Differential Effects of Selenium Compounds on Mitochondrial Function in PRRSV-Infected Porcine Alveolar Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Selenium Compounds
2.2. Viruses
2.3. PRRSV-2 In Vitro Infection Layout
2.4. Cytotoxicity Assay
2.5. Tissue Culture Infectious Dose (TCID50) Analysis
2.6. RT-qPCR
2.7. Evaluation of Mitochondrial Fitness
2.8. Measurement of Reactive Oxygen Species (ROS) and Nitric Oxide (NO)
2.9. Gene Expression Analysis via NanoString Technology
2.10. Statistical Analysis
3. Results
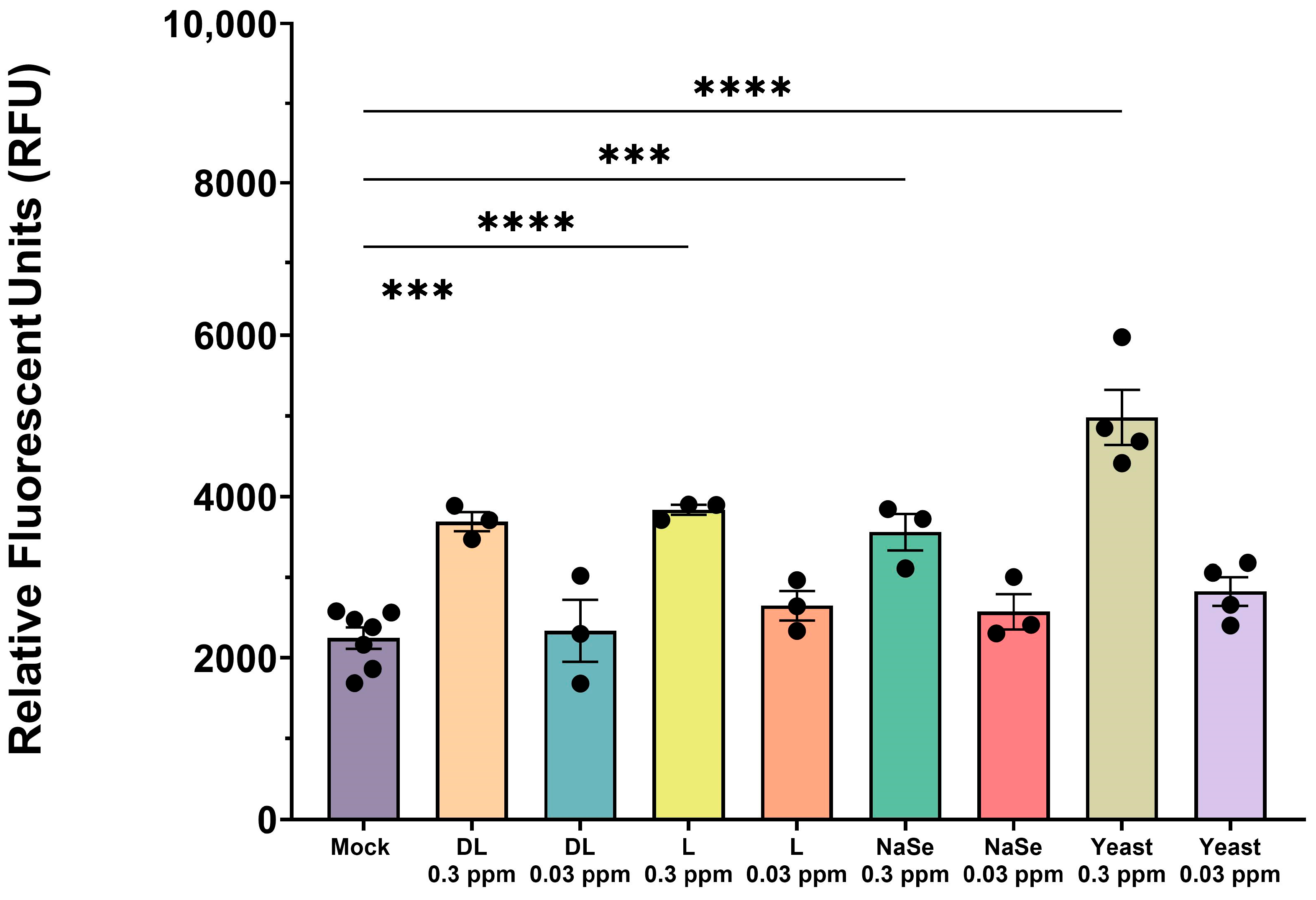
3.1. Selenium Compounds Exhibit Concentration-Dependent Cytotoxicity in PAMs
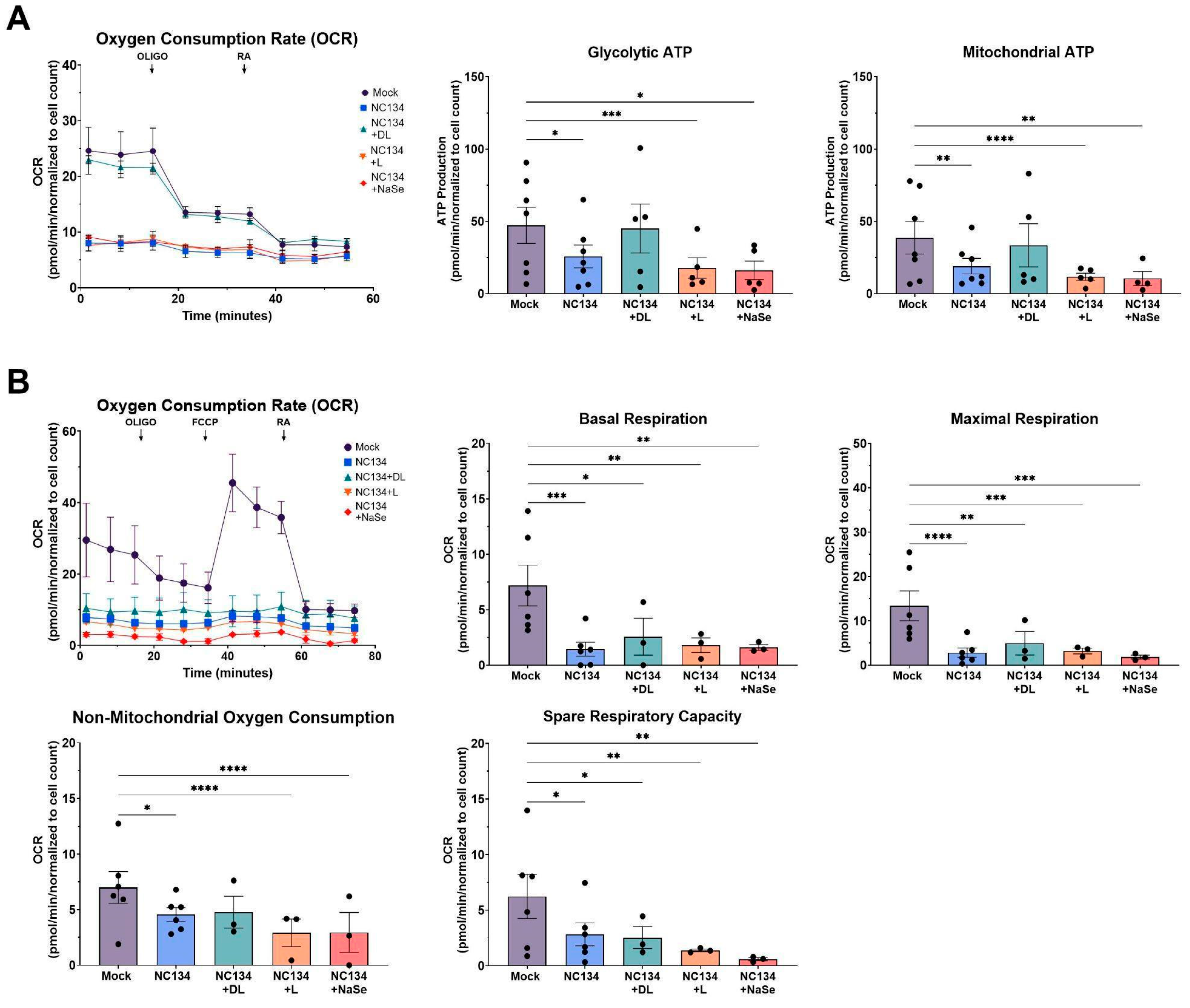
3.2. DL-Selenomethionine Treatment Modulates ATP Production in PRRSV-2 Infected Macrophages and Restores Mitochondrial Function
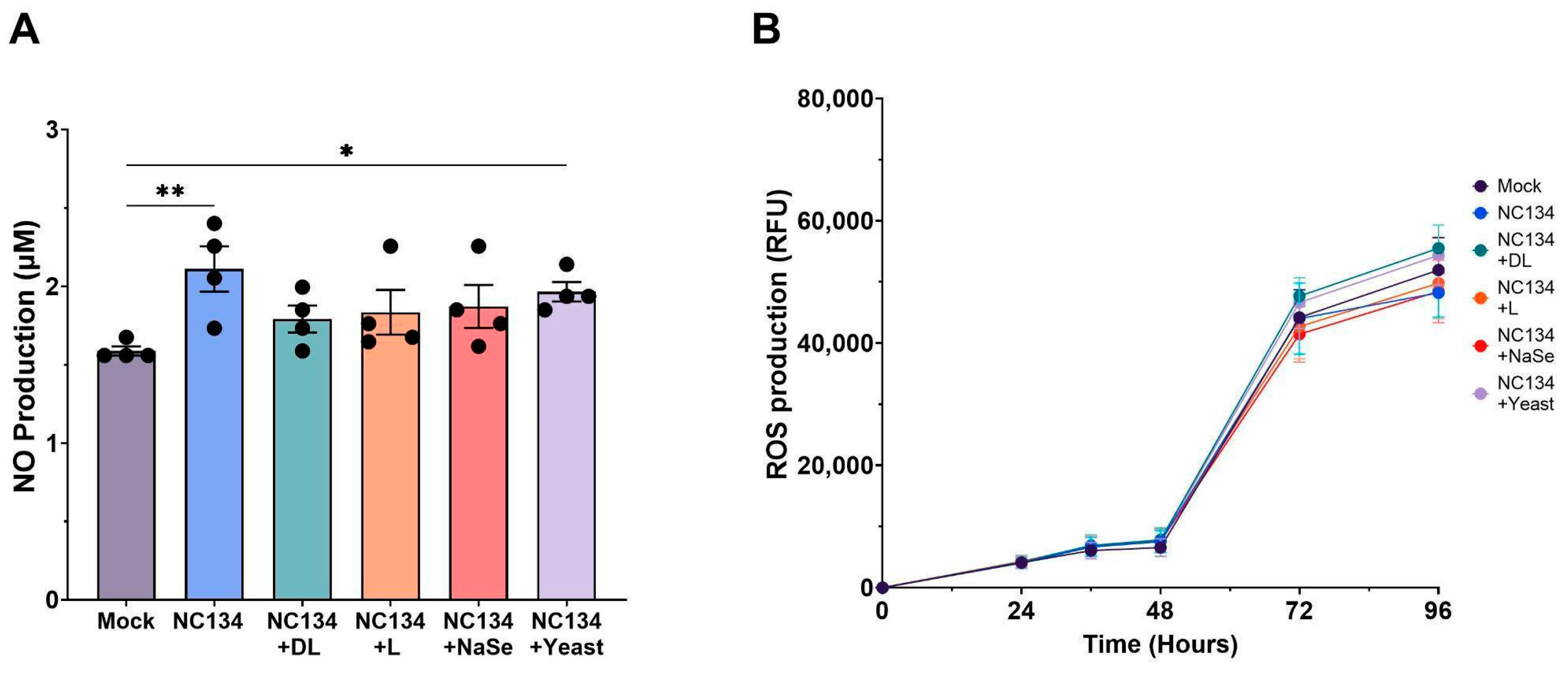
3.3. PRRSV-Associated Oxidative Stress Persists Despite Se Treatment in PAMs
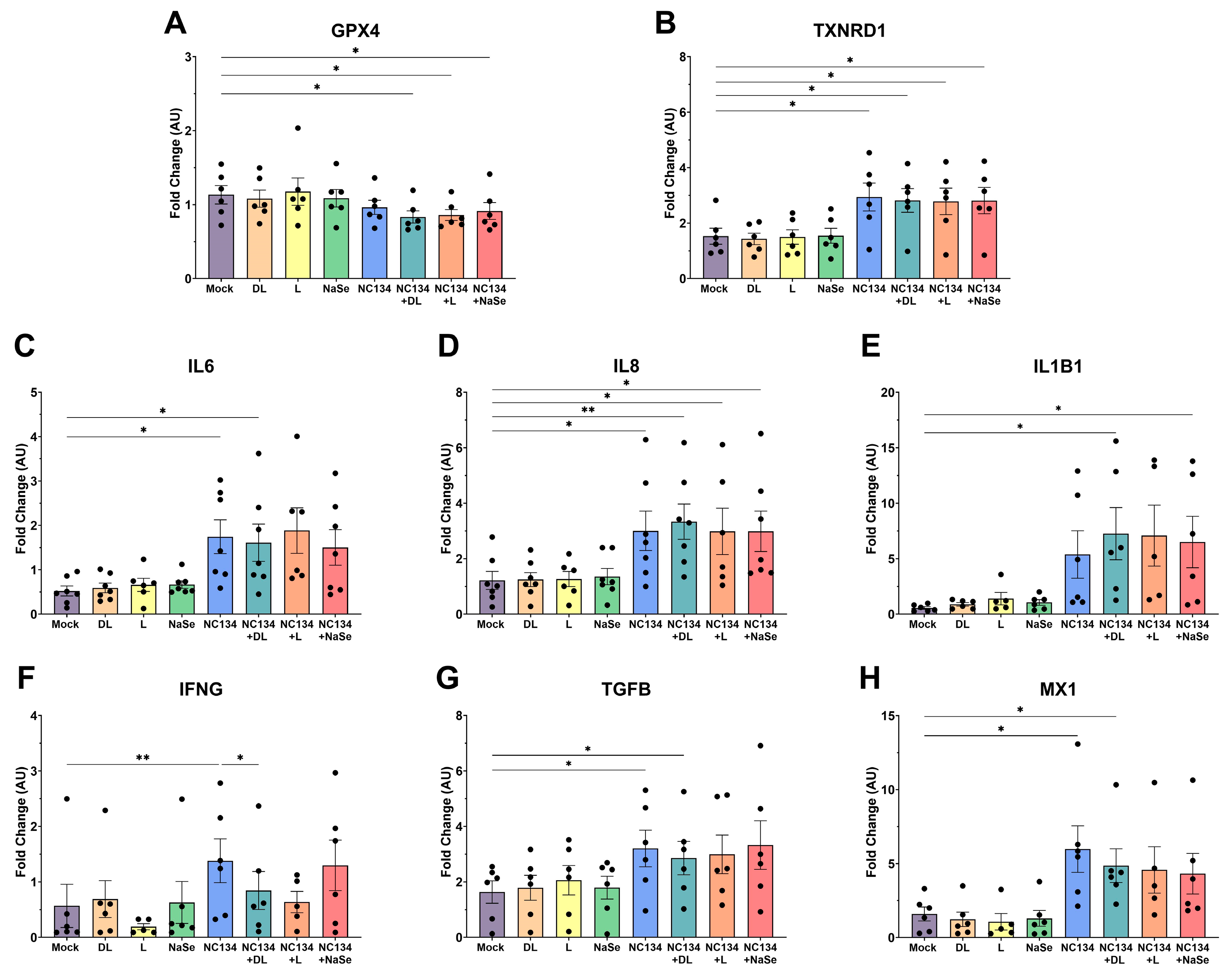
3.4. Transcriptomic Data upon Se Treatment of Infected PAMs Reveals Limited Modulation of Immune Cellular Responses
3.5. Se Treatment Fails to Confer Antiviral Protection in PRRSV-2 Infected PAMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DL | DL-Selenomethionine |
| FCCP | carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone |
| L | L-Selenomethionine |
| MOI | Multiplicity of Infection |
| NaSe | Sodium Selenite |
| NO | Nitric Oxide |
| OCR | Oxygen Consumption Rate |
| Oligo | Oligomycin |
| PAM | Porcine alveolar macrophage |
| PIM | Pulmonary interstitial macrophage |
| Ppm | Parts per million |
| PRRSV | Porcine Reproductive and Respiratory Syndrome Virus |
| RA | Rotenone/AA |
