The Host Immune Response to Enterovirus A71 (EV-A71): From Viral Immune Evasion to Immunopathology and Prognostic Biomarkers of Severe Disease—A Narrative Review
Abstract
1. Introduction
2. Characteristics of Enterovirus A71
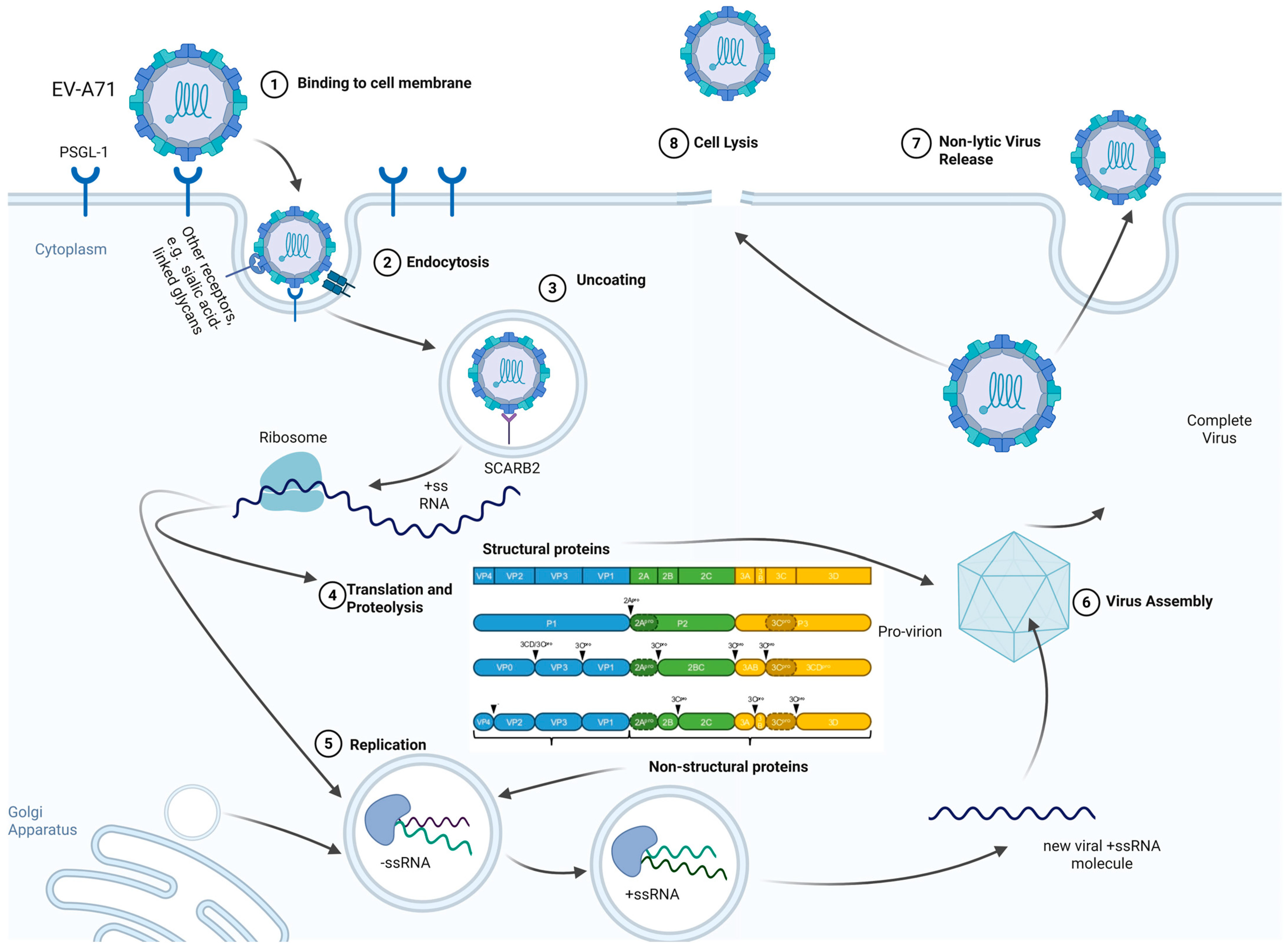
2.1. Viral Structure and Life Cycle
2.2. Tissue Tropism with Emphasis on the Central Nervous System (CNS)
2.3. Genotype—Pathogenicity Associations in Human EV-A71 Infections
3. Innate Immune Response to EV-A71
3.1. Early Recognition and Evasion of Host Immune Sensors
3.2. Type I and III Interferons and Proinflammatory Cytokines
3.3. Natural Killer Cell Responses and Monocyte Dysfunction in EV-A71 Infection
4. Adaptive Immune Response to EV-71
4.1. Humoral Response
4.2. Cellular Response
4.3. Dysfunction of Regulatory T Cells (Treg) and cAMP Reduction
5. Clinical Implications and Biomarkers of Severe EV-A71 Infection
5.1. Pathogenic Cytokine Storm and Clinical Correlates
5.2. Biomarkers and Predictors of Immune Dysfunction
6. Discussion
6.1. Vaccine Efficacy and Real-World Effectiveness
6.2. Cross-Protection, Genotype Shift, and Multivalent Strategies
6.3. Safety Profile and Immunological Risks
6.4. Therapeutic Strategies: Risk/Benefit Analysis and Pediatric Safety
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.M.; Wang, C.S.; Yu, X.W. Insights into the mechanisms of apoptosis and pathogenesis in enterovirus 71 infections A review. Medicine 2025, 104, e42183. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tijsma, A.; Mirabelli, C.; Baggen, J.; Wahedi, M.; Franco, D.; De Palma, A.; Leyssen, P.; Verbeken, E.; van Kuppeveld, F.J.M.; et al. Intra-host emergence of an enterovirus A71 variant with enhanced PSGL1 usage and neurovirulence. Emerg. Microbes Infect. 2019, 8, 1076–1085. [Google Scholar] [CrossRef]
- Lu, J.; Tao, Z.; Zhang, Y. Progressing our knowledge of enterovirus: Epidemiology, diagnosis, prevention, control, and beyond. Biosaf. Health 2024, 6, 3–4. [Google Scholar] [CrossRef]
- Chang, L.Y.; Lin, H.Y.; Gau, S.S.F.; Lu, C.Y.; Hsia, S.H.; Huang, Y.C.; Huang, L.M.; Lin, T.Y. Enterovirus A71 neurologic complications and long-term sequelae. J. Biomed. Sci. 2019, 26, 57. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.K.; Zainol, M.I.; Sam, I.C.; Chan, Y.F. Recent advances in the understanding of enterovirus A71 infection: A focus on neuropathogenesis. Expert Rev. Anti-Infect. Ther. 2021, 19, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shen, L.; Wu, J.; Zou, X.; Gu, J.; Chen, J.; Mao, L. Enterovirus A71 proteins: Structure and function. Front. Microbiol. 2018, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Lv, L.; Wang, T.; Gu, W.; Luo, Y.; Feng, H. Recent Progress in Innate Immune Responses to Enterovirus A71 and Viral Evasion Strategies. Int. J. Mol. Sci. 2024, 25, 5688. [Google Scholar] [CrossRef]
- Liu, Q.; Long, J.E. Insight into the Life Cycle of Enterovirus-A71. Viruses 2025, 17, 181. [Google Scholar] [CrossRef]
