Abstract
Cytomegalovirus (CMV) infection during pregnancy is the leading cause of congenital infection subsequent to viral transplacental transmission. CMV placental infection can contribute to the development of adverse outcomes likely through placental dysfunction. This case report shows the potential utility of angiogenic markers, such as placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1), in assessing CMV-related placental involvement and monitoring the effect of antiviral therapy on placental function, and highlights the possibility of integrating these markers into the clinical management of CMV infection.
Keywords:
cytomegalovirus; infection; placental dysfunction; angiogenic markers; PlGF; sFlt-1; sFlt-1/PlGF 1. Introduction
Cytomegalovirus (CMV), a DNA herpesvirus, is the leading cause of congenital infection worldwide, with an estimated overall prevalence of 0.67% [1]. This condition represents a significant global health concern due to its potential to cause severe long-term disabilities in affected neonates, including hearing loss and neurological impairment [2]. Neonatal adverse outcomes in congenital CMV (cCMV) infection are related to the timing of maternal infection during pregnancy. In particular, infections acquired during the periconceptional period and the first trimester are at the highest risk of fetal and neonatal sequelae [3,4].
In the absence of a universal maternal serological screening, suspicion of cCMV infection often arises from fetal ultrasound findings, such as growth restriction, and can be confirmed through amniocentesis [5]. Such diagnosis is crucial for timely intervention with the antiviral drug Valacyclovir, which can improve neonatal outcomes [6].
cCMV occurs when the virus infects and crosses the placenta, thus reaching the fetus. Recent studies have shown that placental infection can indirectly play a role in fetal injury, as CMV infects and replicates in cytotrophoblasts [7] and disrupts their function. This can trigger inflammation, leading to villitis, deciduitis, and ischemia [8]. These conditions cause placental edema and fibrosis, reducing oxygen and nutrient supply to the fetus, thus possibly contributing to the development of fetal growth restriction (FGR) [9].
Given the potential role of placental dysfunction in the pathogenesis of cCMV-related FGR, monitoring angiogenic factors such as placental growth factor (PlGF) and soluble Fms-like tyrosine kinase 1 (sFlt-1) could provide valuable insights into CMV-induced placental pathology.
PlGF is a pro-angiogenic protein from the vascular endothelial growth factor (VEGF) family that supports placental angiogenesis and vascular homeostasis. sFlt-1, a soluble VEGF receptor, antagonizes the positive effects of PlGF, leading to endothelial dysfunction; it is released in response to hypoxia and oxidative stress [10] (Figure 1). The assessment of the sFlt1/PlGF ratio is well-established in the diagnosis and management of the obstetric syndrome of preeclampsia (preE) [11]. Imbalanced angiogenesis is characteristic of normal placental maturation but becomes pathognomonic of placental dysfunction when excessive or premature [12]. Elevated maternal serum levels of sFlt-1 are associated with the onset and severity of preE, while low levels of PlGF are linked to both preE and FGR [13].
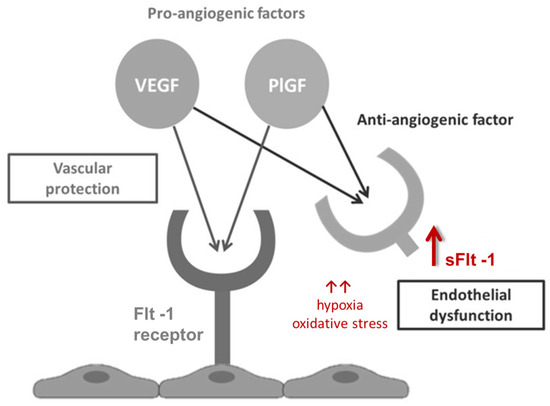
Figure 1.
Angiogenic factor interactions.
This case report explores the potential utility of angiogenic markers, sFlt-1 and PlGF, in assessing placental function in the case of cCMV infection complicated by FGR and undergoing antiviral treatment with Valacyclovir.
2. Detailed Case Description
A 19-year-old primigravida with an unremarkable medical and surgical history and spontaneous conception was diagnosed with FGR according to Delphi criteria [14] at 276/7 weeks’ gestation.
The woman had a pre-pregnancy body mass index of 18.1 kg/m2 and was a non-smoker. Her partner, non-consanguineous, was in good health. Family history was negative for hereditary diseases or congenital malformations. She had worked as a babysitter for a toddler up to the second trimester.
