Monitoring Dengue Virus in Aedes aegypti to Improve Dengue Surveillance and Control in Puerto Rico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Dengue Case Data
2.3. Ethics Statement
2.4. Entomological Surveillance
2.5. Detecting Dengue Viruses in Ae. aegypti
2.6. Statistical Methods
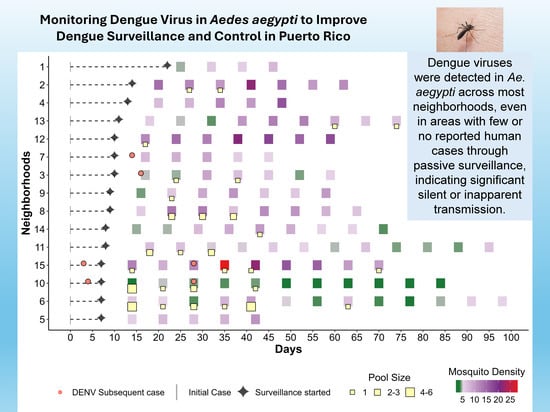
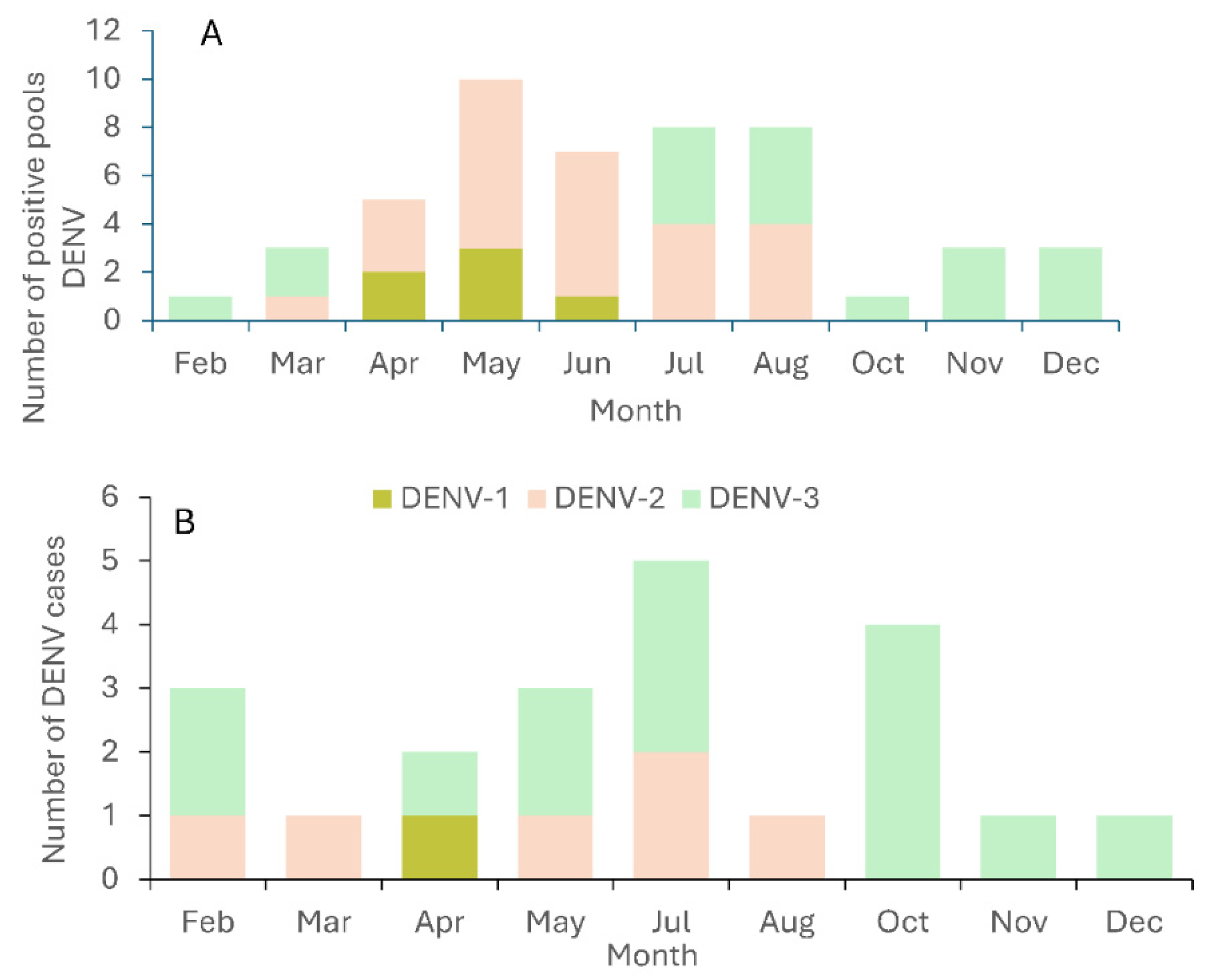
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Argaez-Sierra, D.G.; Baak-Baak, C.M.; Garcia-Rejon, J.E.; Cetina-Trejo, R.C.; Tzuc-Dzul, J.C.; Acosta-Viana, K.Y.; Chan-Perez, J.I.; Cigarroa-Toledo, N. Entomo-virological surveillance of Flavivirus in mosquitoes in Yucatan State, Mexico. Rev. Inst. Med. Trop. Sao Paulo 2024, 66, e56. [Google Scholar] [CrossRef] [PubMed]
- Tomashek, K.M.; Margolis, H.S. Dengue: A potential transfusion-transmitted disease. Transfusion 2011, 51, 1654–1660. [Google Scholar] [CrossRef]
- Haby, M.M.; Pinart, M.; Elias, V.; Reveiz, L. Prevalence of asymptomatic Zika virus infection: A systematic review. Bull. World Health Organ. 2018, 96, 402–413d. [Google Scholar] [CrossRef]
- Mitchell, P.K.; Mier, Y.T.-R.L.; Biggerstaff, B.J.; Delorey, M.J.; Aubry, M.; Cao-Lormeau, V.M.; Lozier, M.J.; Cauchemez, S.; Johansson, M.A. Reassessing Serosurvey-Based Estimates of the Symptomatic Proportion of Zika Virus Infections. Am. J. Epidemiol. 2019, 188, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.; Lambrechts, L.; Paul, R.E.; Ly, S.; Lay, R.S.; Long, K.C.; Huy, R.; Tarantola, A.; Scott, T.W.; Sakuntabhai, A.; et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 14688–14693. [Google Scholar] [CrossRef]
- Teixeira, M.D.; Barreto, M.L.; Costa, M.D.N.; Ferreira, L.D.A.; Vasconcelos, P.F.C.; Cairncross, S. Dynamics of dengue virus circulation: A silent epidemic in a complex urban area. Trop. Med. Int. Health 2002, 7, 757–762. [Google Scholar] [CrossRef]
- Bos, S.; Zambrana, J.V.; Duarte, E.; Graber, A.L.; Huffaker, J.; Montenegro, C.; Premkumar, L.; Gordon, A.; Kuan, G.; Balmaseda, A.; et al. Serotype-specific epidemiological patterns of inapparent versus symptomatic primary dengue virus infections: A 17-year cohort study in Nicaragua. Lancet Infect. Dis. 2025, 25, 346–356. [Google Scholar] [CrossRef]