| RFU | Relative Fluorescent Units |
| ROS | Reactive Oxygen Species |
| RSP24 | Ribosomal Protein S24 |
| TCID50 | Tissue Culture Infectious Dose |
| Yeast | Yeast-Selenium |
References
- Roepke, D. Growing Losses from PRRS Cost Pork Producers $1.2 Billion Per Year, New Study Shows; Iowa State Research: Ames, Iowa, 2024. [Google Scholar]
- Osemeke, O.; Silva, G.S.; Corzo, C.A.; Kikuti, M.; Vadnais, S.; Yue, X.; Linhares, D.; Holtkamp, D. Economic Impact of Productivity Losses Attributable to Porcine Reproductive And Respiratory Syndrome Virus in United States Pork Production, 2016 to 2020. Prev. Vet. Med. 2025, 244, 106627. [Google Scholar] [CrossRef] [PubMed]
- VanderWaal, K.; Pamornchainavakul, N.; Kikuti, M.; Zhang, J.; Zeller, M.; Trevisan, G.; Rossow, S.; Schwartz, M.; Linhares, D.C.L.; Holtkamp, D.J.; et al. PRRSV-2 variant classification: A dynamic nomenclature for enhanced monitoring and surveillance. mSphere 2025, 10, e0070924. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.A.; Chang, J.; Trevisan, G.; Main, R.G.; Gauger, P.C.; Zhang, J. Rapid PRRSV-2 ORF5-based lineage classification using Nextclade. Front. Vet. Sci. 2024, 11, 1419340. [Google Scholar] [CrossRef]
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. ICTV Virus Taxonomy Profile: Arteriviridae 2021. J. Gen. Virol. 2021, 102, 001632. [Google Scholar] [CrossRef]
- Butler, J.E.; Lager, K.M.; Golde, W.; Faaberg, K.S.; Sinkora, M.; Loving, C.; Zhang, Y.I. Porcine reproductive and respiratory syndrome (PRRS): An immune dysregulatory pandemic. Immunol. Res. 2014, 59, 81–108. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, H.; Cheng, H.; Liu, M.; Wen, S.; Ren, J. Progress in PRRSV Infection and Adaptive Immune Response Mechanisms. Viruses 2023, 15, 1442. [Google Scholar] [CrossRef]
