Molecular Survey and Genetic Characterization of Hop Stunt Viroid (HSVd) in Fruit Trees in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
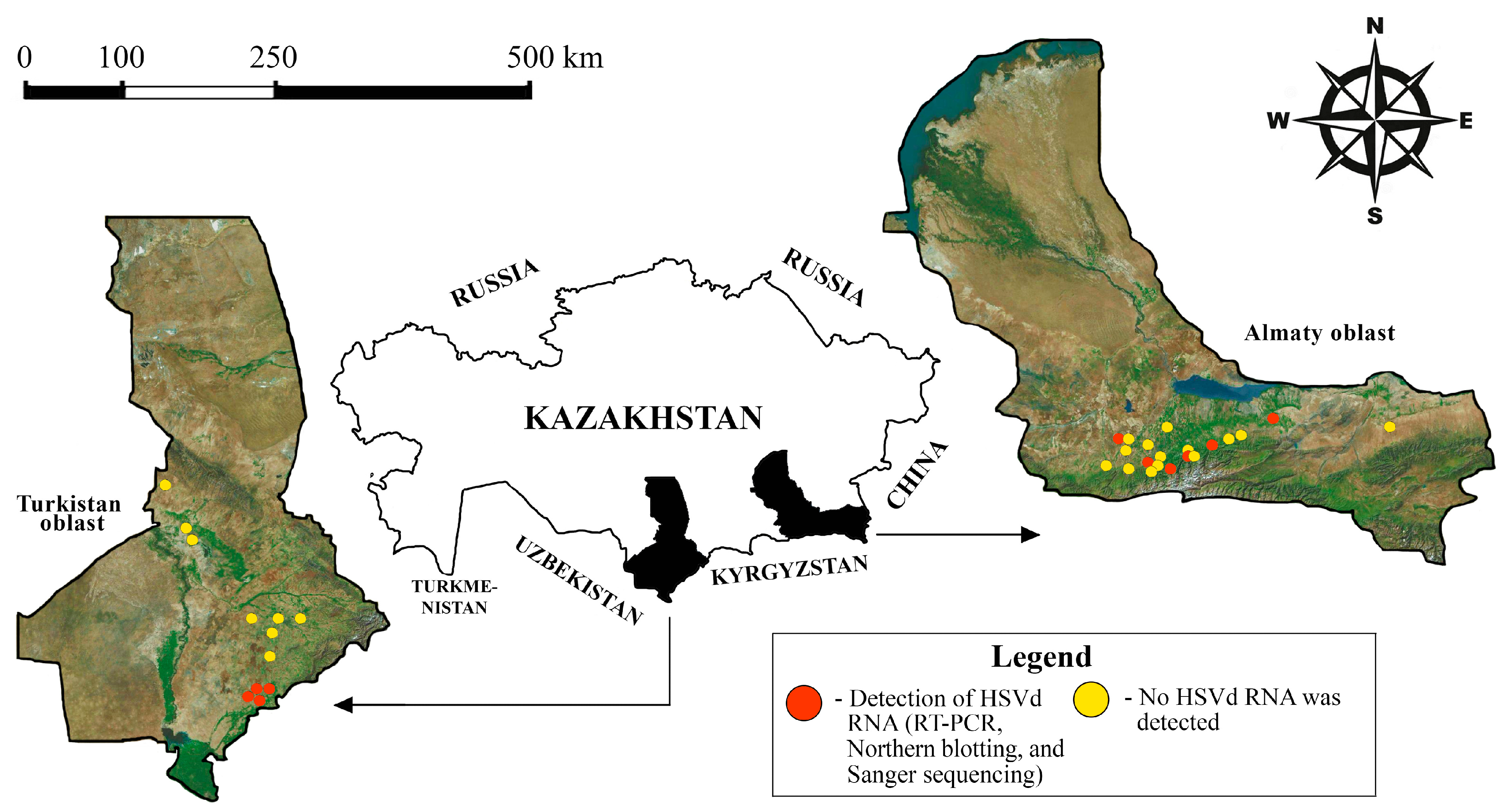
2.1. Study Area
2.2. Plant Material and RNA Isolation
2.3. Reverse Transcription, PCR, and cDNA Cloning
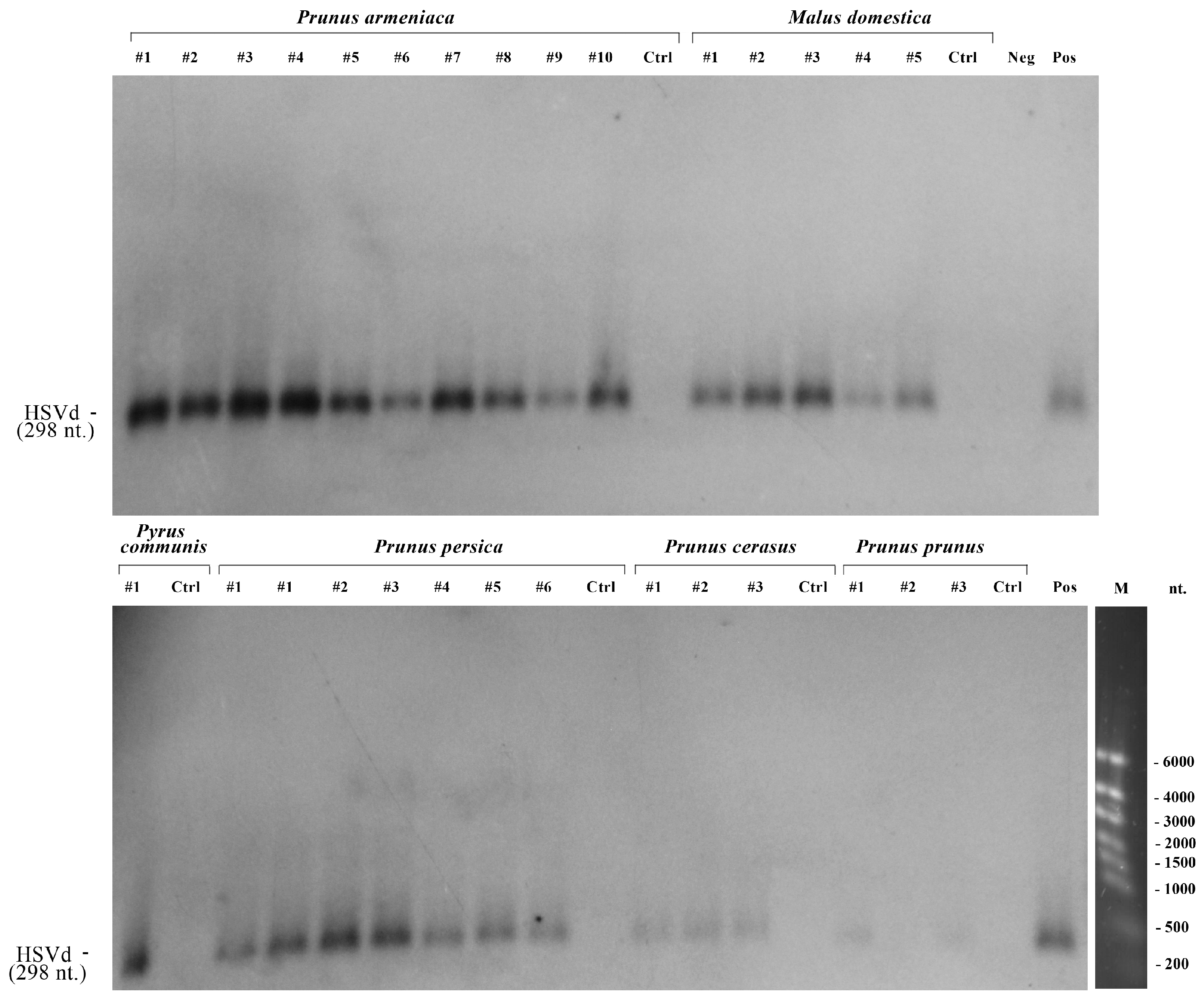
2.4. Northern Blotting
2.5. Sequencing and Phylogenetic Analysis
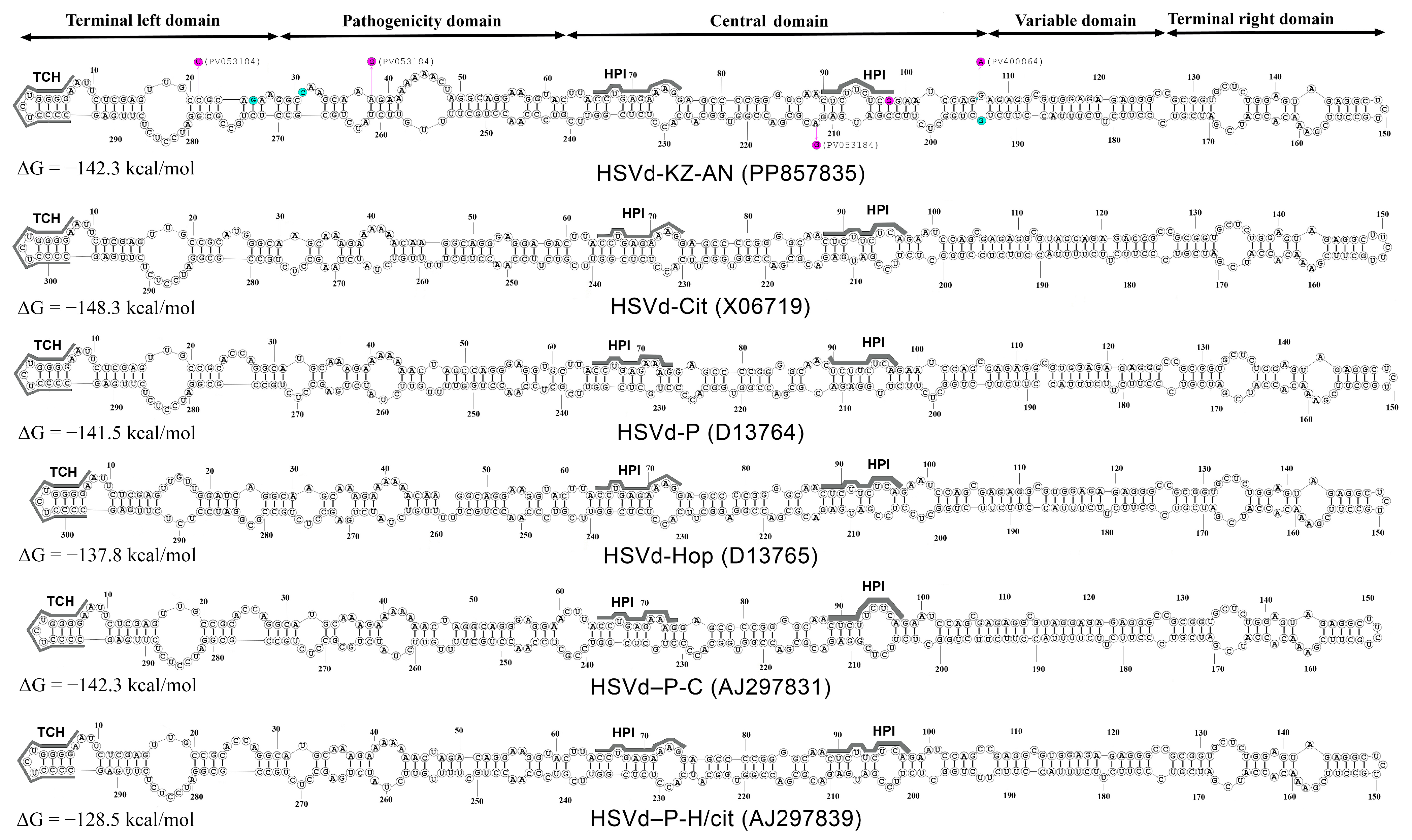
2.6. Prediction of Secondary Structures of Viroid RNAs
3. Results
3.1. Samples
3.2. Identification of HSVd from Fruit Trees
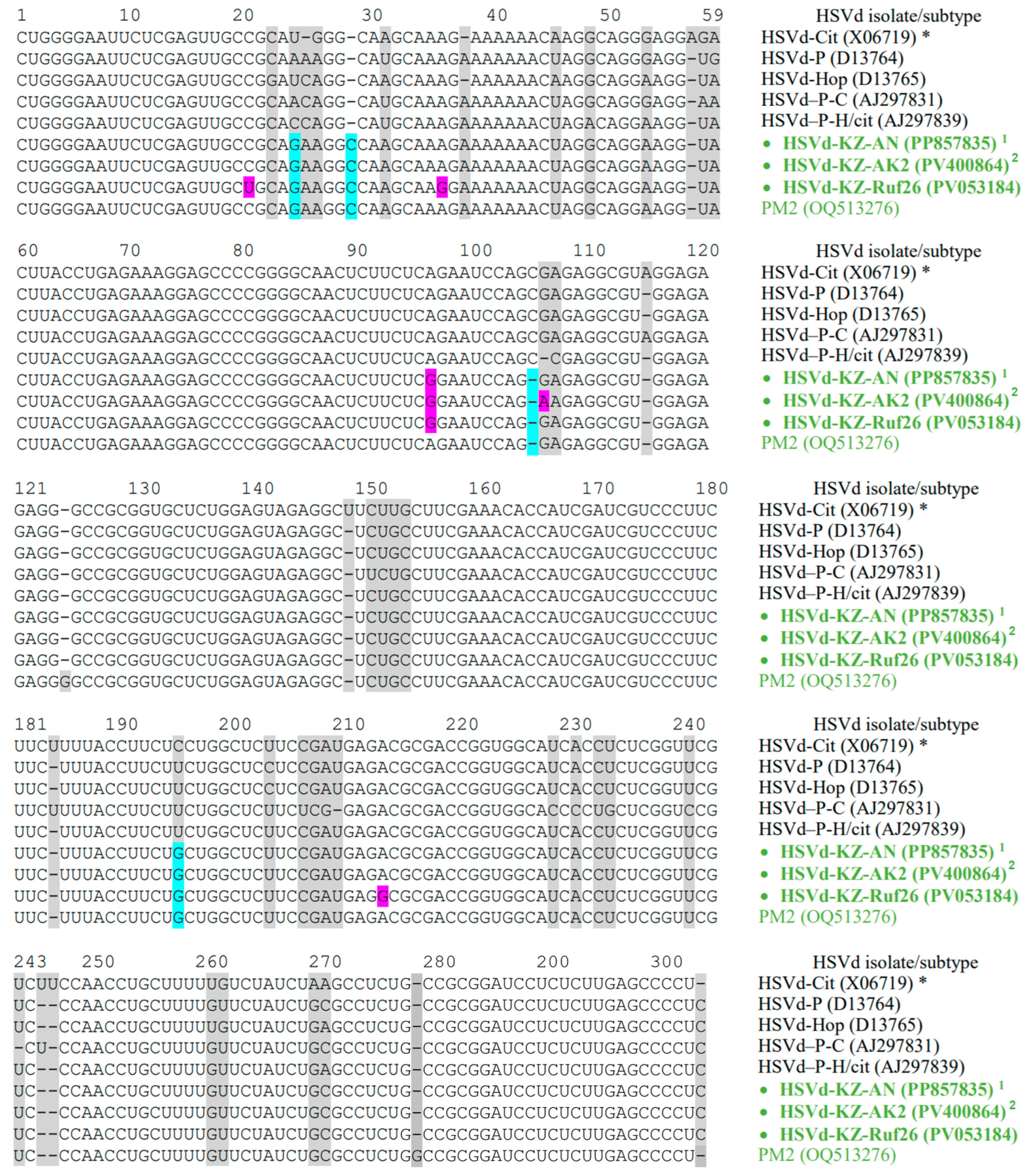
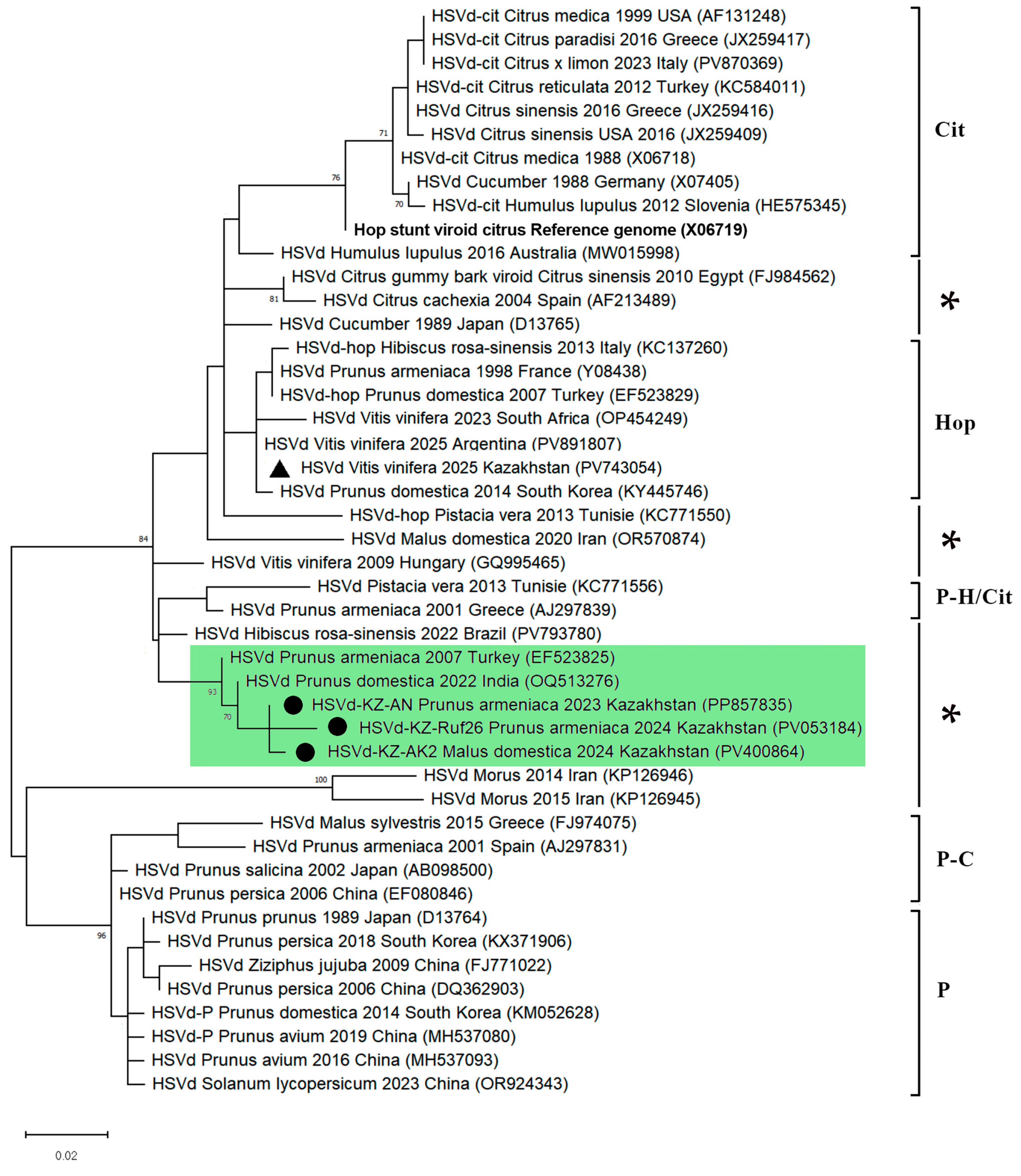
3.3. Phylogenetic Analysis of the Identified HSVd Variants
3.4. RNA Secondary Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLAST | Basic GenBank Local Alignment Search Tool |
| 95% CI | 95% confidence interval |
| EAEU | The Eurasian Economic Union |
| HSVd | Hop stunt viroid |
| MEGA | Molecular Evolutionary Genetics Analysis |
| SNP | Single nucleotide polymorphism |
| ΔG | The Gibbs free energy |
References
- Wang, Y. Current view and perspectives in viroid replication. Curr. Opin. Virol. 2021, 47, 32–37. [Google Scholar] [CrossRef]
- Flores, R.; Daròs, J.-A.; Hernández, C.; Navarro, B.; Di Serio, F. Viroids. Encycl. Life Sci. 2020, 1, 192–203. [Google Scholar] [CrossRef]