- Eisen, L.; Beaty, B.J.; Morrison, A.C.; Scott, T.W. Proactive Vector Control Strategies and Improved Monitoring and Evaluation Practices for Dengue Prevention. J. Med. Entomol. 2009, 46, 1245–1255. [Google Scholar] [CrossRef]
- Huerta, H.; Gonzalez-Roldan, J.F.; Sanchez-Tejeda, G.; Correa-Morales, F.; Romero-Contreras, F.E.; Cardenas-Flores, R.; Rangel-Martinez, M.L.; Mata-Rivera, J.M.; Siller-Martinez, J.J.; Vazquez-Prokopec, G.M.; et al. Detection of Zika virus in Aedes mosquitoes from Mexico. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 328–331. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.; Peña-García, V.H.; Calle-Tobón, A.; Quimbayo-Forero, M.; Rojo, R.; Henao, E.; Shragai, T.; Rúa-Uribe, G. Entomovirological surveillance in schools: Are they a source for arboviral diseases transmission? Int. J. Environ. Res. Public Health 2021, 18, 6137. [Google Scholar] [CrossRef]
- Morlan, H.B.; Hayes, R.O. Urban dispersal and activity of Aedes aegypti. Mosq. News 1958, 18, 137–144. [Google Scholar]
- Vazquez-Prokopec, G.M.; Montgomery, B.L.; Horne, P.; Clennon, J.A.; Ritchie, S.A. Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Sci. Adv. 2017, 3, e1602024. [Google Scholar] [CrossRef]
- Salje, H.; Lessler, J.; Maljkovic Berry, I.; Melendrez, M.C.; Endy, T.; Kalayanarooj, S.; A-Nuegoonpipat, A.; Chanama, S.; Sangkijporn, S.; Klungthong, C.; et al. Dengue diversity across spatial and temporal scales: Local structure and the effect of host population size. Science 2017, 355, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Amador, M.; Acevedo, V.; Beltran, M.; Muñoz, J.L. A comparison of mosquito densities, weather and infection rates of Aedes aegypti during the first epidemics of Chikungunya (2014) and Zika (2016) in areas with and without vector control in Puerto Rico. Med. Vet. Entomol. 2019, 33, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rejon, J.; Lorono-Pino, M.A.; Farfan-Ale, J.A.; Flores-Flores, L.; Del Pilar Rosado-Paredes, E.; Rivero-Cardenas, N.; Najera-Vazquez, R.; Gomez-Carro, S.; Lira-Zumbardo, V.; Gonzalez-Martinez, P.; et al. Dengue virus-infected Aedes aegypti in the home environment. Am. J. Trop. Med. Hyg. 2008, 79, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Liew, J.W.K.; Selvarajoo, S.; Lim, X.Y.; Foo, C.J.; Refai, W.F.; Robson, N.; Othman, S.; Hadi, H.A.; Mydin, F.H.M.; et al. Inapparent dengue in a community living among dengue-positive Aedes mosquitoes and in a hospital in Klang Valley, Malaysia. Acta Trop. 2020, 204, 105330. [Google Scholar] [CrossRef]