- Ang, P.Y.; Chong, C.W.H.; Alonso, S. Viral determinants that drive Enterovirus-A71 fitness and virulence. Emerg. Microbes Infect. 2021, 10, 713–724. [Google Scholar] [CrossRef]
- Chen, K.R.; Ling, P. Interplays between Enterovirus A71 and the innate immune system. J. Biomed. Sci. 2019, 26, 95. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shimojima, M.; Tano, Y.; Miyamura, T.; Wakita, T.; Shimizu, H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 2009, 15, 794–797. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Zheng, M. Enterovirus A71 antivirals: Past, present, and future. Acta Pharm. Sin. B 2022, 12, 1542–1566. [Google Scholar] [CrossRef]
- Liu, Y.; Maisimu, M.; Ge, Z.; Xiao, S.; Wang, H. The Pathogenesis and Virulence of the Major Enterovirus Pathogens Associated with Severe Clinical Manifestations: A Comprehensive Review. Cells 2025, 14, 1617. [Google Scholar] [CrossRef]
- Shingler, K.L.; Yoder, J.L.; Carnegie, M.S.; Ashley, R.E.; Makhov, A.M.; Conway, J.F.; Hafenstein, S. The Enterovirus 71 A-particle Forms a Gateway to Allow Genome Release: A CryoEM Study of Picornavirus Uncoating. PLoS Pathog. 2013, 9, e1003240. [Google Scholar] [CrossRef]
- Majer, A.; McGreevy, A.; Booth, T.F. Molecular Pathogenicity of Enteroviruses Causing Neurological Disease. Front. Microbiol. 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Kingston, N.J.; Snowden, J.S.; Grehan, K.; Hall, P.K.; Hietanen, E.V.; Passchier, T.C.; Polyak, S.J.; Filman, D.J.; Hogle, J.M.; Rowlands, D.J.; et al. Mechanism of enterovirus VP0 maturation cleavage based on the structure of a stabilised assembly intermediate. PLoS Pathog. 2024, 20, e1012511. [Google Scholar] [CrossRef]
- van der Linden, L.; Wolthers, K.C.; van Kuppeveld, F.J.M. Replication and inhibitors of enteroviruses and parechoviruses. Viruses 2015, 7, 4529–4562. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Wong, K.T. Understanding enterovirus 71 neuropathogenesis and its impact on other neurotropic enteroviruses. Brain Pathol. 2015, 25, 614–624. [Google Scholar] [CrossRef]
- Peters, C.E.; Carette, J.E. Return of the neurotropic enteroviruses: Co-opting cellular pathways for infection. Viruses 2021, 13, 166. [Google Scholar] [CrossRef]
- Mielcarska, M.B.; Rouse, B.T. Viruses and the Brain—A Relationship Prone to Trouble. Viruses 2025, 17, 203. [Google Scholar] [CrossRef] [PubMed]
- Gaume, L.; Chabrolles, H.; Bisseux, M.; Lopez-Coqueiro, I.; Dehouck, L.; Mirand, A.; Henquell, C.; Gosselet, F.; Archimbaud, C.; Bailly, J.-L. Enterovirus A71 crosses a human blood–brain barrier model through infected immune cells. Microbiol. Spectr. 2024, 12, e0069024. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Wang, X.; Li, N.; Kuang, D.X.; Tong, P.F.; Lu, C.X.; Han, Y.Y.; Sun, X.M.; Dai, J.J.; Liu, L.D. EV-A71 invades the central nervous system and affects the blood-brain barrier in a tree shrew model. Front. Immunol. 2025, 16, 1583768. [Google Scholar] [CrossRef] [PubMed]
