Lipids, Tetraspanins, and Exosomes: Cell Factors in Orthoflavivirus Replication and Propagation
Abstract
1. Introduction
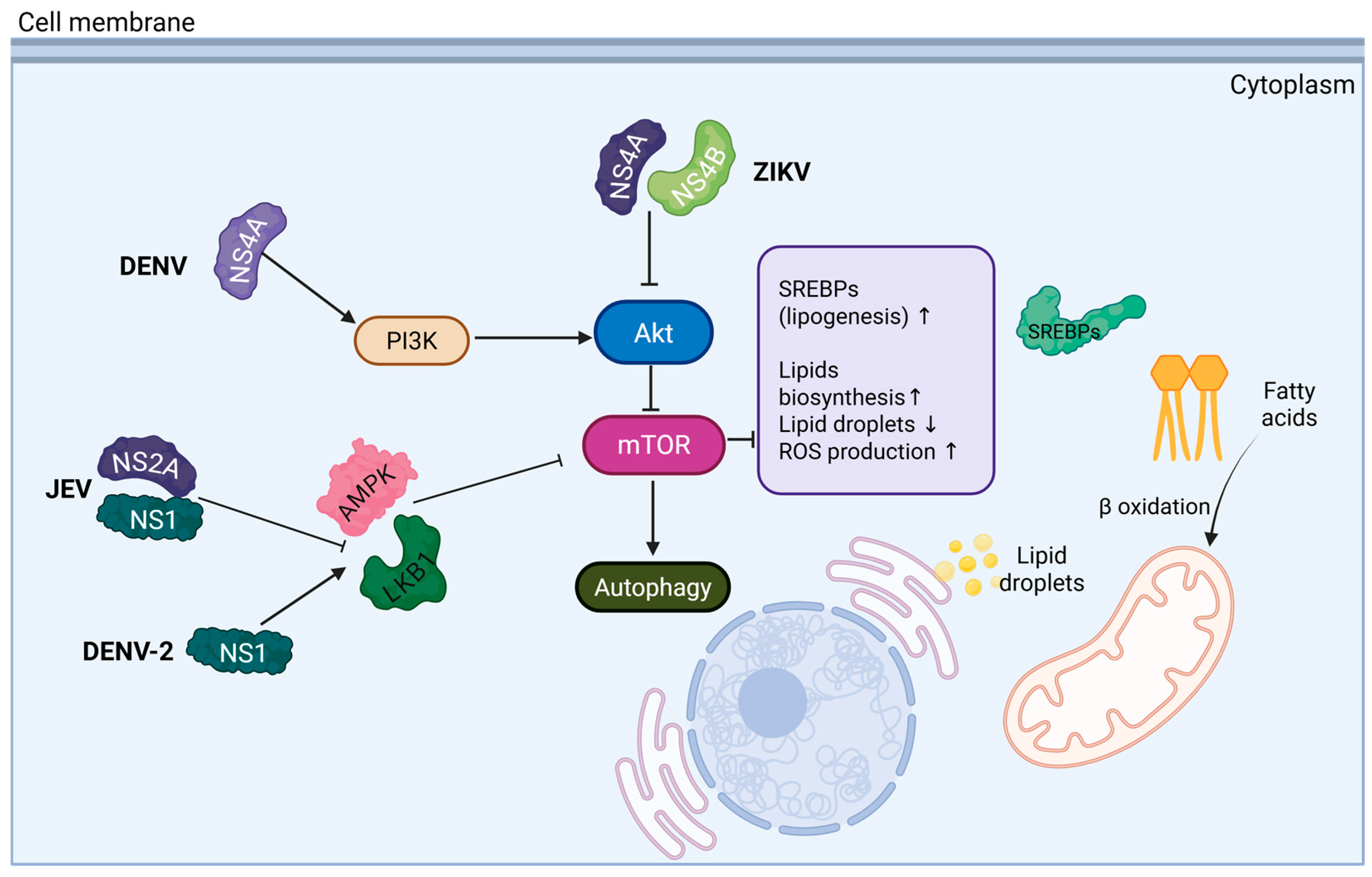
2. Lipid Involvement in Orthoflavivirus Replicative Cycle
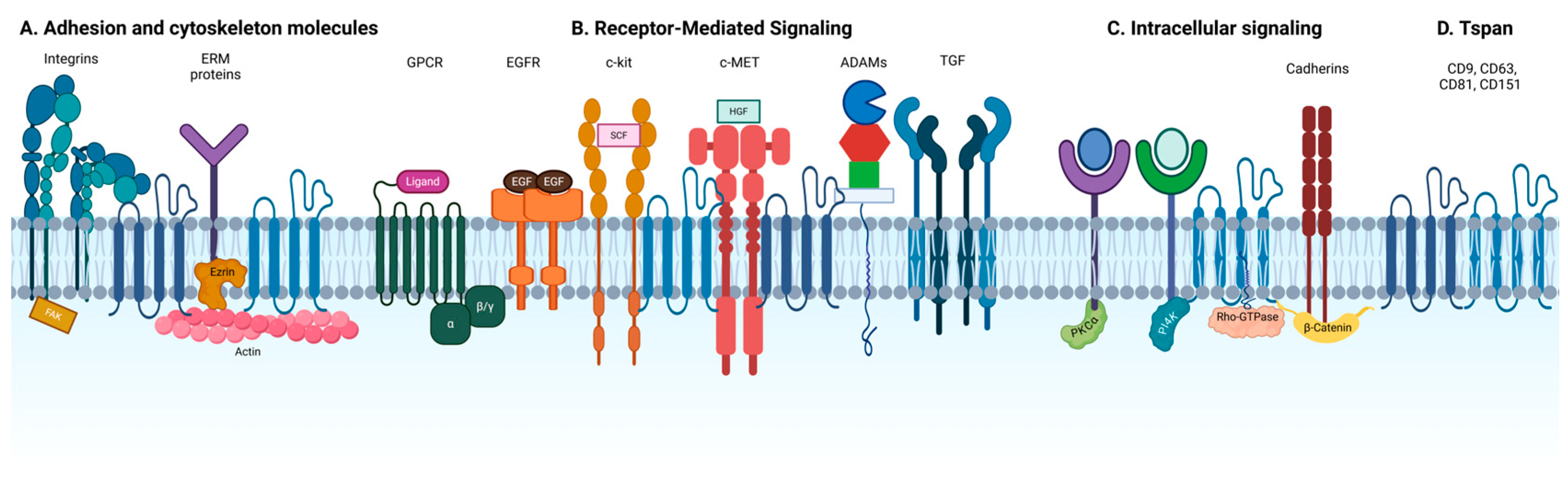
3. Tetraspanin-Enriched Microdomains
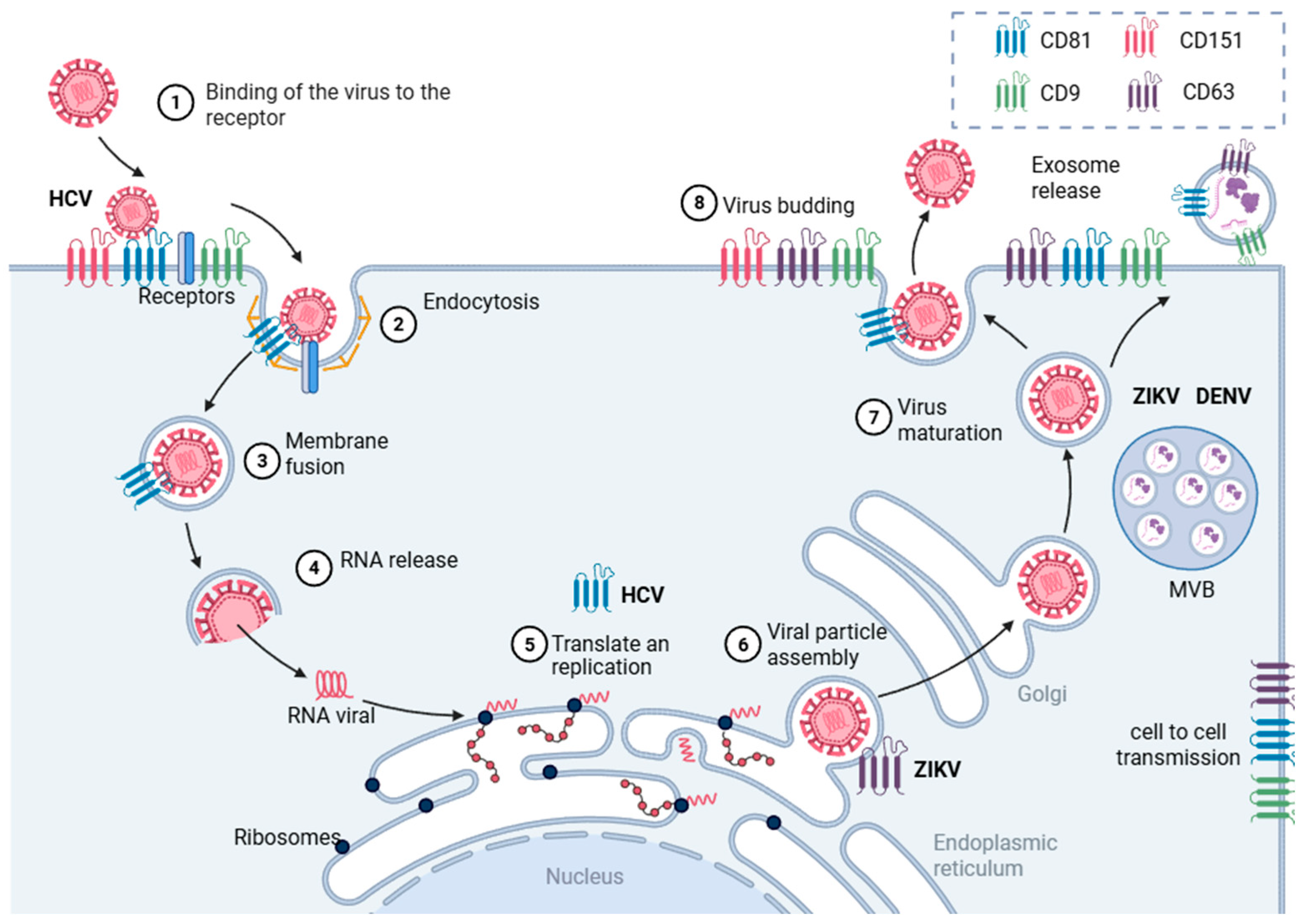
4. TEMs in the Replicative Cycle
5. Tetraspanins
6. Tetraspanins in Viral Infections
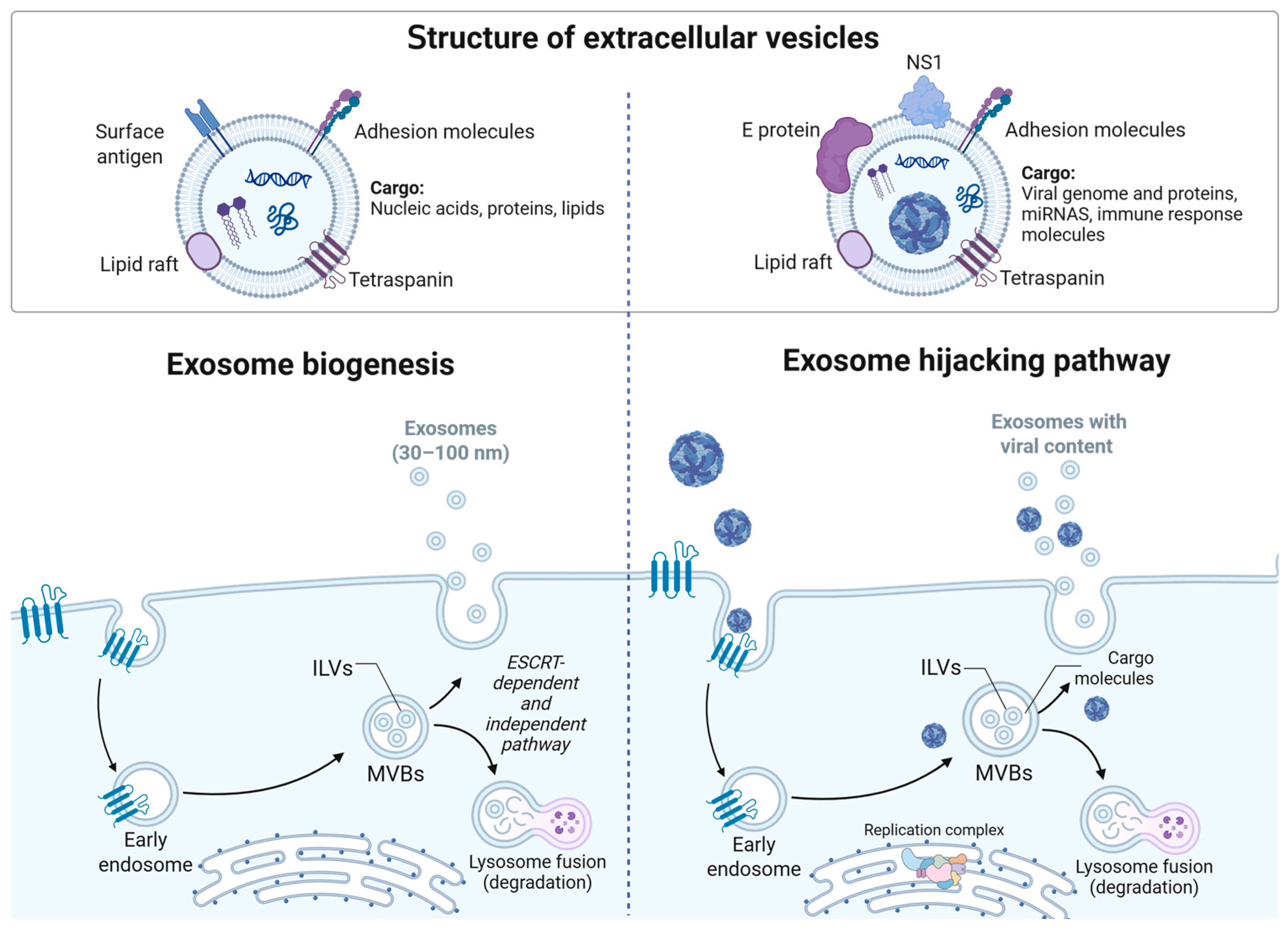
7. Tetraspanins in Extracellular Vesicles
8. Extracellular Vesicles During Orthoflavivirus Infection
9. Therapeutic Targeting of Tetraspanins and Lipid Metabolism in Orthoflavivirus Infections
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- San Martín, J.L.; Brathwaite, O.; Zambrano, B.; Solórzano, J.O.; Bouckenooghe, A.; Dayan, G.H.; Guzmán, M.G. The Epidemiology of Dengue in the Americas Over the Last Three Decades: A Worrisome Reality. Am. Soc. Trop. Med. Hyg. 2010, 82, 128–135. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Cucunubá, Z.M.; Dorigatti, I.; Nedjati-Gilani, G.L.; Donnelly, C.A.; Basáñez, M.G.; Nouvellet, P.; Lessler, J. Countering the Zika epidemic in Latin America. Science 2016, 353, 353–354. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Liao, K.C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.L.; Horovitz, D.D.G.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.R.; Neri, J.I.; Neto, J.M.D.P.; Wanderley, H.Y.C.; et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Vázquez-Calvo, Á.; Saiz, J.C. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog. Lipid Res. 2016, 64, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Jiménez De Oya, N.; Saiz, J.C. Lipid Metabolism as a Source of Druggable Targets for Antiviral Discovery against Zika and Other Flaviviruses. Pharmaceuticals 2019, 12, 97. [Google Scholar] [CrossRef]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Reyes-Ruiz, J.M.; Hurtado-Monzón, A.M.; Osuna-Ramos, J.F.; González-González, A.M.; De Jesús-González, L.A.; Palacios-Rápalo, S.N.; Del Ángel, R.M. Anti-flavivirus Properties of Lipid-Lowering Drugs. Front. Physiol. 2021, 12, 749770. [Google Scholar]
- Martín-Acebes, M.A.; Merino-Ramos, T.; Blázquez, A.B.; Casas, J.; Escribano-Romero, E.; Sobrino, F.; Saiz, J.-C. The Composition of West Nile Virus Lipid Envelope Unveils a Role of Sphingolipid Metabolism in Flavivirus Biogenesis. J. Virol. 2014, 88, 12041–12054. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef]
- Richard, A.S.; Zhang, A.; Park, S.J.; Farzan, M.; Zong, M.; Choe, H. Virion-associated phosphatidylethanolamine promotes TIM1-mediated infection by Ebola, dengue, and West Nile viruses. Proc. Natl. Acad. Sci. USA 2015, 112, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Riley, C.; Isaac, G.; Hopf-Jannasch, A.S.; Moore, R.J.; Weitz, K.W.; Pasa-Tolic, L.; Metz, T.O.; Adamec, J.; Kuhn, R.J. Dengue Virus Infection Perturbs Lipid Homeostasis in Infected Mosquito Cells. PLoS Pathog. 2012, 8, e1002584. [Google Scholar] [CrossRef] [PubMed]
- Samsa, M.M.; Mondotte, J.A.; Iglesias, N.G.; Assunção-Miranda, I.; Barbosa-Lima, G.; Da Poian, A.T.; Bozza, P.T.; Gamarnik, A.V. Dengue Virus Capsid Protein Usurps Lipid Droplets for Viral Particle Formation. PLoS Pathog. 2009, 5, e1000632. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V. Lipid Droplets and Cellular Lipid Metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef] [PubMed]