- Schwab, S.R.; Stone, C.M.; Fonseca, D.M.; Fefferman, N.H. The importance of being urgent: The impact of surveillance target and scale on mosquito-borne disease control. Epidemics 2018, 23, 55–63. [Google Scholar] [CrossRef]
- Barrera, R. Surveillance and Control of Dengue Vectors in the United States and Territories; Open Book Publishers: Cambridge, UK, 2025; p. 127. [Google Scholar]
- Barrera, R.; Acevedo-Soto, V.; Ruiz-Valcarcel, J.; Medina, J.; Rivera, R.; Otero, L.; Tosado, R.; Medina, F.A.; Saavedra, R.; Miranda, J.; et al. Defining Aedes aegypti density thresholds for preventing human arboviral infections. Acta Trop. 2025, 267, 107688. [Google Scholar] [CrossRef]
- Felix, G.E.; Barrera, R.; Vazquez, J.; Ryff, K.; Munoz, J.L.; Matias, K.Y.; Hemme, R.R. Entomological investigation of Aedes aegypti in neighborhoods with confirmed human arbovirus infection in Puerto Rico. J. Am. Mosq. Control Assoc. 2018; in press. [Google Scholar] [CrossRef]
- Medeiros, A.S.; Costa, D.M.P.; Branco, M.S.D.; Sousa, D.M.C.; Monteiro, J.D.; Galvão, S.P.M.; Azevedo, P.R.M.; Fernandes, J.V.; Jeronimo, S.M.B.; Araújo, J.M.G. Dengue virus in Aedes aegypti and Aedes albopictus in urban areas in the state of Rio Grande do Norte, Brazil: Importance of virological and entomological surveillance. PLoS ONE 2018, 13, e0194108. [Google Scholar] [CrossRef]
- Guedes, D.R.D.; Cordeiro, M.T.; Melo-Santos, M.A.V.; Magalhaes, T.; Marques, E.; Regis, L.; Furtado, A.F.; Ayres, C.F.J. Patient-based dengue virus surveillance in Aedes aegypti from Recife, Brazil. J. Vector Borne Dis. 2010, 47, 67–75. [Google Scholar]
- Ware-Gilmore, F.; Rodriguez, D.M.; Ryff Mph, K.; Torres, J.M.; Velez, M.P.; Torres-Toro, C.T.; Santiago, G.A.; Rivera, A.; Madewell, Z.J.; Maldonado, Y.; et al. Dengue outbreak and response—Puerto Rico, 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 54–60. [Google Scholar] [CrossRef]
- CDC. Dengue Virus Infections: 2015 Case Definition: Centers for Disease Control and Prevention. 2015. Available online: https://ndc.services.cdc.gov/case-definitions/dengue-virus-infections-2015/ (accessed on 25 August 2025).