- Di Serio, F.; Li, S.-F.; Pallás, V.; Owens, R.A.; Randles, J.W.; Sano, T.; Verhoeven, J.T.J.; Vidalakis, G.; Flores, R. Chapter 13-Viroid Taxonomy. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: Cambridge, MA USA, 2017; pp. 135–146. [Google Scholar] [CrossRef]
- Di Serio, F.; Owens, R.A.; Li, S.F.; Matoušek, J.; Pallás, V.; Randles, J.W.; Sano, T.; Verhoeven, J.T.J.; Vidalakis, G.; Flores, R.; et al. ICTV virus taxonomy profile: Pospiviroidae. J. Gen. Virol. 2021, 102, 001543. [Google Scholar] [CrossRef]
- Marquez-Molins, J.; Gomez, G.; Pallas, V. Hop stunt viroid: A polyphagous pathogenic RNA that has shed light on viroid-host interactions. Mol. Plant Pathol. 2021, 22, 153–162. [Google Scholar] [CrossRef]
- CABI Compendium. Hop Stunt Viroid. Wallingford, UK. 2022. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.27694 (accessed on 5 May 2025).
- EPPO Global Database. Hostuviroid impedihumuli (HSVD00). 2025. Available online: https://gd.eppo.int/taxon/HSVD00/distribution (accessed on 5 May 2025).
- Guo, L.; Liu, S.; Wu, Z.; Mu, L.; Xiang, B.; Li, S. Hop stunt viroid (HSVd) newly reported from hop in Xinjiang, China. Plant Pathol. 2008, 57, 764. [Google Scholar] [CrossRef]
- Sano, T.; Mimura, R.; Ohshima, K. Phylogenetic analysis of hop and grapevine isolates of hop stunt viroid supports a grapevine origin for hop stunt disease. Virus Genes 2001, 22, 53–59. [Google Scholar] [CrossRef]