- Hsia, J.Z.; Liu, D.; Haynes, L.; Cruz-Cosme, R.; Tang, Q. Lipid Droplets: Formation, Degradation, and Their Role in Cellular Responses to Flavivirus Infections. Microorganisms 2024, 12, 647. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Ren, H.; Yao, Q.; Ba, J.; Luan, J.; Zhao, P.; Qin, Z.; Qi, Z. mTOR signaling regulates Zika virus replication bidirectionally through autophagy and protein translation. J. Med. Virol. 2023, 95, e28422. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.S.; Lee, S.A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef]
- McLean, J.E.; Wudzinska, A.; Datan, E.; Quaglino, D.; Zakeri, Z. Flavivirus NS4A-induced Autophagy Protects Cells against Death and Enhances Virus Replication. J. Biol. Chem. 2011, 286, 22147–22159. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Ji, J.; Gou, X.; Hu, P.; Cheng, Y.; Liu, Y.; Wang, Y.; Zhang, Q.; Zuo, L. DENV-2 NS1 promotes AMPK-LKB1 interaction to activate AMPK/ERK/mTOR signaling pathway to induce autophagy. Virol. J. 2023, 20, 231. [Google Scholar] [CrossRef]
- Zhang, Y.; Ba, J.; Luan, J.; Qi, Z.; Liu, B. Orthoflavivirus infection and the mTOR signaling pathway. Front. Microbiol. 2025, 16, 1565350. [Google Scholar] [CrossRef]
- Zhou, J.-F.; Zhang, M.-R.; Wang, Q.; Li, M.-Z.; Bai, J.-S.; Dai, Q.; Zhang, Y.; Yan, M.; Li, X.; Chen, J.; et al. Two novel compounds inhibit Flavivirus infection in vitro and in vivo by targeting lipid metabolism. J. Virol. 2024, 98, e00635-e24. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tu, S.; Ding, L.; Jin, M.; Chen, H.; Zhou, H. The role of autophagy in viral infections. J. Biomed. Sci. 2023, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, E.; Mateo, R.; Majzoub, K.; Van Buuren, N.; Bird, S.W.; Carette, J.E.; Kirkegaard, K. Differential and convergent utilization of autophagy components by positive-strand RNA viruses. PLoS Biol. 2019, 17, e2006926. [Google Scholar] [CrossRef]
- Song, M.H.; Sun, Y.; Qiu, X.B. Hijacking autophagy for infection by flaviviruses. Virus Res. 2024, 347, 199422. [Google Scholar] [CrossRef]
- Stoyanova, G.; Jabeen, S.; Landazuri Vinueza, J.; Ghosh Roy, S.; Lockshin, R.A.; Zakeri, Z. Zika virus triggers autophagy to exploit host lipid metabolism and drive viral replication. Cell Commun. Signal. 2023, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Randall, G. Dengue Virus-Induced Autophagy Regulates Lipid Metabolism. Cell Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef]
- Chermahini, F.A.; Arvejeh, P.M.; Marincola, F.M.; Ahmad, S.; Naderian, R.; Pajand, O.; Eslami, M.; Hasannia, M.; Sanami, S. Investigating how dengue virus-induced metabolic changes affect the host immune response and how to develop Immunomodulatory strategies. Virol. J. 2025, 22, 117. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef]
- Regen, S.L. The Origin of Lipid Rafts. Biochemistry 2020, 59, 4617–4621. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, Y. Virus Infection and Lipid Rafts. Biol. Pharm. Bull. 2006, 29, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Diwaker, D.; Mishra, K.P.; Ganju, L.; Singh, S.B. Protein Disulfide Isomerase Mediates Dengue Virus Entry in Association with Lipid Rafts. Viral Immunol. 2015, 28, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Neelakanta, G.; Sultana, H. Tetraspanins as Potential Therapeutic Candidates for Targeting Flaviviruses. Front. Immunol. 2021, 12, 630571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef]