- Fiers, J.; Cay, A.B.; Maes, D.; Tignon, M. A Comprehensive Review on Porcine Reproductive and Respiratory Syndrome Virus with Emphasis on Immunity. Vaccines 2024, 12, 942. [Google Scholar] [CrossRef]
- Panahipoor Javaherdehi, A.; Ghanbari, S.; Mahdavi, P.; Zafarani, A.; Razizadeh, M.H. The role of alveolar macrophages in viral respiratory infections and their therapeutic implications. Biochem. Biophys. Rep. 2024, 40, 101826. [Google Scholar] [CrossRef]
- Melo, E.M.; Oliveira, V.L.S.; Boff, D.; Galvão, I. Pulmonary macrophages and their different roles in health and disease. Int. J. Biochem. Cell Biol. 2021, 141, 106095. [Google Scholar] [CrossRef]
- Allard, B.; Panariti, A.; Martin, J.G. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front. Immunol. 2018, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Crisci, E.; Fraile, L.; Montoya, M. Cellular Innate Immunity against PRRSV and Swine Influenza Viruses. Vet. Sci. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Zhou, A.; Khan, F.A.; Hu, L.; Zhang, S. Porcine reproductive and respiratory syndrome virus triggers mitochondrial fission and mitophagy to attenuate apoptosis. Oncotarget 2016, 7, 56002–56012. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, Z.; Deng, J.; Zhang, Z.; Chen, C.; Fan, Y.; Peng, G.; Cao, S.; Hu, Y.; Yu, S.; et al. Protective Role of Selenium in Immune-Relevant Cytokine and Immunoglobulin Production by Piglet Splenic Lymphocytes Exposed to Deoxynivalenol. Biol. Trace Elem. Res. 2018, 184, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Davis, D.; Fang, Y. Intercellular transfer of mitochondria rescues virus-induced cell death but facilitates cell-to-cell spreading of porcine reproductive and respiratory syndrome virus. Virology 2018, 517, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef]