- Barrera, R.; Amador, M.; Acevedo, V.; Hemme, R.R.; Felix, G. Sustained, area-wide control of Aedes aegypti using CDC autocidal gravid ovitraps. Am. J. Trop. Med. Hyg. 2014, 91, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme JVazquez, J.; Medina, J.F.; Medina, F.; Colon, C.; Margolis, H.; Munoz-Jordan, J.L. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Santiago, G.A.; Vazquez, J.; Courtney, S.; Matias, K.Y.; Andersen, L.E.; Colon, C.; Butler, A.E.; Roulo, R.; Bowzard, J.; Villanuea, J.M.; et al. Performance of the trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat. Commun. 2018, 9, 1391. [Google Scholar] [CrossRef] [PubMed]
- Chisenhall, D.M.; Vitek, C.J.; Richards, S.L.; Mores, C.N. A method to increase efficiency in testing pooled field-collected mosquitoes. J. Am. Mosq. Control Assoc. 2008, 24, 311–314. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975; p. 165. [Google Scholar]
- Ten Bosch, Q.A.; Clapham, H.E.; Lambrechts, L.; Duong, V.; Buchy, P.; Althouse, B.M.; Lloyd, A.L.; Waller, L.A.; Morrison, A.C.; Kitron, U.; et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018, 14, e1006965. [Google Scholar] [CrossRef]
- Coatsworth, H.; Lippi, C.A.; Vasquez, C.; Ayers, J.B.; Stephenson, C.J.; Waits, C.; Florez, M.; Wilke, A.B.B.; Unlu, I.; Medina, J.; et al. A molecular surveillance-guided vector control response to concurrent dengue and West Nile virus outbreaks in a COVID-19 hotspot of Florida. Lancet Reg. Health Am. 2022, 11, 100231. [Google Scholar] [CrossRef]
- Narvaez, F.; Montenegro, C.; Juarez, J.G.; Zambrana, J.V.; Gonzalez, K.; Videa, E.; Arguello, S.; Barrios, F.; Ojeda, S.; Plazaola, M.; et al. Dengue severity by serotype and immune status in 19 years of pediatric clinical studies in Nicaragua. PLoS Negl Trop Dis. 2025, 19, e0012811. [Google Scholar] [CrossRef]
- Morrison, A.C.; Santiago, M.; Reiter, P.; RigauPerez, J.G.; Clark, G.G. Clustering patterns of dengue cases during an outbreak in Puerto Rico (1991–1992) and their relationship to Aedes aegypti dispersal and blood-feeding behavior. J. Am. Mosq. Control Assoc. 1996, 12, 466. [Google Scholar]