- Ishikawa, T.; Narita, K.; Matsuyama, K.; Masuda, M. Dissemination of the Flavivirus Subgenomic Replicon Genome and Viral Proteins by Extracellular Vesicles. Viruses 2024, 16, 524. [Google Scholar] [CrossRef]
- Poveda, J.A.; Fernández, A.M.; Encinar, J.A.; González-Ros, J.M. Protein-promoted membrane domains. Biochim. Biophys. Acta BBA—Biomembr. 2008, 1778, 1583–1590. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Dietrich, C.; Bagatolli, L.A.; Volovyk, Z.N.; Thompson, N.L.; Levi, M.; Jacobson, K.; Gratton, E. Lipid Rafts Reconstituted in Model Membranes. Biophys. J. 2001, 80, 1417–1428. [Google Scholar] [CrossRef]
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The ins and outs of lipid rafts: Functions in intracellular cholesterol homeostasis, microparticles, and cell membranes. J. Lipid Res. 2020, 61, 676–686. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998, 14, 111–136. [Google Scholar] [CrossRef]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. Functional domains in tetraspanin proteins. Trends Biochem. Sci. 2003, 28, 106–112. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin Proteins Mediate Cellular Penetration, Invasion, and Fusion Events and Define a Novel Type of Membrane Microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Florin, L.; Lang, T. Tetraspanin Assemblies in Virus Infection. Front. Immunol. 2018, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Hantak, M.P.; Qing, E.; Earnest, J.T.; Gallagher, T. Tetraspanins: Architects of Viral Entry and Exit Platforms. J. Virol. 2019, 93, e01429-17. [Google Scholar] [CrossRef] [PubMed]
- New, C.; Lee, Z.Y.; Tan, K.S.; Wong, A.H.P.; Wang, D.Y.; Tran, T. Tetraspanins: Host Factors in Viral Infections. Int. J. Mol. Sci. 2021, 22, 11609. [Google Scholar] [CrossRef]
- Marsh, M.; Helenius, A. Virus Entry: Open Sesame. Cell 2006, 124, 729–740. [Google Scholar] [CrossRef]
- Brito, A.F.; Pinney, J.W. Protein–Protein Interactions in Virus–Host Systems. Front. Microbiol. 2017, 8, 1557. [Google Scholar] [CrossRef]
- Cordero-Rivera, C.D.; De Jesús-González, L.A.; Osuna-Ramos, J.F.; Palacios-Rápalo, S.N.; Farfan-Morales, C.N.; Reyes-Ruiz, J.M.; Del Ángel, R.M. The importance of viral and cellular factors on flavivirus entry. Curr. Opin. Virol. 2021, 49, 164–175. [Google Scholar] [CrossRef]
- White, J.M.; Whittaker, G.R. Fusion of Enveloped Viruses in Endosomes. Traffic 2016, 17, 593–614. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Barreiro, O.; Gordon-Alonso, M.; Sala-Valdés, M.; Sánchez-Madrid, F. Tetraspanin-enriched microdomains: A functional unit in cell plasma membranes. Trends Cell Biol. 2009, 19, 434–446. [Google Scholar] [CrossRef]
- Dharan, R.; Sorkin, R. Tetraspanin proteins in membrane remodeling processes. J. Cell Sci. 2024, 137, jcs261532. [Google Scholar] [CrossRef]
- Kummer, D.; Steinbacher, T.; Schwietzer, M.F.; Thölmann, S.; Ebnet, K. Tetraspanins: Integrating cell surface receptors to functional microdomains in homeostasis and disease. Med. Microbiol. Immunol. 2020, 209, 397–405. [Google Scholar] [CrossRef]
- Kovalenko, O.V.; Metcalf, D.G.; DeGrado, W.F.; Hemler, M.E. Structural organization and interactions of transmembrane domains in tetraspanin proteins. BMC Struct. Biol. 2005, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.; Kelly, B.; McMillan, B.J.; Seegar, T.C.M.; Dror, R.O.; Kruse, A.C.; Blacklow, S.C. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket. Cell 2016, 167, 1041–1051.e11. [Google Scholar] [PubMed]