- Too, I.H.K.; Bonne, I.; Tan, E.L.; Chu, J.J.H.; Alonso, S. Prohibitin plays a critical role in Enterovirus 71 neuropathogenesis. PLoS Pathog. 2018, 14, e1006778. [Google Scholar] [CrossRef]
- Aknouch, I.; García-Rodríguez, I.; Giugliano, F.P.; Calitz, C.; Koen, G.; van Eijk, H.; Johannessson, N.; Rebers, S.; Brouwer, L.; Muncan, V.; et al. Amino acid variation at VP1-145 of enterovirus A71 determines the viral infectivity and receptor usage in a primary human intestinal model. Front. Microbiol. 2023, 14, 1045587. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Koike, S. Cellular receptors for enterovirus A71. J. Biomed. Sci. 2020, 27, 23. [Google Scholar] [CrossRef]
- Huang, S.W.; Cheng, D.; Wang, J.R. Enterovirus A71: Virulence, antigenicity, and genetic evolution over the years. J. Biomed. Sci. 2019, 26, 81. [Google Scholar] [CrossRef]
- Hu, K.; Onintsoa Diarimalala, R.; Yao, C.; Li, H.; Wei, Y. EV-A71 Mechanism of Entry: Receptors/Co-Receptors, Related Pathways and Inhibitors. Viruses 2023, 15, 785. [Google Scholar] [CrossRef]
- Pathinayake, P.S.; Hsu, A.C.Y.; Wark, P.A.B. Innate immunity and immune evasion by enterovirus 71. Viruses 2015, 7, 6613–6630. [Google Scholar] [CrossRef]
- Lei, X.; Xiao, X.; Wang, J. Innate immunity evasion by enteroviruses: Insights into virus-host interaction. Viruses 2016, 8, 22. [Google Scholar] [CrossRef]
- Hsiao, H.B.; Chou, A.H.; Lin, S.I.; Chen, I.H.; Lien, S.P.; Liu, C.C.; Chong, P.; Liu, S.J. Toll-Like Receptor 9-Mediated Protection of Enterovirus 71 Infection in Mice Is Due to the Release of Danger-Associated Molecular Patterns. J. Virol. 2014, 88, 11658–11670. [Google Scholar] [CrossRef]
- Song, J.; Hu, Y.; Li, J.; Zheng, H.; Wang, J.; Guo, L.; Shi, H.; Liu, L. Suppression of the toll-like receptor 7-dependent type I interferon production pathway by autophagy resulting from enterovirus 71 and coxsackievirus A16 infections facilitates their replication. Arch. Virol. 2018, 163, 135–144. [Google Scholar] [CrossRef]
- Rui, Y.; Su, J.; Wang, H.; Chang, J.; Wang, S.; Zheng, W.; Cai, Y.; Wei, W.; Gordy, J.Y.; Markham, R.; et al. Disruption of MDA5-Mediated Innate Immune Responses by the 3C Proteins of Coxsackievirus A16, Coxsackievirus A6, and Enterovirus D68. J. Virol. 2017, 91, 546–563. [Google Scholar] [CrossRef]
- Wells, A.I.; Grimes, K.A.; Coyne, C.B. Enterovirus Replication and Dissemination Are Differentially Controlled by Type I and III Interferons in the Gastrointestinal Tract. mBio 2022, 13, e0044322. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Shereen, M.A.; Zeng, X.; Liang, Y.; Li, W.; Ruan, Z.; Li, Y.; Liu, W.; Liu, Y.; Wu, K.; et al. The tlr3/irf1/type iii ifn axis facilitates antiviral responses against enterovirus infections in the intestine. mBio 2020, 11, e02540-20. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, J.; Lu, N.; Zhang, C. Enterovirus 71 Antagonizes Antiviral Effects of Type III Interferon and Evades the Clearance of Intestinal Intraepithelial Lymphocytes. Front. Microbiol. 