- Di Serio, F.; Ambrós, S.; Sano, T.; Flores, R.; Navarro, B. Viroid Diseases in pome and stone fruit trees and Koch’s postulates: A critical assessment. Viruses 2018, 10, 612. [Google Scholar] [CrossRef]
- Amari, K.; Ruiz, D.; Gómez, G.; Sánchez-Pina, M.A.; Pallás, V.; Egea, J. An important new apricot disease in Spain is associated with hop stunt viroid infection. Eur. J. Plant Pathol. 2007, 118, 173–181. [Google Scholar] [CrossRef]
- Hataya, T.; Tsushima, T.; Sano, T. Hop stunt viroid. In Viroids and Satellites; Hadidi, A., Randles, J., Flores, R., Palukaitis, P., Eds.; Academic Press: Cambridge, MA USA, 2017; pp. 199–210. [Google Scholar] [CrossRef]
- Lemmetty, A.; Werkman, A.W.; Soukainen, M. First report of Hop stunt viroid in greenhouse cucumber in Finland. Plant Disease 2011, 95, 615. [Google Scholar] [CrossRef] [PubMed]
- Alaxin, P.; Predajňa, L.; Achs, A.; Šubr, Z.; Mrkvová, M.; Glasa, M. Analysis of Hop stunt viroid diversity in grapevine (Vitis vinifera L.) in Slovakia: Coexistence of Two Particular Genetic Groups. Pathogens 2023, 12, 205. [Google Scholar] [CrossRef]
- Wüsthoff, K.P.; Steger, G. Conserved Motifs and Domains in Members of Pospiviroidae. Cells 2022, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Kolonko, N.; Bannach, O.; Aschermann, K.; Hu, K.H.; Moors, M.; Schmitz, M.; Steger, G.; Riesner, D. Transcription of potato spindle tuber viroid by RNA polymerase II starts in the left terminal loop. Virology 2006, 347, 392–404. [Google Scholar] [CrossRef]