- Lang, T.; Hochheimer, N. Tetraspanins. Curr. Biol. 2020, 30, R204–R206. [Google Scholar] [CrossRef]
- Berditchevski, F.; Odintsova, E. Tetraspanins as Regulators of Protein Trafficking. Traffic 2007, 8, 89–96. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, more than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef]
- Hung, A.Y.; Sheng, M. PDZ Domains: Structural Modules for Protein Complex Assembly. J. Biol. Chem. 2002, 277, 5699–5702. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.M.H.; Amoussou, N.G.; Mai, H.L.; Logé, C.; Brouard, S. Tetraspanins: Useful multifunction proteins for the possible design and development of small-molecule therapeutic tools. Drug Discov. Today 2021, 26, 56–68. [Google Scholar] [CrossRef]
- Yauch, R.L.; Berditchevski, F.; Harler, M.B.; Reichner, J.; Hemler, M.E. Highly Stoichiometric, Stable, and Specific Association of Integrin α3β1 with CD151 Provides a Major Link to Phosphatidylinositol 4-Kinase, and May Regulate Cell Migration. Mol. Biol. Cell 1998, 9, 2751–2765. [Google Scholar] [CrossRef]
- Berditchevski, F.; Tolias, K.F.; Wong, K.; Carpenter, C.L.; Hemler, M.E. A Novel Link between Integrins, Transmembrane-4 Superfamily Proteins (CD63 and CD81), and Phosphatidylinositol 4-Kinase. J. Biol. Chem. 1997, 272, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Roth, D.M.; Jans, D.A.; Pouton, C.W.; Partridge, L.J.; Monk, P.N.; Moseley, G.W. Tetraspanins in viral infections: A fundamental role in viral biology? J. Virol. 2005, 79, 10839–10851. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, E.; Bujny, M.V.; Kim, D.; Davidson, M.W.; Zhuang, X. Dual function of CD81 in influenza virus uncoating and budding. PLoS Pathog. 2013, 9, e1003701. [Google Scholar] [CrossRef]
- Earnest, J.T.; Hantak, M.P.; Li, K.; McCray, P.B.; Perlman, S.; Gallagher, T. The tetraspanin CD9 facilitates MERS-coronavirus entry by scaffolding host cell receptors and proteases. PLoS Pathog. 2017, 13, e1006546. [Google Scholar] [CrossRef]
- Mikuličić, S.; Fritzen, A.; Scheffer, K.; Strunk, J.; Cabañas, C.; Sperrhacke, M.; Reiss, K.; Florin, L. Tetraspanin CD9 affects HPV16 infection by modulating ADAM17 activity and the ERK signalling pathway. Med. Microbiol. Immunol. 2020, 209, 461–471. [Google Scholar] [CrossRef]
- Healy, E.F. How tetraspanin-mediated cell entry of SARS-CoV-2 can dysregulate the shedding of the ACE2 receptor by ADAM17. Biochem. Biophys. Res. Commun. 2022, 593, 52–56. [Google Scholar] [CrossRef]
- Malla, R.; Kamal, M.A. Tetraspanin-enriched Microdomain Containing CD151, CD9, and TSPAN 8—Potential Mediators of Entry and Exit Mechanisms in Respiratory Viruses Including SARS-CoV-2. Curr. Pharm. Des. 2022, 28, 3649–3657. [Google Scholar] [CrossRef]
- Anwar, M.N.; Akhtar, R.; Abid, M.; Khan, S.A.; Rehman, Z.U.; Tayyub, M.; Malik, M.I.; Shahzad, M.K.; Mubeen, H.; Qadir, M.S.; et al. The interactions of flaviviruses with cellular receptors: Implications for virus entry. Virology 2022, 568, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef]
- Cosset, F.L.; Lavillette, D. Cell Entry of Enveloped Viruses. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 121–183. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780123808608000045 (accessed on 21 May 2023).