2022, 12, 806084. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Hiscox, J.A.; Solomon, T.; Ooi, M.H.; Ng, L.F.P. Immunopathogenesis and virus-host interactions of enterovirus 71 in patients with hand, foot and mouth disease. Front. Microbiol. 2017, 8, 2249. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Qi, G.; Liu, N.; Sheng, L.; Shang, L.; Qi, B. The immune mechanism of intestinal tract Toll-like receptor in mediating EV71 virus type severe handfoot-and-mouth disease and the MAPK pathway. Exp. Ther. Med. 2017, 13, 2263–2266. [Google Scholar] [CrossRef]
- Wang, S.M.; Lei, H.Y.; Su, L.Y.; Wu, J.M.; Yu, C.K.; Wang, J.R.; Liu, C.C. Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin. Microbiol. Infect. 2007, 13, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.I.; Shih, S.R. Neurotropic enterovirus infections in the central nervous system. Viruses 2015, 7, 6051–6066. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Gan, X.; Song, J.; Sun, P.; Dong, X.P. Serum cytokine profiles of children with human enterovirus 71-associated hand, foot, and mouth disease. J. Med. Virol. 2014, 86, 1377–1385. [Google Scholar] [CrossRef]
- Coffman, J.A. Enteroviruses Activate Cellular Innate Immune Responses Prior to Adaptive Immunity and Tropism Contributes to Severe Viral Pathogenesis. Microorganisms 2025, 13, 870. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Deng, H.; Zhang, Y.; Liu, C.; Chen, Y.; Zhang, C.; Zhang, W.; Jia, X.; Dang, S.; et al. Integrative scRNA-seq and transcriptomic analysis initially reveals monocyte/macrophage activation drives EV-A71-induced immune dysregulation and neural injury in severe HFMD. Front. Immunol. 2025, 16, 1620633. [Google Scholar] [CrossRef]
- Pei, X.; Fan, X.; Zhang, H.; Duan, H.; Xu, C.; Xie, B.; Wang, L.; Li, X.; Peng, Y.; Shen, T. Low frequency, weak MCP-1 secretion and exhausted immune status of peripheral monocytes were associated with progression of severe enterovirus A71-infected hand, foot and mouth disease. Clin. Exp. Immunol. 2019, 196, 353–363. [Google Scholar] [CrossRef]
- Kalam, N.; Balasubramaniam, V. Targeting HMGB1 and Inflammasome Pathways: A Novel Approach to Mitigate Inflammation in Non-Polio Enterovirus Infections. 2024. Available online: https://www.authorea.com/users/818499/articles/1218210-targeting-hmgb1-and-inflammasome-pathways-a-novel-approach-to-mitigate-inflammation-in-non-polio-enterovirus-infections?commit=f7bc9ee4d79183cbe063ef37e23bd5d0d0a1c23b (accessed on 22 October 2025).
- Zhu, K.; Yang, J.; Luo, K.; Yang, C.; Zhang, N.; Xu, R.; Chen, J.; Jin, M.; Xu, B.; Guo, N.; et al. TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection. PLoS Pathog. 2015, 11, e1004613. [Google Scholar] [CrossRef]
- Cisternas, I.S.; Salazar, J.C.; García-Angulo, V.A. Overview on the bacterial iron-riboflavin metabolic axis. Front. Microbiol. 2018, 9, 1478. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Yao, L.; Kollmann, T.R.; Casper, C.; Zhang, J.; Self, S.G. Implications of age-dependent immune responses to enterovirus 71 infection for disease pathogenesis and vaccine design. J. Pediatr. Infect. Dis. Soc. 2013, 2, 163–170. [Google Scholar] [CrossRef]