Pregnancy dating was based on her last menstrual period (regular cycles), with a positive pregnancy test one month after her last period and an ultrasound at 125/7 weeks showing a crown-rump length consistent with 12 weeks. The woman decided not to undergo any non-invasive prenatal diagnosis testing.
At the 203/7 week ultrasound, fetal anatomy was normal; however, fetal biometrics were at the lower limits according to Hadlock’s curves [15,16]: abdominal circumference (AC) was at the 6th centile, biparietal diameter (BPD) and head circumference (HC) were below the 10th centile, and the femur length (FL) was at the 20th centile. Maternal–fetal Doppler velocimetry values were normal. Amniocentesis was not performed, in line with the mother’s will; also, maternal CMV serology was not investigated.
At 276/7 weeks, FGR was diagnosed with an AC and estimated fetal weight (792 g) below the 1st centile. Despite a generally low growth trend, fetal growth continued until 354/7 weeks when a plateau was detected, associated with vasodilation in the middle cerebral artery, prompting hospital admission for more intensive monitoring. The ultrasound showed no additional abnormalities, and maternal Doppler velocimetry values were normal. Maternal blood pressure was regular.
During hospital stay, blood pressure remained normal, and routine laboratory tests showed only mild anemia.
To gain a better understanding of the potential underlying cause of FGR, both angiogenic markers (Cobas e601 platform, Roche Diagnostics—Basel, Switzerland) and maternal CMV serology and virology (PCR, CMV ELITe MGB Kit, ELITechGroup—Puteaux, France) were assessed to investigate the placental and infectious cause, respectively. The sFlt-1/PlGF ratio at 356/7 weeks was 106.18, an intermediate value according to Verlohren’s categories [17] (Table 1). PlGF was 94.8 pg/mL, slightly below the 10th centile for gestational age in an uncomplicated singleton pregnancy, but above the 10th centile compared to a term singleton pregnancy (68.6 pg/mL). In contrast, sFlt-1 levels were 10,006 pg/mL, above the 90th percentile for both gestational age and an uncomplicated singleton term pregnancy (7901 pg/mL) [18] (Table 2). Maternal CMV serology showed positive IgG and IgM (IgG 99.6 U/mL, CLIA, positive ≥ 22; IgM 28 U/mL, CLIA, positive ≥ 14), with IgM positivity confirmed with an alternative test (IgM 0.94 index, ELFA, positive >0.9) and intermediate IgG avidity (0.189 index; low avidity < 0.150, high avidity > 0.250). Viral DNA was identified in urine (1350 UI/mL), whereas blood and saliva were negative. These laboratory findings were suggestive of a maternal infection in pregnancy, which prompted the execution of an amniocentesis at 365/7 weeks to assess the fetal infectious status and its karyotype. The virological assessment of the amniotic fluid revealed CMV DNA at high levels (206,000,000 copies/mL); the molecular karyotype was normal. A detailed ultrasound examination did not identify any additional fetal abnormalities.

Table 1.
Current categories for sFlt-1/PlGF ratio with relative cut-offs distinguished by gestational age, < or ≥34th week [17].

Table 2.
Serum reference range 10th–90th percentile for PlGF (pg/mL) and sFlt-1 (pg/mL) across different weeks of pregnancy (Roche Diagnostics) [18].
Considering the diagnosis of symptomatic cCMV infection (i.e., FGR) and in line with the Italian Drug Agency statement on the use of Valacyclovir in pregnancy [19], the woman was started on antiviral therapy (2 g every 6 h) until childbirth.
At 376/7 weeks’ gestation, 8 days after starting Valacyclovir, angiogenic markers were assessed again, revealing an improvement in the angiogenic profile with both PlGF and sFlt-1 within the range for an uncomplicated singleton term pregnancy (114.0 pg/mL and 7303.0 pg/mL, respectively), and an intermediate sFlt-1/PlGF ratio according to Verlohren’s categories (64.06) [17,18] (Table 3).

Table 3.
Trends of angiogenic markers sFlt-1, PlGF, and sFlt-1/ PlGF before and after antiviral therapy.
The 380/7 week-ultrasound evaluation showed an estimated fetal weight of 1866 g (<1st centile according to Hadlock) [16], and a persistent middle cerebral artery vasodilation. Thus, at 382/7 weeks of gestation, labor was medically induced, leading to a vaginal birth of a female newborn weighting 1900 g (<1st centile according to INTERGROWTH-21 charts [20]), with a regular Apgar score and umbilical artery pH.