- Más, V.; Melero, J.A. Entry of enveloped viruses into host cells: Membrane fusion. In Structure and Physics of Viruses; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2013; Volume 68, pp. 467–487. [Google Scholar]
- Banse, P.; Moeller, R.; Bruening, J.; Lasswitz, L.; Kahl, S.; Khan, A.; Marcotrigiano, J.; Pietschmann, T.; Gerold, G. CD81 Receptor Regions outside the Large Extracellular Loop Determine Hepatitis C Virus Entry into Hepatoma Cells. Viruses 2018, 10, 207. [Google Scholar] [CrossRef]
- Pileri, P.; Uematsu, Y.; Campagnoli, S.; Galli, G.; Falugi, F.; Petracca, R.; Weiner, A.J.; Houghton, M.; Rosa, D.; Grandi, G.; et al. Binding of Hepatitis C Virus to CD81. Science 1998, 282, 938–941. [Google Scholar] [CrossRef]
- Palor, M.; Stejskal, L.; Mandal, P.; Lenman, A.; Alberione, M.P.; Kirui, J.; Moeller, R.; Ebner, S.; Meissner, F.; Gerold, G.; et al. Cholesterol sensing by CD81 is important for hepatitis C virus entry. J. Biol. Chem. 2020, 295, 16931–16948. [Google Scholar] [CrossRef] [PubMed]
- Bruening, J.; Lasswitz, L.; Banse, P.; Kahl, S.; Marinach, C.; Vondran, F.W.; Kaderali, L.; Silvie, O.; Pietschmann, T.; Meissner, F.; et al. Hepatitis C virus enters liver cells using the CD81 receptor complex proteins calpain-5 and CBLB. PLoS Pathog. 2018, 14, e1007111. [Google Scholar] [CrossRef] [PubMed]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Biol. 2017, 5, 34. [Google Scholar] [CrossRef]
- Maecker, H.T.; Todd, S.C.; Levy, S. The tetraspanin superfamily: Molecular facilitators. FASEB J. 1997, 11, 428–442. [Google Scholar] [CrossRef]
- Harris, H.J.; Davis, C.; Mullins, J.G.L.; Hu, K.; Goodall, M.; Farquhar, M.J.; Mee, C.J.; McCaffrey, K.; Young, S.; Drummer, H.; et al. Claudin Association with CD81 Defines Hepatitis C Virus Entry. J. Biol. Chem. 2010, 285, 21092–21102. [Google Scholar] [CrossRef]
- Zoladek, J.; Burlaud-Gaillard, J.; Chazal, M.; Desgraupes, S.; Jeannin, P.; Gessain, A.; Pardigon, N.; Hubert, M.; Roingeard, P.; Jouvenet, N.; et al. Human Claudin-Derived Peptides Block the Membrane Fusion Process of Zika Virus and Are Broad Flavivirus Inhibitors. Microbiol. Spectr. 2022, 10, e02989-22. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.S.; Shin, H.J. Role of Virus-Induced EGFR Trafficking in Proviral Functions. Biomolecules 2023, 13, 1766. [Google Scholar] [CrossRef]
- Diao, J.; Pantua, H.; Ngu, H.; Komuves, L.; Diehl, L.; Schaefer, G.; Kapadia, S.B. Hepatitis C Virus Induces Epidermal Growth Factor Receptor Activation via CD81 Binding for Viral Internalization and Entry. J. Virol. 2012, 86, 10935–10949. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Chen, H.W.; Zhang, H.X.; Wang, K.; Su, J.; Chen, Y.R.; Wang, X.-R.; Fu, Z.-F.; Cui, M. EGFR Activation Impairs Antiviral Activity of Interferon Signaling in Brain Microvascular Endothelial Cells During Japanese Encephalitis Virus Infection. Front. Microbiol. 2022, 13, 894356. [Google Scholar] [CrossRef]
- Xu, R.; Ji, Z.; Xu, C.; Zhu, J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: A systematic review and meta-analysis. Medicine 2018, 97, e12912. [Google Scholar] [CrossRef]
- Medigeshi, G.R.; Hirsch, A.J.; Brien, J.D.; Uhrlaub, J.L.; Mason, P.W.; Wiley, C.; Nikolich-Zugich, J.; Nelson, J.A. West Nile Virus Capsid Degradation of Claudin Proteins Disrupts Epithelial Barrier Function. J. Virol. 2009, 83, 6125–6134. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.N.; Sukumaran, B.; Pal, U.; Agaisse, H.; Murray, J.L.; Hodge, T.W.; Fikrig, E. Rab 5 Is Required for the Cellular Entry of Dengue and West Nile Viruses. J. Virol. 2007, 81, 4881–4885. [Google Scholar] [CrossRef]
- Tang, W.C.; Lin, R.J.; Liao, C.L.; Lin, Y.L. Rab18 Facilitates Dengue Virus Infection by Targeting Fatty Acid Synthase to Sites of Viral Replication. J. Virol. 2014, 88, 6793–6804. [Google Scholar] [CrossRef]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, L.; Xing, J.; Zhang, J.; Li, H.; Liu, L.; Hu, C.; Liao, M.; Yue, J.; Qi, W. Japanese encephalitis virus manipulates lysosomes membrane for RNA replication and utilizes autophagy components for intracellular growth. Vet. Microbiol. 2021, 255, 109025. [Google Scholar] [CrossRef]
- Grützkau, A.; Smorodchenko, A.; Lippert, U.; Kirchhof, L.; Artuc, M.; Henz, B.M. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytom. Part A 2004, 61, 62–68. [Google Scholar] [CrossRef]
- Tabata, K.; Arimoto, M.; Arakawa, M.; Nara, A.; Saito, K.; Omori, H.; Arai, A.; Ishikawa, T.; Konishi, E.; Suzuki, R.; et al. Unique Requirement for ESCRT Factors in Flavivirus Particle Formation on the Endoplasmic Reticulum. Cell Rep. 2016, 16, 2339–2347. [Google Scholar] [CrossRef]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. EWI-2 Is a Major CD9 and CD81 Partner and Member of a Novel Ig Protein Subfamily. J. Biol. Chem. 2001, 276, 40545–40554. [Google Scholar] [CrossRef] [PubMed]
- Montpellier, C.; Tews, B.A.; Poitrimole, J.; Rocha-Perugini, V.; D’Arienzo, V.; Potel, J.; Zhang, X.A.; Rubinstein, E.; Dubuisson, J.; Cocquerel, L. Interacting Regions of CD81 and Two of Its Partners, EWI-2 and EWI-2wint, and Their Effect on Hepatitis C Virus Infection. J. Biol. Chem. 2011, 286, 13954–13965. [Google Scholar] [CrossRef]
- Lammerding, J.; Kazarov, A.R.; Huang, H.; Lee, R.T.; Hemler, M.E. Tetraspanin CD151 regulates α6β1 integrin adhesion strengthening. Proc. Natl. Acad. Sci. USA 2003, 100, 7616–7621. [Google Scholar] [CrossRef]
- Reis, V.P.D.; Keller, M.; Schmidt, K.; Ulrich, R.G.; Groschup, M.H. αVβ3 Integrin Expression Is Essential for Replication of Mosquito and Tick-Borne Flaviviruses in Murine Fibroblast Cells. Viruses 2021, 14, 18. [Google Scholar] [CrossRef]
- Wolff, G.; Melia, C.E.; Snijder, E.J.; Bárcena, M. Double-Membrane Vesicles as Platforms for Viral Replication. Trends Microbiol. 2020, 28, 1022–1033. [Google Scholar] [CrossRef]