- Zhang, Y.; Roh, Y.J.; Han, S.-J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.-R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Jain, V.K.; Priyadarsini, K.I. Selenium compounds as promising antiviral agents. New J. Chem. 2024, 48, 6534–6552. [Google Scholar] [CrossRef]
- Pila-Castellanos, I.; Molino, D.; McKellar, J.; Lines, L.; Da Graca, J.; Tauziet, M.; Chanteloup, L.; Mikaelian, I.; Meyniel-Schicklin, L.; Codogno, P.; et al. Mitochondrial morphodynamics alteration induced by influenza virus infection as a new antiviral strategy. PLoS Pathog. 2021, 17, e1009340. [Google Scholar] [CrossRef]
- Duan, C.; Ma, R.; Zeng, X.; Chen, B.; Hou, D.; Liu, R.; Li, X.; Liu, L.; Li, T.; Huang, H. SARS-CoV-2 Achieves Immune Escape by Destroying Mitochondrial Quality: Comprehensive Analysis of the Cellular Landscapes of Lung and Blood Specimens From Patients With COVID-19. Front. Immunol. 2022, 13, 946731. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Chen, Y.; Zhang, Y.; Yang, W.; Feng, Y.; Jing, H.; Kang, R.; Chen, D.; Liu, Y.; et al. Zinc Oxide–Selenium Nanoparticles for Inhibiting the Proliferation of Porcine Reproductive and Respiratory Syndrome Virus. ACS Appl. Nano Mater. 2024, 7, 3734–3747. [Google Scholar] [CrossRef]
- Chen, X.; Ren, F.; Hesketh, J.; Shi, X.; Li, J.; Gan, F.; Huang, K. Selenium blocks porcine circovirus type 2 replication promotion induced by oxidative stress by improving GPx1 expression. Free Radic. Biol. Med. 2012, 53, 395–405. [Google Scholar] [CrossRef]