- Aw-Yong, K.L.; NikNadia, N.M.N.; Tan, C.W.; Sam, I.C.; Chan, Y.F. Immune responses against enterovirus A71 infection: Implications for vaccine success. Rev. Med. Virol. 2019, 29, e2073. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.T.I.; Poh, C.L. T cell immunity to enterovirus 71 infection in humans and implications for vaccine development. Int. J. Med. Sci. 2018, 15, 1143–1152. [Google Scholar] [CrossRef]
- Swain, S.K.; Panda, S.; Sahu, B.P.; Sarangi, R. Activation of Host Cellular Signaling and Mechanism of Enterovirus 71 Viral Proteins Associated with Hand, Foot and Mouth Disease. Viruses 2022, 14, 2190. [Google Scholar] [CrossRef]
- Wang, L.C.; Kao, C.M.; Ling, P.; Su, I.J.; Chang, T.M.; Chen, S.H. CD4 T-cell-independent antibody response reduces enterovirus 71 lethality in mice by decreasing tissue viral loads. Clin. Dev. Immunol. 2012, 2012, 580696. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Su, S.B.; Chen, K.T. A review of enterovirus-associated hand-foot and mouth disease: Preventive strategies and the need for a global enterovirus surveillance network. Pathog. Glob. Health 2024, 118, 538–548. [Google Scholar] [CrossRef]
- Bopegamage, S. Enterovirus infections: Pivoting role of the adaptive immune response. Virulence 2016, 7, 495–497. [Google Scholar] [CrossRef]
- Bifani, A.M.; Tee, H.K.; Antanasijevic, A.; Clément, S.; Tapparel, C. Enterovirus A71 priorities, challenges, and future opportunities in humoral immunity and vaccine development. npj Vaccines 2025, 10, 194. [Google Scholar] [CrossRef]
- Ke, Y.; Nam Liu, W.; Her, Z.; Liu, M.; Yee Tan, S.; Wah Tan, Y.; Chan, X.Y.; Fan, Y.; Huang, E.K.; Chen, H.; et al. Enterovirus A71 Infection Activates Human Immune Responses and Induces Pathological Changes in Humanized Mice. J. Virol. 2019, 93, 1066–1084. [Google Scholar] [CrossRef]
- Lim, H.X.; Poh, C.L. Insights into innate and adaptive immune responses in vaccine development against EV-A71. Ther. Adv. Vaccines Immunother. 2019, 7, 2515135519888998. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Gao, F.; Li, D.; Ji, S.; Zhang, S.; Guo, W.; Li, B.; Wang, F.E. The Effect of FOXP3+Regulatory T Cells on Infectious and Inflammatory Diseases. Infect. Microbes Dis. 2021, 3, 187–197. [Google Scholar] [CrossRef]
- Wang, S.M.; Chen, I.C.; Liao, Y.T.; Liu, C.C. The clinical correlation of regulatory T cells and cyclic adenosine monophosphate in enterovirus 71 infection. PLoS ONE 2014, 9, e102025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, Z.; Zhang, J.; Guo, H.; Li, J.; Nie, X.; Wang, S.; Zhang, L. Enterovirus 71 Activates Plasmacytoid Dendritic Cell–Dependent PSGL-1 Binding Independent of Productive Infection. J. Immunol. 2024, 212, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Z.; Huang, M.; Zeng, J. Predicting Severe Enterovirus 71-Infected Hand, Foot, and Mouth Disease: Cytokines and Chemokines. Mediat. Inflamm. 2020, 2020, 9273241. [Google Scholar] [CrossRef]