The patient’s postpartum course was uncomplicated, and she was discharged on the third day after birth, while the neonate was admitted to the neonatal post-intensive care unit for further evaluation. PCR testing for CMV DNA in neonatal blood, urine, and saliva was positive (blood, 4161 copies/mL; urine, 212,000,000 copies/mL; saliva 20,575,137 copies/mL), confirming cCMV infection. Laboratory results revealed mild, transitory thrombocytopenia. Cerebral ultrasound and MRI were negative, with an unremarkable neurological clinical examination. Similarly, additional exams, including abdominal ultrasound, ophthalmological examination with fundus oculi, and hearing screening with otoacustic emission and auditory brainstem response were normal. Thus, postnatal antiviral treatment was not administered. Follow-up at 24 months was regular, with appropriate neurodevelopment score (Bayley-III) and normal hearing.
Placental histology revealed mild chronic villitis with a stromal infiltrate of CD3-positive lymphocytes and rare CD138-positive plasmacytic cells, along with microclots in villous vessels and some fibrotic, avascular villi.
3. Discussion
This case highlights the potential role of angiogenic markers in the context of intrauterine CMV infection. Although these markers provide valuable insights into placental function, their routine clinical use remains inconsistent due to ongoing questions regarding interpretation, cut-off values, and performance in conditions such as infections [21].
High sFlt-1 levels have been observed in maternal infections such as COVID-19, where they are associated with a preE-like syndrome [22]. Additionally, elevated sFlt-1 levels have been documented in cases of maternal infection with viruses that replicate in the placenta [23]. Three case reports described women with mirror syndrome, two of which were associated with CMV infection and one with Parvovirus B19 infection, highlighting the potential connection between viral placental infection and altered angiogenic marker levels. Collectively, these cases suggest that an infected, hydropic placenta can secrete exceptionally high levels of sFlt-1 [24,25,26].
In our case, the initial angiogenic profile was characterized mainly by high levels of sFlt-1 (10,006 pg/mL), which was above the 90th centile for both gestational age and an uncomplicated singleton term pregnancy (7901 pg/mL) [18], alongside with a slightly decreased PlGF value (94.8 pg/mL) for gestational age (<10th but above the 5th centile). This angiogenic profile is not typical of placental-related FGR cases, which are usually characterized by very low levels of PlGF [13].
It is possible that PlGF is not substantially impacted by placental CMV infection, since CMV replicates in the cytotrophoblast whereas PlGF is produced by the syncytiotrophoblast. In addition, placental CMV infection has been shown to directly and indirectly enhance all stages of angiogenesis, including the induction of the release of angiogenic factors like VEGF and IL-6, thus leading to pathological angiogenesis [27]. In turn, virally induced placental abnormalities, like villitis, deciduitis, and ischemia, could induce elevated sFlt-1 levels, as this anti-angiogenic molecule is released in response to hypoxia and oxidative stress [10].
Altogether, these findings suggest that angiogenic markers could be used in cases of FGR to aid in the differential diagnosis and better define the extent of CMV-induced placental damage. Of note, sFlt-1 concentration in maternal blood has been recently suggested as a potential predictive biomarker of transplacental CMV transmission in cases of maternal infection in pregnancy [28]. This study showed significant differences in sFlt-1 levels between CMV-transmitting and non-transmitting women; in addition, the authors reported that the sFlt1/PlGF ratio was not altered by transmission, although some cases of transmission were associated with elevated values >38, thus confirming our observation that PlGF does not undergo substantial changes in the case of placental CMV infection. Our case report emphasizes the importance of analyzing both markers and not just the sFlt-1/PlGF ratio and comparing their values with those of a singleton uncomplicated pregnancy at the same gestational age and at term [29,30].
Assessing sFlt-1 levels enables the identification of patients at high risk for preE. The excessive production of sFlt-1 leads to maternal endothelial injury, which contributes to the development of preE manifestations [31], and serves as a strong predictor of both preE severity and adverse outcomes. There appears to be a threshold beyond which elevated sFlt-1 levels become toxic to the mother. Our research demonstrated that increased sFlt-1 levels (≥15,802 pg/mL) are associated with severe obstetric complications in multiple pregnancies with hypertensive disorders of pregnancy (HDP) and FGR, irrespective of gestational age and chorionicity [32]. These findings emphasize the importance of monitoring sFlt-1 levels to predict potential adverse outcomes.