- Benayas, B.; Sastre, I.; López-Martín, S.; Oo, A.; Kim, B.; Bullido, M.J.; Aldudo, J.; Yáñez-Mó, M. Tetraspanin CD81 regulates HSV-1 infection. Med. Microbiol. Immunol. 2020, 209, 489–498. [Google Scholar] [CrossRef]
- Lasswitz, L.; Zapatero-Belinchón, F.J.; Moeller, R.; Hülskötter, K.; Laurent, T.; Carlson, L.A.; Goffinet, C.; Simmons, G.; Baumgärtner, W.; Gerold, G. The Tetraspanin CD81 Is a Host Factor for Chikungunya Virus Replication. Mbio 2022, 13, e00731-22. [Google Scholar] [CrossRef]
- Umotoy, J.C.; Kroon, P.Z.; Man, S.; Van Dort, K.A.; Atabey, T.; Schriek, A.I.; Dekkers, G.; Herrera-Carrillo, E.; Geijtenbeek, T.B.H.; Heukers, R.; et al. Inhibition of HIV-1 replication by nanobodies targeting tetraspanin CD9. iScience 2024, 27, 110958. [Google Scholar] [CrossRef] [PubMed]
- Duven, M.; Friedrichs, A.; Tomlinson, M.G.; Steffen, I.; Gerold, G. Tetraspanins 10 and 15 support Venezuelan equine encephalitis virus replication in astrocytoma cells. Mol. Biol. Cell 2025, 36, ar35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhang, B.H.; Ishii, K.; Liang, T.J. Novel Function of CD81 in Controlling Hepatitis C Virus Replication. J. Virol. 2010, 84, 3396–3407. [Google Scholar] [CrossRef]
- Bunz, M.; Eisele, M.; Hu, D.; Ritter, M.; Kammerloher, J.; Lampl, S.; Schindler, M. CD81 suppresses NF-κB signaling and is downregulated in hepatitis C virus expressing cells. Front. Cell. Infect. Microbiol. 2024, 14, 1338606. [Google Scholar] [CrossRef]
- York, S.B.; Sun, L.; Cone, A.S.; Duke, L.C.; Cheerathodi, M.R.; Meckes, D.G. Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-to-Cell Transmission. Msphere 2021, 6, e00192-21. [Google Scholar] [CrossRef]
- Vora, A.; Zhou, W.; Londono-Renteria, B.; Woodson, M.; Sherman, M.B.; Colpitts, T.M.; Neelakanta, G.; Sultana, H. Arthropod EVs mediate dengue virus transmission through interaction with a tetraspanin domain containing glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. USA 2018, 115, E6604–E6613. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, C.F.; Tu, C.H.; Huang, C.G.; Shih, Y.T.; Chuang, C.K.; Chen, W.-J. A novel tetraspanin C189 upregulated in C6/36 mosquito cells following dengue 2 virus infection. Virus Res. 2007, 124, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Tu, C.H.; Lo, Y.P.; Cheng, C.C.; Chen, W.J. Involvement of Tetraspanin C189 in Cell-to-Cell Spreading of the Dengue Virus in C6/36 Cells. PLoS Negl. Trop. Dis. 2015, 9, e0003885. [Google Scholar] [CrossRef] [PubMed]
- Neupane, D. Mosquito Exosomal Tetraspanin CD151 Facilitates Flaviviral Transmission and Interacts with ZIKV and DENV2 Viral Proteins. Int. J. Mol. Sci. 2025, 26, 7394. [Google Scholar] [CrossRef]
- van den Elsen, K.; Quek, J.P.; Luo, D. Molecular Insights into the Flavivirus Replication Complex. Viruses 2021, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Hurtado-Monzón, A.M.; Gallardo-Flores, C.E.; Alcaraz-Estrada, S.L.; Salas-Benito, J.S.; et al. The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell–Cell Communication: New Insights on Virus–Host Interactions. Viruses 2020, 12, 765. [Google Scholar] [CrossRef]
- Rubinstein, E.; Théry, C.; Zimmermann, P. Tetraspanins affect membrane structures and the trafficking of molecular partners: What impact on extracellular vesicles? Biochem. Soc. Trans. 2025, 53, 371–382. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.-J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Anderson, M.R.; Kashanchi, F.; Jacobson, S. Exosomes in Viral Disease. Neurotherapeutics 2016, 13, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Taïeb, J.; André, F.; Zitvogel, L. The potential of exosomes in immunotherapy. Expert Opin. Biol. Ther. 2005, 5, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rojas, P.P.; Monroy-Martínez, V.; Ruiz-Ordaz, B.H. Role of extracellular vesicles in the pathogenesis of mosquito-borne flaviviruses that impact public health. J. Biomed. Sci. 2025, 32, 4. [Google Scholar] [CrossRef]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Hurtado-Monzón, A.M.; Farfan-Morales, C.N.; Cervantes-Salazar, M.; Bolaños, J.; Cigarroa-Mayorga, O.E.; Martín-Martínez, E.S.; Medina, F.; et al. Isolation and characterization of exosomes released from mosquito cells infected with dengue virus. Virus Res. 2019, 266, 1–14. [Google Scholar] [CrossRef]
- Martínez-Rojas, P.P.; Quiroz-García, E.; Monroy-Martínez, V.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ruiz-Ordaz, B.H. Participation of Extracellular Vesicles from Zika-Virus-Infected Mosquito Cells in the Modification of Naïve Cells’ Behavior by Mediating Cell-to-Cell Transmission of Viral Elements. Cells 2020, 9, 123. [Google Scholar] [CrossRef]
- Martínez-Rojas, P.P.; Monroy-Martínez, V.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ruiz-Ordaz, B.H. Zika Virus-Infected Monocyte Exosomes Mediate Cell-to-Cell Viral Transmission. Cells 2024, 13, 144. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Liu, N.; Lv, D.; Chen, Y.; Liu, Z.; Jin, X.; Xiao, M.; Lavillette, D.; Zhong, J.; et al. Extracellular vesicles from Zika virus-infected cells display viral E protein that binds ZIKV-neutralizing antibodies to prevent infection enhancement. EMBO J. 2023, 42, e112096. [Google Scholar] [CrossRef]
- Safadi, D.E.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; Begue, F.; Hoareau, M.; Veeren, B.; Roche, M.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses 2023, 15, 364. [Google Scholar] [CrossRef]
- Slonchak, A.; Clarke, B.; Mackenzie, J.; Amarilla, A.A.; Setoh, Y.X.; Khromykh, A.A. West Nile virus infection and interferon alpha treatment alter the spectrum and the levels of coding and noncoding host RNAs secreted in extracellular vesicles. BMC Genom. 2019, 20, 474. [Google Scholar] [CrossRef]