- Zhu, P.; Ji, W.; Li, D.; Li, Z.; Chen, Y.; Dai, B.; Han, S.; Chen, S.; Jin, Y.; Duan, G. Current status of hand-foot-and-mouth disease. J. Biomed. Sci. 2023, 30, 15. [Google Scholar] [CrossRef]
- Chen, J.; Tong, J.; Liu, H.; Liu, Y.; Su, Z.; Wang, S.; Shi, Y.; Zheng, D.; Sandoghchian, S.; Geng, J.; et al. Increased frequency of Th17 cells in the peripheral blood of children infected with enterovirus 71. J. Med. Virol. 2012, 84, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Ruohtula, T.; Kondrashova, A.; Lehtonen, J.; Oikarinen, S.; Hämäläinen, A.M.; Niemelä, O.; Peet, A.; Tillmann, V.; Nieminen, J.K.; Ilonen, J.; et al. Immunomodulatory Effects of Rhinovirus and Enterovirus Infections During the First Year of Life. Front. Immunol. 2020, 11, 567046. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, C.; Feng, J.; Li, X.; Wang, Y.; Yang, J.; Chen, Z. Peripheral T lymphocyte subset imbalances in children with enterovirus 71-induced hand, foot and mouth disease. Virus Res. 2014, 180, 84–91. [Google Scholar] [CrossRef]
- He, Y.; Feng, Z.; Wang, W.; Chen, Y.; Cheng, J.; Meng, J.; Yang, H.; Wang, Y.; Yao, X.; Feng, Q.; et al. Global cytokine/chemokine profile identifies potential progression prediction indicators in hand-foot-and-mouth disease patients with Enterovirus A71 infections. Cytokine 2019, 123, 154765. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chang, L.Y.; Huang, Y.C.; Hsu, K.H.; Chiu, C.H.; Yang, K.D. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: Implications for early recognition and therapy. Acta Paediatr. 2002, 91, 632–635. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Chen, F.; Chang, Z.; Zhang, Z.; Pons-Salort, M.; Grassly, N.C. The efficacy and effectiveness of enterovirus A71 vaccines against hand, foot, and mouth disease: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0323782. [Google Scholar] [CrossRef]
- Hu, Q.; Xie, Y.; Ji, F.; Zhao, F.; Song, X.; Lu, S.; Li, Z.; Geng, J.; Yang, H.; Long, J.; et al. Effectiveness of EV-A71 Vaccine and Its Impact on the Incidence of Hand, Foot and Mouth Disease: A Systematic Review. Vaccines 2024, 12, 1028. [Google Scholar] [CrossRef]
- Mbani, C.J.; Morvan, C.; Nekoua, M.P.; Debuysschere, C.; Alidjinou, E.K.; Moukassa, D.; Hober, D. Enterovirus Antibodies: Friends and Foes. Rev. Med. Virol. 2024, 34, e70004. [Google Scholar] [CrossRef]
- Yang, D.; Liu, W.; Wang, W.; Deng, P.; Ye, C.; Yang, L.; Xue, C. Analysis of the Impact of Inactivated A71 Vaccine on the Incidence of Hand–Foot–Mouth Disease in Pudong New Area, Shanghai. Vaccines 2024, 12, 962. [Google Scholar] [CrossRef]