In our case, the sFlt-1 levels were above the 90th centile for both gestational age and an uncomplicated singleton term pregnancy, but below 15,802 pg/mL, and the patient did not develop preE. Interestingly, several observational and experimental studies have reported that CMV infection can constitute a risk factor for the development of preE, hypertension, and cardiovascular disease [33].
Another interesting aspect of this case report is the effect of the antiviral therapy with Valacyclovir on placental angiogenesis. After only 8 days of treatment, the angiogenic placental markers were already within the range for gestational age, thus suggesting that these markers’ levels can vary over time with appropriate therapies. By reducing sFlt-1 levels, antiviral therapy could reduce placental damage, thus possibly averting the development of preE [34].
4. Conclusions
This case report provides new insights into the role of angiogenic markers, PlGF and sFlt-1, in maternal and fetal CMV infections. Specifically, it supports their role in the differential diagnosis of FGR and in providing meaningful insights into the severity of CMV-induced placental damage. It also highlights their potential ability in monitoring the effects of the antiviral therapy on placental function. Additionally, this case report underscores the importance of assessing both angiogenic markers separately, and not only their ratio, and comparing them with values from an uncomplicated pregnancy at the same gestational age and at term.
However, it is important to acknowledge that the findings are based on a single case report, and therefore, further research is needed to explore placental angiogenesis in greater depth. In the meantime, it is crucial to educate obstetricians and neonatologists on the interpretation and clinical application of these markers.
Author Contributions
Conceptualization, writing—original draft preparation and investigation, V.G.; writing—review and editing, V.G., R.C., S.O., M.C. and A.L.; data collection, V.G., R.C. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The patient has provided informed consent for publication.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries With Universal Screening: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2120736. [Google Scholar] [CrossRef]
- Salomè, S.; Corrado, F.R.; Mazzarelli, L.L.; Maruotti, G.M.; Capasso, L.; Blazquez-Gamero, D.; Raimondi, F. Congenital cytomegalovirus infection: The state of the art and future perspectives. Front. Pediatr. 2023, 11, 1276912. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Ville, Y.; Makrydimas, G.; Dinas, K.; Zavlanos, A.; Sotiriadis, A. Timing of primary maternal cytomegalovirus infection and rates of vertical transmission and fetal consequences. Am. J. Obstet. Gynecol. 2020, 223, 870–883.e11. [Google Scholar] [CrossRef]
- Naing, Z.W.; Scott, G.M.; Shand, A.; Hamilton, S.T.; Van Zuylen, W.J.; Basha, J.; Hall, B.; Craig, M.E.; Rawlinson, W.D. Congenital cytomegalovirus infection in pregnancy: A review of prevalence, clinical features, diagnosis and prevention. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 9–18. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM); Hughes, B.L.; Gyamfi-Bannerman, C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am. J. Obstet. Gynecol. 2016, 214, B5–B11. [Google Scholar] [CrossRef]
- Pinninti, S.; Boppana, S. Antiviral Treatment of Maternal and Congenital Cytomegalovirus (CMV) Infections. Viruses 2023, 15, 2116. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Maidji, E. Cytomegalovirus infection in the human placenta: Maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr. Top. Microbiol. Immunol. 2008, 325, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.M.; Chow, S.S.W.; Craig, M.E.; Pang, C.N.I.; Hall, B.; Wilkins, M.R.; Jones, C.A.; Lloyd, A.R.; Rawlinson, W.D. Cytomegalovirus infection during pregnancy with maternofetal transmission induces a proinflammatory cytokine bias in placenta and amniotic fluid. J. Infect. Dis. 2012, 205, 1305–1310. [Google Scholar] [CrossRef]
- Tanimura, K.; Uchida, A.; Uenaka, M.; Imafuku, H.; Tairaku, S.; Hashimura, H.; Ueno, Y.; Kido, T.; Fujioka, K. Fetal Ultrasound and Magnetic Resonance Imaging Abnormalities in Congenital Cytomegalovirus Infection Associated with and without Fetal Growth Restriction. Diagnostics 2023, 13, 306. [Google Scholar] [CrossRef]
- Murphy, S.R.; Cockrell, K. Regulation of soluble fms-like tyrosine kinase-1 production in response to placental ischemia/hypoxia: Role of angiotensin II. Physiol. Rep. 2015, 3, e12310. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Brennecke, S.P.; Galindo, A.; Karumanchi, S.A.; Mirkovic, L.B.; Schlembach, D.; Stepan, H.; Vatish, M.; Zeisler, H.; Rana, S. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022, 27, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shinar, S.; Tigert, M.; Agrawal, S.; Parks, W.A.; Kingdom, J.C. Placental growth factor as a diagnostic tool for placental mediated fetal growth restriction. Pregnancy Hypertens. 2021, 25, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Deter, R.L.; Harrist, R.B.; Park, S.K. Estimating fetal age: Computer-assisted analysis of multiple fetal growth parameters. Radiology 1984, 152, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 2012, 206, 58.e1–58.e8. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase–specific cutoff values for the use of the soluble fms-Like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Agenzia Italiana del Farmaco (AIFA). Valaciclovir—Uso Terapeutico per la Prevenzione e il Trattamento Dell’Infezione da CMV in Gravidanza. 2021. Available online: https://www.aifa.gov.it/documents/20142/1288746/Allegato-1_Valaciclovir_04.01.2021.pdf (accessed on 22 January 2025).