- Rao, Z.-X.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D.; Gebhardt, J.T. Evaluation of selenium source on nursery pig growth performance, serum and tissue selenium concentrations, and serum antioxidant status. Transl. Anim. Sci. 2023, 7, txad049. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef]
- Dalgaard, T.S.; Briens, M.; Engberg, R.M.; Lauridsen, C. The influence of selenium and selenoproteins on immune responses of poultry and pigs. Anim. Feed Sci. Technol. 2018, 238, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Shojadoost, B.; Kulkarni, R.R.; Yitbarek, A.; Laursen, A.; Taha-Abdelaziz, K.; Negash Alkie, T.; Barjesteh, N.; Quinteiro-Filho, W.M.; Smith, T.K.; Sharif, S. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2. Vet. Immunol. Immunopathol. 2019, 207, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Frias-De-Diego, A.; Gilbertie, J.M.; Scholle, F.; Dejarnette, S.; Crisci, E. Effect of BIO-PLYTM, a Platelet-Rich Plasma Derived Biologic on PRRSV-2-Infected Macrophages. Viruses 2022, 14, 2666. [Google Scholar] [CrossRef]
- Bordet, E.; Blanc, F.; Tiret, M.; Crisci, E.; Bouguyon, E.; Renson, P.; Maisonnasse, P.; Bourge, M.; Leplat, J.-J.; Giuffra, E.; et al. Porcine Reproductive and Respiratory Syndrome Virus Type 1.3 Lena Triggers Conventional Dendritic Cells 1 Activation and T Helper 1 Immune Response Without Infecting Dendritic Cells. Front. Immunol. 2018, 9, 2299. [Google Scholar] [CrossRef]
- Paploski, I.A.D.; Corzo, C.; Rovira, A.; Murtaugh, M.P.; Sanhueza, J.M.; Vilalta, C.; Schroeder, D.C.; VanderWaal, K. Temporal Dynamics of Co-circulating Lineages of Porcine Reproductive and Respiratory Syndrome Virus. Front. Microbiol. 2019, 10, 2486. [Google Scholar] [CrossRef]