- Le, T.H.L.; Weng, T.Y.; Yen, H.; Chia, M.Y.; Lee, M.S. Development of a novel EV-A71 monoclonal antibody for monitoring vaccine potency. PLoS Neglected Trop. Dis. 2025, 19, e0013127. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cheng, S.; Li, L. Evaluation of enterovirus A71 vaccine effectiveness against pediatric hand, foot, and mouth disease caused by enterovirus A71 virus in Tianjin, China, 2016–2023: A test-negative design case-control study. Hum. Vaccin. Immunother. 2025, 21, 2572181. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Wang, J.; Wang, Y.; Sun, L.; Cui, S.; Li, J.; Guo, Y. Adverse events following immunization surveillance on two types of enterovirus 71 vaccines: A real-world comparative study in China. Hum. Vaccin. Immunother. 2025, 21, 2458831. [Google Scholar] [CrossRef]
- Han, J.F.; Cao, R.Y.; Deng, Y.Q.; Tian, X.; Jiang, T.; Qin, E.D.; Qin, C.-F. Antibody dependent enhancement infection of enterovirus 71 in vitro and in vivo. Virol. J. 2011, 8, 106. [Google Scholar] [CrossRef]
- Cao, R.Y.; Dong, D.Y.; Liu, R.J.; Han, J.F.; Wang, G.C.; Zhao, H.; Li, X.F.; Deng, Y.Q.; Zhu, S.Y.; Wang, X.Y.; et al. Human IgG Subclasses against Enterovirus Type 71: Neutralization versus Antibody Dependent Enhancement of Infection. PLoS ONE 2013, 8, e64024. [Google Scholar] [CrossRef]
- You, S.Y.; Chen, S.H.; Wang, S.M. Promising therapeutics of enterovirus 71 infection: Sunshine behind cloud. Front. Pharmacol. 2025, 16, 1652159. [Google Scholar] [CrossRef]
- Khong, W.X.; Foo, D.G.W.; Trasti, S.L.; Tan, E.L.; Alonso, S. Sustained High Levels of Interleukin-6 Contribute to the Pathogenesis of Enterovirus 71 in a Neonate Mouse Model. J. Virol. 2011, 85, 3067–3076. [Google Scholar] [CrossRef]
- Wang, L.C.; Yao, H.W.; Chang, C.F.; Wang, S.W.; Wang, S.M.; Chen, S.H. Suppression of interleukin-6 increases enterovirus A71 lethality in mice. J. Biomed. Sci. 2017, 24, 94. [Google Scholar] [CrossRef]
- Kimmig, L.M.; Wu, D.; Gold, M.; Pettit, N.N.; Pitrak, D.; Mueller, J.; Husain, A.N.; Mutlu, E.A.; Mutlu, G.M. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Front. Med. 2020, 7, 583897. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Malhotra, A.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Savic, S.; Douglas, I.S.; et al. Tocilizumab in patients hospitalised with COVID-19 pneumonia: Efficacy, safety, viral clearance, and antibody response from a randomised controlled trial (COVACTA). EClinicalMedicine 2022, 47, 101409. [Google Scholar] [CrossRef] [PubMed]
- Koritala, T.; Pattan, V.; Tirupathi, R.; Rabaan, A.A.; Al Mutair, A.; Alhumaid, S.; Adhikari, R.; Deepika, K.; Jain, N.K.; Bansal, V.; et al. Infection risk with the use of interleukin inhibitors in hospitalized patients with COVID-19: A narrative review. Infez. Med. 2021, 29, 495–503. [Google Scholar] [PubMed]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Huang, S.; Zhu, H.; Zhang, B.; Wu, X. Interaction between PHB2 and enterovirus A71 VP1 induces autophagy and affects EV-A71 infection. Viruses 2020, 12, 414. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Specific Marker | Correlation with Severe HFMD/CNS Involvement | Sample Type | Study Type | Pathological Implication | Supporting References |