- Sharp, T.M.; Lorenzi, O.; Torres-Velasquez, B.; Acevedo, V.; Perez-Padilla, J.; Rivera, A.; Munoz-Jordan, J.; Margolis, H.S.; Waterman, S.H.; Biggerstaff, B.J.; et al. Autocidal gravid ovitraps protect humans from chikungunya virus infection by reducing Aedes aegypti mosquito populations. PLoS Negl. Trop. Dis. 2019, 13, e0007538. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.E.; Martin, S.W.; Lindsey, N.P.; Lehman, J.A.; Rivera, A.; Kolsin, J.; Landry, K.; Staples, J.E.; Sharp, T.M.; Paz-Bailey, G.; et al. Epidemiology of dengue, chikungunya, and Zika virus disease in U.S. States and territories, 2017. Am. J. Trop. Med. Hyg. 2019, 101, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.M.; Ryff, K.R.; Alvarado, L.; Shieh, W.J.; Zaki, S.R.; Margolis, H.S.; Rivera-Garcia, B. Surveillance for chikungunya and dengue during the first year of chikungunya virus circulation in Puerto Rico. J. Infect. Dis. 2016, 214 (Suppl. S5), S475–S481. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.K.; Pang, F.Y. Dengue virus infection rate in field populations of female Aedes aegypti and Aedes albopictus in Singapore. Trop. Med. Int. Health 2002, 7, 322–330. [Google Scholar] [CrossRef]
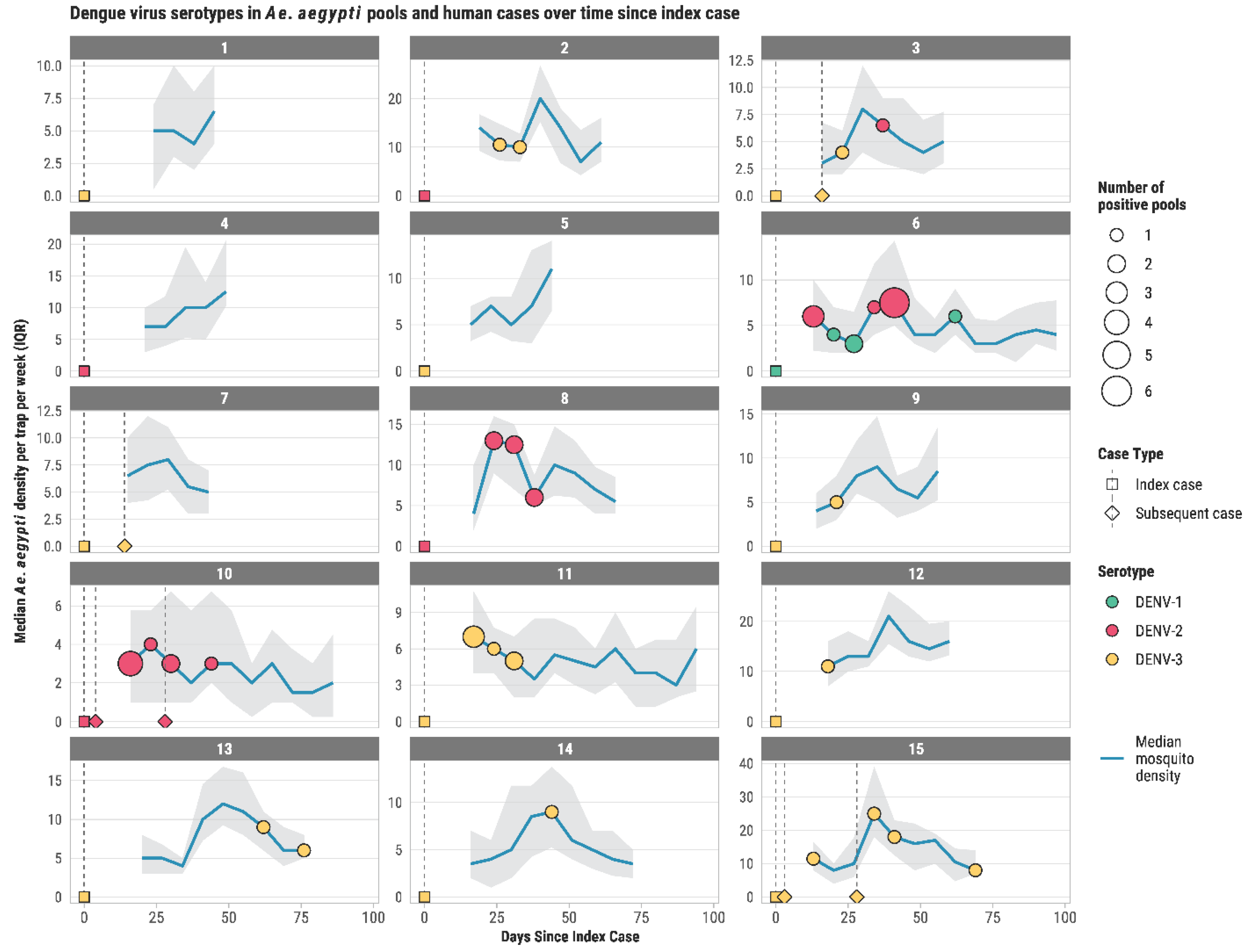
| Neighborhood | Initial Case DENV Serotype | Subsequent Dengue Cases and Their Serotypes | Positive Mosquito Pools and Serotypes | ||
|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | |||
| 1 | DENV-3 | 0 | 0 | 0 | 0 |
| 2 | DENV-2 | 0 | 0 | 0 | 2 |
| 3 | DENV-3 | 1 DENV-3 | 0 | 1 | 1 |
| 4 | DENV-2 | 0 | 0 | 0 | 0 |
| 5 | DENV-3 | 0 | 0 | 0 | 0 |
| 6 | DENV-1 | 0 | 6 | 10 | 0 |
| 7 | DENV-3 | 1 DENV-3 | 0 | 0 | 0 |
| 8 | DENV-2 | 0 | 0 | 6 | 0 |
| 9 | DENV-3 | 0 | 0 | 0 | 1 |
| 10 | DENV-2 | 2 DENV-2 | 0 | 8 | 0 |
| 11 | DENV-3 | 0 | 0 | 0 | 6 |
| 12 | DENV-3 | 0 | 0 | 0 | 1 |
| 13 | DENV-3 | 0 | 0 | 0 | 20 |
| 14 | DENV-3 | 0 | 0 | 0 | 1 |
| 15 | DENV-3 | 2 DENV-3 | 0 | 0 | 4 |
| Total | 15 | 6 | 6 | 25 | 18 |
| Neighborhood | Total Pools Analyzed | Positive Pools | Analyzed Mosquitoes | Average Mosquito Density (±S.E.) | Pool Infection Rate (95% C.I.) | Vector Index (95% C.I.) |
|---|---|---|---|---|---|---|
| 1 | 170 | 0 | 634 | 6.1 ± 0.5 | 0 | 0 |