- Schnölzer, M.; Haas, B.; Raam, K.; Hofmann, H.; Sänger, H.L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985, 4, 2181–2190. [Google Scholar] [CrossRef]
- Kitabayashi, S.; Tsushima, D.; Adkar-Purushothama, C.R.; Sano, T. Identification and molecular mechanisms of key nucleotides causing attenuation in pathogenicity of Dahlia isolate of Potato spindle tuber viroid. Int. J. Mol. Sci. 2020, 21, 7352. [Google Scholar] [CrossRef] [PubMed]
- Baumstark, T.; Schröder, A.R.; Riesner, D. Viroid processing: Switch from cleavage to ligation is driven by a change from a tetraloop to a loop E conformation. EMBO J. 1997, 16, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Sano, T. Progress in 50 years of viroid research-Molecular structure, pathogenicity, and host adaptation. PJA Series B 2021, 97, 371–401. [Google Scholar] [CrossRef]
- Diener, T. Viroid processing: A model involving the central conserved region and hairpin I. Proc. Natl. Acad. Sci. USA 1986, 83, 58–62. [Google Scholar] [CrossRef]
- Qi, Y.; Ding, B. Inhibition of cell growth and shoot development by a specific nucleotide sequence in a noncoding viroid RNA. Plant Cell 2003, 15, 1360–1374. [Google Scholar] [CrossRef]
