah, T.; Eveno, E.; Pavio, N.; Rose, N. Risk Factors Associated with the Presence of Hepatitis E Virus in Livers and Seroprevalence in Slaughter-Age Pigs: A Retrospective Study of 90 Swine Farms in France. Epidemiol. Infect. 2014, 142, 1934–1944. [Google Scholar] [CrossRef]
- Meester, M.; Rademaker, A.; Bouwknegt, M.; Hakze-van der Honing, R.W.; Stegeman, A.; van der Poel, W.H.M.; Tobias, T.J. Evaluation of Non-Invasive Sampling Methods for Detection of Hepatitis E Virus Infected Pigs in Pens. Microorganisms 2023, 11, 500. [Google Scholar] [CrossRef]
- Linden, A.; Licoppe, A.; Volpe, R.; Paternostre, J.; Lesenfants, C.; Cassart, D.; Garigliany, M.; Tignon, M.; van den Berg, T.; Desmecht, D.; et al. Summer 2018: African Swine Fever Virus Hits North-Western Europe. Transbound. Emerg. Dis. 2019, 66, 54–55. [Google Scholar] [CrossRef]
- ANSES. AVIS de l’Anses Relatif Aux Mesures de Biosécurité En Zones Réglementées Vis-à-Vis de La Peste Porcine Africaine (PPA); ANSES: Paris, France, 2020. [Google Scholar]
- Licoppe, A.; De Waele, V.; Malengreaux, C.; Paternostre, J.; Van Goethem, A.; Desmecht, D.; Herman, M.; Linden, A. Management of a Focal Introduction of ASF Virus in Wild Boar: The Belgian Experience. Pathogens 2023, 12, 152. [Google Scholar] [CrossRef]
- Wolff, A.; Günther, T.; Albert, T.; Schilling-Loeffler, K.; Gadicherla, A.K.; Johne, R. Stability of Hepatitis E Virus at Different PH Values. Int. J. Food Microbiol. 2020, 325, 108625. [Google Scholar] [CrossRef] [PubMed]
- Wißmann, J.E.; Brüggemann, Y.; Todt, D.; Steinmann, J.; Steinmann, E. Survival and Inactivation of Hepatitis E Virus on Inanimate Surfaces. J. Hosp. Infect. 2023, 134, 57–62. [Google Scholar] [CrossRef]
- Wolff, A.; Günther, T.; Johne, R. Stability of Hepatitis E Virus After Drying on Different Surfaces. Food Environ. Virol. 2022, 14, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Andraud, M.; Dumarest, M.; Cariolet, R.; Aylaj, B.; Barnaud, E.; Eono, F.; Pavio, N.; Rose, N. Direct Contact and Environmental Contaminations Are Responsible for HEV Transmission in Pigs. Vet. Res. 2013, 44, 102. [Google Scholar] [CrossRef] [PubMed]
- Kasorndorkbua, C.; Guenette, D.K.; Huang, F.F.; Thomas, P.J.; Meng, X.J.; Halbur, P.G. Routes of Transmission of Swine Hepatitis E Virus in Pigs. J. Clin. Microbiol. 2004, 42, 5047–5052. [Google Scholar] [CrossRef]
- Berto, A.; Martelli, F.; Grierson, S.; Banks, M. Hepatitis E Virus in Pork Food Chain, United Kingdom, 2009–2010. Emerg. Infect. Dis. 2012, 18, 1358. [Google Scholar] [CrossRef]
- de Oliveira-Filho, E.F.; Lopes, K.G.S.; Cunha, D.S.; Silva, V.S.; Barbosa, C.N.; Brandespim, D.F.; Junior, J.W.P.; Bertani, G.R.; Gil, L.H.V.G. Risk Analysis and Occurrence of Hepatitis E Virus (HEV) in Domestic Swine in Northeast Brazil. Food Environ. Virol. 2017, 9, 256–259. [Google Scholar] [CrossRef]
| Variable | Category | N | Univariate Model | |
|---|---|---|---|---|
| OR (95% CI) | p-Value | |||
| Piglets’ purchase | Yes | 160 | - | - |
| No | 107 | 8.03 (4.51–14.34) | <0.01 *** | |
| Variable | Category | N | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Availability of a free-range system | Yes | 19 | 1.41 (1.12–1.77) | 0.004 *** | 1.39 (1.10–1.74) | 0.005 *** |
| No | 248 | - | - | - | - | |
| Presence of a footbath/boot-washer or changing of boots between compartments | Yes | 109 | - | - | - | - |
| No | 158 | 1.17 (1.04–1.32) | 0.01 ** | 1.16 (1.03–1.31) | 0.016 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielick, C.; Ludwig-Begall, L.; Ribbens, S.; Thiry, É.; Faes, C.; Saegerman, C. Biosecurity Risk Factors and Predictive Index for Hepatitis E Virus Serological Status in Belgian Pig Farms: Conventional and Free-Range Systems. Viruses 2025, 17, 432. https://doi.org/10.3390/v17030432
Wielick C, Ludwig-Begall L, Ribbens S, Thiry É, Faes C, Saegerman C. Biosecurity Risk Factors and Predictive Index for Hepatitis E Virus Serological Status in Belgian Pig Farms: Conventional and Free-Range Systems. Viruses. 2025; 17(3):432. https://doi.org/10.3390/v17030432
Chicago/Turabian StyleWielick, Constance, Louisa Ludwig-Begall, Stefaan Ribbens, Étienne Thiry, Christel Faes, and Claude Saegerman. 2025. "Biosecurity Risk Factors and Predictive Index for Hepatitis E Virus Serological Status in Belgian Pig Farms: Conventional and Free-Range Systems" Viruses 17, no. 3: 432. https://doi.org/10.3390/v17030432
APA StyleWielick, C., Ludwig-Begall, L., Ribbens, S., Thiry, É., Faes, C., & Saegerman, C. (2025). Biosecurity Risk Factors and Predictive Index for Hepatitis E Virus Serological Status in Belgian Pig Farms: Conventional and Free-Range Systems. Viruses, 17(3), 432. https://doi.org/10.3390/v17030432