| 2 | 361 | 2 | 2739 | 13.7 ± 0.7 | 0.73 (0.13–2.39) | 10.00 |
| 3 | 332 | 2 | 1246 | 6.1 ± 0.4 | 1.61 (0.29–5.24) | 9.82 |
| 4 | 167 | 0 | 1720 | 11.1 ± 0.8 | 0 | 0 |
| 5 | 154 | 0 | 1220 | 7.8 ± 0.5 | 0 | 0 |
| 6 | 366 | 16 | 2191 | 5.7 ± 0.2 | 7.52 (4.47–11.92) | 42.86 |
| 7 | 151 | 0 | 1105 | 7.4 ± 0.4 | 0 | 0 |
| 8 | 248 | 6 | 2291 | 9.6 ± 0.5 | 2.67 (1.09–5.54) | 25.63 |
| 9 | 211 | 1 | 1637 | 7.8 ± 0.4 | 0.61 (0.04–2.95) | 4.76 |
| 10 | 278 | 8 | 1187 | 3.6 ± 0.2 | 6.89 (3.23–13.05) | 24.80 |
| 11 | 338 | 6 | 2060 | 5.7 ± 0.2 | 2.94 (1.2–6.08) | 16.78 |
| 12 | 255 | 1 | 3303 | 16.0 ± 0.6 | 0.30 (0.02–1.47) | 4.80 |
| 13 | 265 | 2 | 2184 | 8.2 ± 0.3 | 0.92 (0.16–2.99) | 7.54 |
| 14 | 264 | 1 | 1773 | 6.7 ± 0.3 | 0.56 (0.03–2.72) | 3.75 |
| 15 | 318 | 4 | 3921 | 15.1 ± 0.7 | 1.03 (0.33–2.47) | 15.55 |
| Total | 3878 | 49 | 29,211 | 8.4 ± 0.13 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, L.M.; Medina, J.; Ruiz-Valcarcel, J.; Rivera, R.; Maldonado, Y.; Torres, J.; Madewell, Z.J.; Adams, L.; Paz-Bailey, G.; Barrera, R. Monitoring Dengue Virus in Aedes aegypti to Improve Dengue Surveillance and Control in Puerto Rico. Viruses 2025, 17, 1539. https://doi.org/10.3390/v17121539
Otero LM, Medina J, Ruiz-Valcarcel J, Rivera R, Maldonado Y, Torres J, Madewell ZJ, Adams L, Paz-Bailey G, Barrera R. Monitoring Dengue Virus in Aedes aegypti to Improve Dengue Surveillance and Control in Puerto Rico. Viruses. 2025; 17(12):1539. https://doi.org/10.3390/v17121539
Chicago/Turabian StyleOtero, Luisa M., Joanelis Medina, Jose Ruiz-Valcarcel, Reinaldo Rivera, Yashira Maldonado, Jomil Torres, Zachary J. Madewell, Laura Adams, Gabriela Paz-Bailey, and Roberto Barrera. 2025. "Monitoring Dengue Virus in Aedes aegypti to Improve Dengue Surveillance and Control in Puerto Rico" Viruses 17, no. 12: 1539. https://doi.org/10.3390/v17121539
APA StyleOtero, L. M., Medina, J., Ruiz-Valcarcel, J., Rivera, R., Maldonado, Y., Torres, J., Madewell, Z. J., Adams, L., Paz-Bailey, G., & Barrera, R. (2025). Monitoring Dengue Virus in Aedes aegypti to Improve Dengue Surveillance and Control in Puerto Rico. Viruses, 17(12), 1539. https://doi.org/10.3390/v17121539