|---|---|---|---|---|---|---|
| Th17/Th Cytokines | IL-17A | Highest levels observed in severe cases. | Serum, Plasma, Peripheral blood mononuclear cells (PBMCs) | Cohort study, case–control study, case–control study | Indicator of critical Th17/Treg imbalance and severe inflammatory promotion. | [62,63,64] |
| IFN-γ | Elevated levels linked to PE pathogenesis. | CSF | Case–control study | Contributes to increased pulmonary vascular permeability. | [38] | |
| Proinflammatory Cytokines | IL-6, IL-1β, TNF-α | Consistently and significantly elevated in CSF/serum in severe/fatal cases. | Serum, serum | Case-control study, cross-sectional clinical case–control study | Primary mediators of systemic cytokine storm and neuroinflammation. | [65,66] |
| Chemokines/Recruitment | IL-8, IP-10, MCP-1 | Crucially elevated in serum and CSF. | Serum | Cross-sectional clinical case–control study | Promotes neutrophil and monocyte recruitment to sites of infection and neurological injury. | [65] |
| Regulatory Signaling | cAMP | Significantly decreased plasma concentration in severe cases. | Plasma, CSF | Case–control study | Indicator of failed inhibitory signaling (PDE pathway dysregulation) leading to uninhibited T cell activation. | [58] |
| Immune Dysfunction | Treg (CD4+CD25+Foxp3+) | Significantly lower frequency observed in severe stages. | Peripheral blood mononuclear cells (PBMCs) | Case–control study | Critical loss of immune control (suppression) over the inflammatory response. | [58] |
| Monocytes (PD-1/PD-L1) | Increased expression of co-inhibitory markers. | Peripheral blood mononuclear cells (PBMCs) | Case–control study | Sign of immune exhaustion and reduced functional capacity. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronik, A.; Lewandowski, D.; Sulik, A.; Toczylowski, K. The Host Immune Response to Enterovirus A71 (EV-A71): From Viral Immune Evasion to Immunopathology and Prognostic Biomarkers of Severe Disease—A Narrative Review. Viruses 2025, 17, 1540. https://doi.org/10.3390/v17121540
Andronik A, Lewandowski D, Sulik A, Toczylowski K. The Host Immune Response to Enterovirus A71 (EV-A71): From Viral Immune Evasion to Immunopathology and Prognostic Biomarkers of Severe Disease—A Narrative Review. Viruses. 2025; 17(12):1540. https://doi.org/10.3390/v17121540
Chicago/Turabian StyleAndronik, Anna, Dawid Lewandowski, Artur Sulik, and Kacper Toczylowski. 2025. "The Host Immune Response to Enterovirus A71 (EV-A71): From Viral Immune Evasion to Immunopathology and Prognostic Biomarkers of Severe Disease—A Narrative Review" Viruses 17, no. 12: 1540. https://doi.org/10.3390/v17121540
APA StyleAndronik, A., Lewandowski, D., Sulik, A., & Toczylowski, K. (2025). The Host Immune Response to Enterovirus A71 (EV-A71): From Viral Immune Evasion to Immunopathology and Prognostic Biomarkers of Severe Disease—A Narrative Review. Viruses, 17(12), 1540. https://doi.org/10.3390/v17121540