- Keese, P.; Symons, R. Domains in viroids: Evidence of intermolecular RNA rearrangement and their contribution to viroid evolution. Proc. Natl. Acad. Sci. USA 1985, 82, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Steger, G. Modelling the three-dimensional structure of the right-terminal domain of pospiviroids. Sci. Rep. 2017, 7, 711. [Google Scholar] [CrossRef]
- Belkina, D.; Karpova, D.; Porotikova, E.; Lifanov, I.; Vinogradova, S. Grapevine Virome of the Don Ampelographic Collection in Russia Has Concealed Five Novel Viruses. Viruses 2023, 15, 2429. [Google Scholar] [CrossRef]
- Yang, Y.A.; Wang, H.Q.; Guo, R.; Cheng, Z.M.; Li, S.F.; Sano, T. First Report of Hop stunt viroid in Apricot in China. Plant Dis. 2006, 90, 828. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.W.; Zhu, D.Z.; Zong, X.J.; Wei, H.R.; Chen, X.; Hammond, R.W.; Liu, Q.Z. First report of hop stunt viroid from sweet cherry with dapple fruit symptoms in China. Plant Dis. 2017, 101, 394. [Google Scholar] [CrossRef]
- Aubakirova, K.P.; Bakytzhanova, Z.N.; Rakhatkyzy, A.; Yerbolova, L.S.; Malakhova, N.P.; Galiakparov, N.N. Nanopore Workflow for Grapevine Viroid Surveillance in Kazakhstan: Bypassing rRNA Depletion Through Non-Canonical Priming. Pathogens 2025, 14, 782. [Google Scholar] [CrossRef]