- VanderWaal, K.; Pamornchainavakul, N.; Kikuti, M.; Linhares, D.C.L.; Trevisan, G.; Zhang, J.; Anderson, T.K.; Zeller, M.; Rossow, S.; Holtkamp, D.J.; et al. Phylogenetic-based methods for fine-scale classification of PRRSV-2 ORF5 sequences: A comparison of their robustness and reproducibility. Front. Virol. 2024, 4, 1433931. [Google Scholar] [CrossRef]
- Kick, A.R.; Amaral, A.F.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Käser, T. The T-Cell Response to Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Viruses 2019, 11, 796. [Google Scholar] [CrossRef]
- Frias-De-Diego, A.; Crisci, E. Use of Crystal Violet to Improve Visual Cytopathic Effect-based Reading for Viral Titration using TCID50 Assays. J. Vis. Exp. (JoVE) 2022, 12, e63063. [Google Scholar]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID(50) for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, C.; Fan, Y.; Chen, C.; He, H.; Wang, X.; Zhang, Z.; Zuo, Z.; Peng, G.; Hu, Y.; et al. Toxicity of DON on GPx1-Overexpressed or Knockdown Porcine Splenic Lymphocytes In Vitro and Protective Effects of Sodium Selenite. Oxid. Med. Cell Longev. 2019, 2019, 5769752. [Google Scholar] [CrossRef]
- Elesela, S.; Lukacs, N.W. Role of Mitochondria in Viral Infections. Life 2021, 11, 232. [Google Scholar] [CrossRef]
- Kim, S.-J.; Khan, M.; Quan, J.; Till, A.; Subramani, S.; Siddiqui, A. Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis. PLoS Pathog. 2013, 9, e1003722. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kleiboeker, S.B. Porcine reproductive and respiratory syndrome virus induces apoptosis through a mitochondria-mediated pathway. Virology 2007, 365, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Kim, H.