- Vedpathak, S.; Sharma, A.; Palkar, S.; Bhatt, V.R.; Patil, V.C.; Kakrani, A.L.; Mishra, A.; Bhosle, D.; Arankalle, V.A.; Shrivastava, S. Platelet derived exosomes disrupt endothelial cell monolayer integrity and enhance vascular inflammation in dengue patients. Front. Immunol. 2023, 14, 1285162. [Google Scholar] [CrossRef]
- Kumari, S.; Bandyopadhyay, B.; Singh, A.; Aggarwal, S.; Yadav, A.K.; Vikram, N.K.; Guchhait, P.; Banerjee, A. Extracellular vesicles recovered from plasma of severe dengue patients induce CD4+ T cell suppression through PD-L1/PD-1 interaction. Mbio 2023, 14, e01823-23. [Google Scholar] [CrossRef]
- Li, X.; Liao, C.; Wu, J.; Yi, B.; Zha, R.; Deng, Q.; Xu, J.; Guo, C.; Lu, J. Distinct serum exosomal miRNA profiles detected in acute and asymptomatic dengue infections: A community-based study in Baiyun District, Guangzhou. Heliyon 2024, 10, e31546. [Google Scholar] [CrossRef]
- Martins, S.D.T.; Kuczera, D.; Lötvall, J.; Bordignon, J.; Alves, L.R. Characterization of Dendritic Cell-Derived Extracellular Vesicles During Dengue Virus Infection. Front. Microbiol. 2018, 9, 1792. [Google Scholar] [CrossRef] [PubMed]
- Bifani, A.M.; Chan, K.W.K.; Borrenberghs, D.; Tan, M.J.A.; Phoo, W.W.; Watanabe, S.; Goethals, O.; Vasudevan, S.G.; Choy, M.M. Therapeutics for flaviviral infections. Antivir. Res. 2023, 210, 105517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, M.; Cao, J.; Shen, J.; Zhou, X.; Wang, D.; Cao, J. Flavivirus: From Structure to Therapeutics Development. Life 2021, 11, 615. [Google Scholar] [CrossRef]
- Hemler, M.E. Targeting of tetraspanin proteins—Potential benefits and strategies. Nat. Rev. Drug Discov. 2008, 7, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Endsley, M.A.; Somasunderam, A.; Gbota, S.L.; Mbaka, M.I.; Murray, J.L.; Ferguson, M.R. The dual role of tetraspanin CD63 in HIV-1 replication. Virol. J. 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Palmulli, R.; Couty, M.; Piontek, M.C.; Ponnaiah, M.; Dingli, F.; Verweij, F.J.; Charrin, S.; Tantucci, M.; Sasidharan, S.; Rubinstein, E.; et al. CD63 sorts cholesterol into endosomes for storage and distribution via exosomes. Nat. Cell Biol. 2024, 26, 1093–1109. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Fenart, L. CD63, a new therapeutical candidate for cholesterol homeostasis regulation through extracellular vesicles? Extracell. Vesicles Circ. Nucleic Acids 2025, 6, 166–170. [Google Scholar] [CrossRef]
- Silvie, O.; Charrin, S.; Billard, M.; Franetich, J.F.; Clark, K.L.; Van Gemert, G.J.; Sauerwein, R.W.; Dautry, F.; Boucheix, C.; Mazier, D.; et al. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J. Cell Sci. 2006, 119, 1992–2002. [Google Scholar] [CrossRef]
- Caparotta, M.; Masone, D. Cholesterol plays a decisive role in tetraspanin assemblies during bilayer deformations. Biosystems 2021, 209, 104505. [Google Scholar] [CrossRef]
- Sahu, V.K.; Lokhande, K.B.; Swamy, V.K.; Basu, S.; Ranjan, A. Role of the transmembrane polar residues on CD151 in cholesterol binding. J. Biomol. Struct. Dyn. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X. Targeting of Tetraspanin CD81 with Monoclonal Antibodies and Small Molecules to Combat Cancers and Viral Diseases. Cancers 2023, 15, 2186. [Google Scholar] [CrossRef]
- Osuna-Ramos, J.F.; Reyes-Ruiz, J.M.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Farfan-Morales, C.N.; De Jesús-González, L.A.; Hurtado-Monzón, A.M.; Del Ángel, R.M. Ezetimibe inhibits dengue virus infection in Huh-7 cells by blocking the cholesterol transporter Niemann-Pick C1-like 1 receptor. Antivir. Res. 2018, 160, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Osuna-Ramos, J.F.; Monroy-Muñoz, I.E.; De Jesús-González, L.A.; Muñoz-Medina, J.E.; Hurtado-Monzón, A.M.; Reyes-Ruiz, J.M.; Del Ángel, R.M. The antiviral effect of metformin on zika and dengue virus infection. Sci. Rep. 2021, 11, 8743. [Google Scholar] [CrossRef]
- Palacios-Rápalo, S.N.; Farfan-Morales, C.N.; Cordero-Rivera, C.D.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Meraz-Ríos, M.A.; Del Ángel, R.M. An ivermectin—Atorvastatin combination impairs nuclear transport inhibiting dengue infection in vitro and in vivo. iScience 2023, 26, 108294. [Google Scholar] [CrossRef]
- Jiménez-Camacho, R.; Bravo-Silva, J.D.J.; Cordero-Rivera, C.D.; Benítez-Vega, M.L.; Hernández-Castillo, J.; Pérez-García, M.; Martínez-Conde, C.; Osuna-Ramos, J.F.; Navarrete-Vázquez, G.; Reyes-Ruiz, J.M.; et al. Antiviral effect of metformin and phenformin analogs against dengue in Huh-7 cells and AG129 mice. Eur. J. Pharmacol. 2025, 1005, 178083. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Bianconi, V.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Pirro, M.; Sahebkar, A. Antiviral effects of statins. Prog. Lipid Res. 2020, 79, 101054. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.W.; Mani, H.; Yen, J.H. Viral Hepatitis, Cholesterol Metabolism, and Cholesterol-Lowering Natural Compounds. Int. J. Mol. Sci. 2022, 23, 3897. [Google Scholar] [CrossRef]
- Vere, C.C.; Streba, C.T.; Streba, L.; Rogoveanu, I. Statins in the Treatment of Hepatitis C. Hepat. Mon. 2012, 12, 369–371. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Zhang, H.; Zhao, R.; Jing, R.; Xu, Y.; He, M.; Peer, J.; Kim, Y.C.; Luo, J.; et al. Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef]
- Sultana, H.; Ahmed, W.; Neelakanta, G. GW4869 inhibitor affects vector competence and tick-borne flavivirus acquisition and transmission by blocking exosome secretion. iScience 2024, 27, 110391. [Google Scholar] [CrossRef] [PubMed]