- World Bank. Kazakhstan, General Water Security Assessment. Available online: http://documents.worldbank.org/curated/en/099062424121021579 (accessed on 5 May 2025).
- Bureau of National Statistics; Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. Annual Data Report: Statistics of Agriculture, Forestry, Hunting and Fisheries. Available online: https://stat.gov.kz/en/industries/business-statistics/stat-forrest-village-hunt-fish/spreadsheets (accessed on 5 May 2025).
- Interactive maps and databases. Official Website of the Hydrometeorological Service of Kazakhstan. Available online: https://www.kazhydromet.kz/en/interactive_cards (accessed on 10 November 2025).
- World Map, Satellite View, Earth Map Online Service. Available online: https://satellites.pro/Kazakhstan_map (accessed on 5 May 2025).
- Li, R.; Mock, R.; Huang, Q.; Abad, J.; Hartung, J.; Kinard, G. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J. Virol. Methods. 2008, 154, 48–55. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). Available online: https://www.ncbi.nlm.nih.gov (accessed on 5 May 2025).
- Zhou, M.-Y.; Gomez-Sanchez, C.E. Universal TA Cloning. Curr. Issues Mol. Biol. 2000, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- RNAstructure, Web Servers for RNA Secondary Structure Prediction. Available online: https://rna.urmc.rochester.edu/RNAstructureWeb/index.html (accessed on 31 May 2025).