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; on behalf of the Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the Diagnosis and Prognosis of Preeclampsia with the Aid of the sFlt-1/ PlGF Ratio in Singleton Pregnancies. Fetal Diagn. Ther. 2017, 43, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Giardini, V.; Ornaghi, S.; Gambacorti-Passerini, C.; Casati, M.; Carrer, A.; Acampora, E.; Vasarri, M.V.; Arienti, F.; Vergani, P. Imbalanced Angiogenesis in Pregnancies Complicated by SARS-CoV-2 Infection. Viruses 2022, 14, 2207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereira, L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Venkatesha, S.; DePaepe, M.; Chien, E.K.; Paglia, M.; Karumanchi, S.A. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet. Gynecol. 2007, 109, 549–552. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.; Sass, N.; Boute, T.; Moron, A.F. sFlt-1 and PlGF levels in a patient with mirror syndrome related to cytomegalovirus infection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Faber, R. Elevated sFlt1 Level and Preeclampsia with Parvovirus-Induced Hydrops. New Engl. J. Med. 2006, 354, 1857–1858. [Google Scholar] [CrossRef]
- Caposio, P.; Orloff, S.L.; Streblow, D.N. The role of cytomegalovirus in angiogenesis. Virus Res. 2011, 157, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Penka, L.; Kagan, K.-O.; Hamprecht, K. Enhanced Serum Levels of sFlt1: Impact on Materno–Fetal CMV Transmission. J. Clin. Med. 2020, 9, 1258. [Google Scholar] [CrossRef] [PubMed]
- Giardini, V.; Casati, M.; Vergani, P.; Locatelli, A. Letter to the editor: Caution: Not all sFlt-1/PlGF ratios are the same. Pregnancy Hypertens. 2024, 37, 101149. [Google Scholar] [CrossRef]
- Giardini, V.; Pelucchi, A.; Daolio, C.; Casati, M.; Vergani, P.; Lattuada, M.; Locatelli, A. Coffin-Siris Syndrome and Unusual Angiogenic Profiles in Pregnancy: A Case Study Emphasizing Caution in Interpreting a Very Low sFlt-1/PlGF Ratio. Am. J. Med Genet. Part A 2024, 197, e63939. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Giardini, V.; Grilli, L.; Terzaghi, A.; Todyrenchuk, L.; Zavettieri, C.; Mazzoni, G.; Cozzolino, S.; Casati, M.; Vergani, P.; Locatelli, A. sFlt-1 Levels as a Predicting Tool in Placental Dysfunction Complications in Multiple Pregnancies. Biomedicines 2023, 11, 2917. [Google Scholar] [CrossRef] [PubMed]
- Geraili, Z.; Riahi, S.M.; Khani, S.; Bayani, M.; Rostami, A.; Hajian-Tilaki, K.; Shiadeh, M.N. Cytomegalovirus infection and risk of preeclampsia: A meta-analysis of observational studies. Casp. J. Intern. Med. 2018, 9, 211–219. [Google Scholar] [CrossRef]
- Giardini, V.; Gambacorti-Passerini, C.; Casati, M.; Carrer, A.; Vergani, P. Analogies between COVID-19 and Preeclampsia: Focus on Therapies. Int. J. Transl. Med. 2023, 3, 203–216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).