- Wichit, S.; Hamel, R.; Bernard, E.; Talignani, L.; Diop, F.; Ferraris, P.; Liegeois, F.; Ekchariyawat, P.; Luplertlop, N.; Surasombatpattana, P.; et al. Imipramine Inhibits Chikungunya Virus Replication in Human Skin Fibroblasts through Interference with Intracellular Cholesterol Trafficking. Sci. Rep. 2017, 7, 3145. [Google Scholar] [CrossRef]
- Álvarez-Fernández, H.; Mingo-Casas, P.; Blázquez, A.B.; Caridi, F.; Saiz, J.C.; Pérez-Pérez, M.J.; Martín-Acebes, M.A.; Priego, E.-M. Allosteric Inhibition of Neutral Sphingomyelinase 2 (nSMase2) by DPTIP: From Antiflaviviral Activity to Deciphering Its Binding Site through In Silico Studies and Experimental Validation. Int. J. Mol. Sci. 2022, 23, 13935. [Google Scholar] [CrossRef]
- Mingo-Casas, P.; Álvarez-Fernández, H.; Blázquez, A.B.; Esteban, A.; Escribano-Romero, E.; de Oya, N.J.; Calvo-Pinilla, E.; Pérez-Pérez, M.-J.; Priego, E.-M.; Martín-Acebes, M.A. Neutral sphingomyelinase 2 inhibition alters inflammatory gene expression signatures in the brain of mice infected with West Nile virus. Int. Immunopharmacol. 2025, 163, 115203. [Google Scholar] [CrossRef]
- Irep, N.; Inci, K.; Tokgun, P.E.; Tokgun, O. Exosome inhibition improves response to first-line therapy in small cell lung cancer. J. Cell Mol. Med. 2024, 28, e18138. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.H.; Baek, M.C. Dissecting exosome inhibitors: Therapeutic insights into small-molecule chemicals against cancer. Exp. Mol. Med. 2022, 54, 1833–1843. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Trindade, R.P.; Thompson, P.R.; Inal, J.M.; Lange, S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. Int. J. Mol. Sci. 2017, 18, 1007. [Google Scholar] [CrossRef]
- Figuera-Losada, M.; Stathis, M.; Dorskind, J.M.; Thomas, A.G.; Bandaru, V.V.R.; Yoo, S.W.; Westwood, N.J.; Rogers, G.W.; McArthur, J.C.; Haughey, N.J.; et al. Cambinol, a Novel Inhibitor of Neutral Sphingomyelinase 2 Shows Neuroprotective Properties. PLoS ONE 2015, 10, e0124481. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Singh, S.; Ahmad, M.; Khanna, K.; Ahmad, T.; Agrawal, A.; Ghosh, B. Simvastatin mediates inhibition of exosome synthesis, localization and secretion via multicomponent interventions. Sci. Rep. 2019, 9, 16373. [Google Scholar] [CrossRef] [PubMed]
- Nieland, T.J.F.; Chroni, A.; Fitzgerald, M.L.; Maliga, Z.; Zannis, V.I.; Kirchhausen, T.; Krieger, M. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J. Lipid Res. 2004, 45, 1256–1265. [Google Scholar] [CrossRef] [PubMed]

| Tetraspanin | Interaction with Cell Factors | Implication in Viral Infection | Reference |
|---|---|---|---|
| CD81 | Claudin-1, SR-BI, EGFR, Integrins | Cofactor in HCV, JEV, WNV, DENV, and ZIKV entry | [74,79,80,81,82,83,84,85,86] |
| CD63 | Rab proteins, ESCRT, LAMP-1 | Involved in endocytosis and multivesicular body formation | [87,88,89,90,91,92] |
| CD9 | Integrins, EWI-2 | Participates in membrane reorganization | [93,94] |
| CD151 | Integrins α3β1, α6β1 | Modulates membrane dynamics and signaling during viral entry | [95,96] |
| Tetraspanin | Viral Protein | Implication in Flavivirus Infection | Reference |
|---|---|---|---|
| CD81 | E2-glicoprotein | HCV entry | [67,69] |
| CD63 | Capsid | ZIKV assembly | [98] |
| Tsp29Fb | Envelope | DENV2 propagation | [99] |
| CD151 | NS2B, capsid | ZIKV and DENV2 Replication and assembly | [102] |
| Inhibitor | Target | Cell Effect | Reference |
|---|---|---|---|
| GW4869 | Neutral sphingomyelinase 2 | Inhibits the formation of intraluminal vesicles (ILVs), reducing exosome biogenesis and release. | [153,154] |
| Imipramine | Acid sphingomyelinase | Inhibits the conversion of acid sphingomyelinase to ceramide and decreases the release of extracellular vesicles. | [155] |
| DPTIP | Neutral Sphingomyelinase 2 | Reduces exosome release. | [156] |
| Simvastatin | HMG-CoA reductase (cholesterol synthesis) | Decreases membrane cholesterol levels, leading to a significant decrease in exosome secretion | [157] |
| Indomethacin | ABCA3 (lipid transporter) | Non-selectively inhibits lipid transporter; prevents exosome release. | [154] |
| Nexinhib20 | Rab27A (vesicle trafficking) | Inhibits the fusion of MVBs with the plasma membrane; combined with cisplatin and etoposide, it enhances the inhibitory effect. | [153] |
| Glibenclamide | ABC transporters | Modulates cholesterol recycling and reduces the release of microvesicles | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benitez-Vega, M.L.; Cordero-Rivera, C.D.; Bravo-Silva, J.D.J.; Jimenez-Camacho, R.; Farfan-Morales, C.N.; Hernández-Castillo, J.; Pérez-García, M.; del Ángel, R.M. Lipids, Tetraspanins, and Exosomes: Cell Factors in Orthoflavivirus Replication and Propagation. Viruses 2025, 17, 1321. https://doi.org/10.3390/v17101321
Benitez-Vega ML, Cordero-Rivera CD, Bravo-Silva JDJ, Jimenez-Camacho R, Farfan-Morales CN, Hernández-Castillo J, Pérez-García M, del Ángel RM. Lipids, Tetraspanins, and Exosomes: Cell Factors in Orthoflavivirus Replication and Propagation. Viruses. 2025; 17(10):1321. https://doi.org/10.3390/v17101321
Chicago/Turabian StyleBenitez-Vega, Magda L., Carlos D. Cordero-Rivera, Jose De Jesus Bravo-Silva, Ricardo Jimenez-Camacho, Carlos Noe Farfan-Morales, Jonathan Hernández-Castillo, Marcos Pérez-García, and Rosa M. del Ángel. 2025. "Lipids, Tetraspanins, and Exosomes: Cell Factors in Orthoflavivirus Replication and Propagation" Viruses 17, no. 10: 1321. https://doi.org/10.3390/v17101321
APA StyleBenitez-Vega, M. L., Cordero-Rivera, C. D., Bravo-Silva, J. D. J., Jimenez-Camacho, R., Farfan-Morales, C. N., Hernández-Castillo, J., Pérez-García, M., & del Ángel, R. M. (2025). Lipids, Tetraspanins, and Exosomes: Cell Factors in Orthoflavivirus Replication and Propagation. Viruses, 17(10), 1321. https://doi.org/10.3390/v17101321