- Reuter, J.S.; Mathews, D.H. RNAstructure: Software for RNA secondary structure prediction and analysis. BMC Bioinform. 2010, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Hataya, T.; Shikata, E. Complete nucleotide sequence of a viroid isolated from Etrog citron, a new member of hop stunt viroid group. Nucleic Acids Res. 1988, 16, 347. [Google Scholar] [CrossRef]
- Kofalvi, S.A.; Marcos, J.F.; Cañizares, M.C.; Pallás, V.; Candresse, T. Hop stunt viroid (HSVd) sequence variants from Prunus species: Evidence for recombination between HSVd isolates. J. Gen. Virol. 1997, 78, 3177–3186. [Google Scholar] [CrossRef]
- Shilpa, N.; Sano, T.; Naoi, T.; Janardhana, J.G. Molecular phylogeny and secondary structure analysis of hop stunt viroid (HSVd) associated with Mulberry (Morus alba) in India. Arch. Microbiol. 2024, 206, 240. [Google Scholar] [CrossRef]
- Amari, K.; Gomez, G.; Myrta, A.; Di Terlizzi, B.; Pallas, V. The molecular characterization of 16 new sequence variants of Hop stunt viroid reveals the existence of invariable regions and a conserved hammerhead-like structure on the viroid molecule. J. Gen. Virol. 2001, 82, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Chu, H.; Kim, H.; Cho, J.K.; Lian, S.; Choi, H.; Kim, S.-M.; Kim, S.-L.; Choon Lee, B.; Cho, W.K. Comprehensive analysis of genomic variation of Hop stunt viroid. Eur. J. Plant Pathol. 2017, 148, 119–127. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Guo, R.; Mu, L.; Yang, Y.; Li, S.; Wang, H. Molecular characterization of Chinese Hop stunt viroid isolates reveals a new phylogenetic group and possible cross transmission between grapevine and stone fruits. Eur. J. Plant Pathol. 2012, 134, 217–225. [Google Scholar] [CrossRef]
- Yang, Y.-A.; Wang, H.-Q.; Wu, Z.-J.; Cheng, Z.-M.; Li, S.-F. Molecular variability of hop stunt viroid: Identification of a unique variant with a tandem 15-nucleotide repeat from naturally infected plum tree. Biochem. Genet. 2008, 46, 113–123. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucle-otide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Gazel, M.; Ulubas Serce, C.; Caglayan, K.; Faggioli, F. Sequence variability of Hop stunt viroid isolates from stone fruits in Turkey. Plant Pathol. J. 2008, 90, 23–28. [Google Scholar]
- Choueiri, E.; Ghanem-Sabanadzovic, N.A.; Khazzaka, K.; Sabanadzovic, S.; di Terlizzi, B.; Myrta, A.; El-Zammar, S.; Jreijiri, F.; Savino, V. First record of Hop stunt viroid in apricot in Lebanon. Plant Pathol. J. 2002, 84, 69. [Google Scholar]
- Naser, S.M.; Alabdallah, O.; Abualrob, A.; Abou Kubaa, R.; Alkowni, R. Molecular detection and identification of citrus bent leaf viroid (CBLVd) and hop stunt viroid (HSVd) in Palestine. Arch. Phytopathol. Pflanzenschutz 2025, 58, 356–364. [Google Scholar] [CrossRef]
- Kaponi, M.; Kyriakopoulou, P.E.; Hadidi, A. Viroids of the Mediterranean Basin. Viruses 2024, 16, 612. [Google Scholar] [CrossRef]
- Vadamalai, G.; Adkar-Purushothama, C.R.; Thanarajoo, S.S.; Iftikhar, Y.; Shruthi, B.; Yanjarappa, S.M.; Sano, T. Chapter 5-Viroids diseases and its distribution in Asia. In Fundamentals of Viroid Biology; Adkar-Purushothama, C.R., Sano, T., Perreault, J.-P., Yanjarappa, S.M., Di Serio, F., Daròs, J.-A., Eds.; Academic Press: Cambridge, MA USA, 2024; pp. 85–107. [Google Scholar] [CrossRef]
- Luigi, M.; Faggioli, F. Development of a quantitative real-time RT-PCR (qRT-PCR) for the detection of hop stunt viroid. Eur. J. Plant. Pathol. 2013, 137, 231–235. [Google Scholar] [CrossRef]
- El-Dougdoug, K.A.; Dawoud, R.A.; Rezk, A.A.; Sofy, A.R. Incidence of fruit trees viroid diseases by tissue print hybridization in Egypt. Int. J. Virol. 2012, 8, 114–120. [Google Scholar] [CrossRef]
- Fekih Hassen, I.; Kummert, J.; Marbot, S.; Fakhfakh, H.; Marrakchi, M.; Jijakli, M.H. First Report of Pear blister canker viroid, Peach latent mosaic viroid, and Hop stunt viroid Infecting Fruit Trees in Tunisia. Plant Dis. 2004, 88, 1164. [Google Scholar] [CrossRef] [PubMed]
- Unified List of Quarantine Objects of the Eurasian Economic Union, Approved in Accordance with Paragraph 3 of Article 59 of the Treaty on the Eurasian Economic Union dated May 29 2014. Available online: https://adilet.zan.kz/rus/docs/H16EV000158 (accessed on 5 May 2025).
| No. | Locality | Species | RT-PCR Positive | Northern Blot Tested/Confirmed | Cloned and Sequenced |
|---|---|---|---|---|---|
| 1 | Ak-Kaiyn | peach | 2 | 2/2 | 1 |
| 2 | Esik | apricot | 2 | 2/2 | 1 |
| 3 | Saymasay | pear | 1 | 1/1 | 1 |
| 4 | Shamalgan | plum | 3 | 3/2 | 1 |
| 5 | Algabas | peach | 1 | 1/1 | 1 |
| 6 | Besagash | cherry | 2 | 2/2 | 1 |
| 7 | apricot | 3 | 3/3 | 1 | |
| 8 | Akniet | cherry | 1 | 1/1 | 1 |
| 9 | Akzhar-1 | apple | 1 | 1/1 | 1 |
| 10 | apricot | 1 | 1/1 | 1 | |
| 11 | Akzhar-2 | apple | 4 | 4/4 | 1 |
| 12 | peach | 1 | 1/1 | 1 | |
| 13 | Jemisty | peach | 3 | 3/3 | 1 |
| 14 | apricot | 3 | 3/3 | 1 | |
| 15 | Yntymak | apricot | 1 | 1/1 | 1 |
| Total | 29 | 29/28 | 15 | ||
| Isolate | Accession Number | Host | Locality | New SNPs | Symptoms 1 |
|---|---|---|---|---|---|
| HSVd-KZ-AN | PP857835 | apricot | Besagash | A96G | DF |
| HSVd-KZ-KEp1 | PV053182 | peach | Algabas | A96G | CB, FD |
| HSVd-KZ-KEp3 | PV053183 | peach | Ak-Kaiyn | A96G | DF; D |
| HSVd-KZ-Ruf26 | PV053184 | apricot | Esik | C21U, A36G, A96G, A213G | |
| HSVd-KZ-SAY | PV400861 | pear | Saymasay | A96G | - |
| HSVd-KZ-ShML | PV400859 | plum | Shamalgan | A96G | - |
| HSVd-KZ-BES | PV400860 | sweet cherry | Besagash | A96G | - |
| HSVd-KZ-Sar72 | PV053185 | apricot | Yntymak | A96G | - |
| HSVd-KZ-Jem35 | PV053186 | apricot | Jemisty | A96G | - |
| HSVd-KZ-Jem2 | PX516286 | peach | Jemisty | A96G | |
| HSVd-KZ-SAR | PV400862 | cherry | Akniet | A96G | - |
| HSVd-KZ-AK1 | PV400863 | apple | Akzhar-1 | A96G | - |
| HSVd-KZ-AK3 | PX516284 | apricot | Akzhar-1 | A96G | |
| HSVd-KZ-AK2 | PV400864 | apple | Akzhar-2 | A96G, G106A | - |
| HSVd-KZ-AK4 | PX516285 | peach | Akzhar-2 | A96G, G106A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadirova, L.T.; Stanbekova, G.E.; Nizkorodova, A.S.; Kryldakov, R.V.; Iskakov, B.K.; Zhigailov, A.V. Molecular Survey and Genetic Characterization of Hop Stunt Viroid (HSVd) in Fruit Trees in Kazakhstan. Viruses 2025, 17, 1547. https://doi.org/10.3390/v17121547
Nadirova LT, Stanbekova GE, Nizkorodova AS, Kryldakov RV, Iskakov BK, Zhigailov AV. Molecular Survey and Genetic Characterization of Hop Stunt Viroid (HSVd) in Fruit Trees in Kazakhstan. Viruses. 2025; 17(12):1547. https://doi.org/10.3390/v17121547
Chicago/Turabian StyleNadirova, Leila T., Gulshan E. Stanbekova, Anna S. Nizkorodova, Ruslan V. Kryldakov, Bulat K. Iskakov, and Andrey V. Zhigailov. 2025. "Molecular Survey and Genetic Characterization of Hop Stunt Viroid (HSVd) in Fruit Trees in Kazakhstan" Viruses 17, no. 12: 1547. https://doi.org/10.3390/v17121547
APA StyleNadirova, L. T., Stanbekova, G. E., Nizkorodova, A. S., Kryldakov, R. V., Iskakov, B. K., & Zhigailov, A. V. (2025). Molecular Survey and Genetic Characterization of Hop Stunt Viroid (HSVd) in Fruit Trees in Kazakhstan. Viruses, 17(12), 1547. https://doi.org/10.3390/v17121547