-T.; Lim, Y.; Kim, M.-J.; Kwon, O.J.; Jeon, K.; Park, H.Y.; Jeong, B.-H.; Koh, W.-J.; Lee, S.-Y. Serum Concentrations of Trace Elements in Patients with Tuberculosis and Its Association with Treatment Outcome. Nutrients 2015, 7, 5969–5981. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.L.; Kumari, S.; Mendelev, N.; Li, P.A. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012, 13, 79. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Liu, X.; Song, Z.; Bai, J.; Nauwynck, H.; Zhao, Y.; Jiang, P. Xanthohumol inhibits PRRSV proliferation and alleviates oxidative stress induced by PRRSV via the Nrf2–HMOX1 axis. Vet. Res. 2019, 50, 61. [Google Scholar] [CrossRef]
- Yan, M.; Hou, M.; Liu, J.; Zhang, S.; Liu, B.; Wu, X.; Liu, G. Regulation of iNOS-Derived ROS Generation by HSP90 and Cav-1 in Porcine Reproductive and Respiratory Syndrome Virus-Infected Swine Lung Injury. Inflammation 2017, 40, 1236–1244. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, J.; Lu, Y.; Huang, C.; Liu, H.; Xu, S. Selenium Deficiency Induces Inflammation via the iNOS/NF-κB Pathway in the Brain of Pigs. Biol. Trace Elem. Res. 2020, 196, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M.K. Role of Selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 23, 280. [Google Scholar] [CrossRef] [PubMed]
- SAPPEY, C.; LEGRAND-POELS, S.; BEST-BELPOMME, M.; FAVIER, A.; RENTIER, B.; PIETTE, J. Stimulation of Glutathione Peroxidase Activity Decreases HIV Type 1 Activation after Oxidative Stress. AIDS Res. Hum. Retroviruses 1994, 10, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Stýblo, M.; Walton, F.S.; Harmon, A.W.; Sheridan, P.A.; Beck, M.A. Activation of superoxide dismutase in selenium-deficient mice infected with influenza virus. J. Trace Elem. Med. Biol. 2007, 21, 52–62. [Google Scholar] [CrossRef]
- Shao, C.; Yu, Z.; Luo, T.; Zhou, B.; Song, Q.; Li, Z.; Yu, X.; Jiang, S.; Zhou, Y.; Dong, W.; et al. Chitosan-Coated Selenium Nanoparticles Attenuate PRRSV Replication and ROS/JNK-Mediated Apoptosis in vitro. Int. J. Nanomed. 2022, 17, 3043–3054. [Google Scholar] [CrossRef]
- Chaudhari, J.; Liew, C.-S.; Riethoven, J.-J.M.; Sillman, S.; Vu, H.L.X. Porcine Reproductive and Respiratory Syndrome Virus Infection Upregulates Negative Immune Regulators and T-Cell Exhaustion Markers. J. Virol. 2021, 95, e0105221. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Badaoui, B.; Tuggle, C.K.; Hu, Z.; Reecy, J.M.; Ait-Ali, T.; Anselmo, A.; Botti, S. Pig immune response to general stimulus and to porcine reproductive and respiratory syndrome virus infection: A meta-analysis approach. BMC Genom. 2013, 14, 220. [Google Scholar] [CrossRef]
- Genini, S.; Delputte, P.L.; Malinverni, R.; Cecere, M.; Stella, A.; Nauwynck, H.J.; Giuffra, E. Genome-wide transcriptional response of primary alveolar macrophages following infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2008, 89, 2550–2564. [Google Scholar] [CrossRef]
- Liu, G.; Yang, G.; Guan, G.; Zhang, Y.; Ren, W.; Yin, J.; Aguilar, Y.M.; Luo, W.; Fang, J.; Yu, X. Effect of dietary selenium yeast supplementation on porcine circovirus type 2 (PCV2) infections in mice. PLoS ONE 2015, 10, e0115833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ren, Z.; Jia, G.; He, H.; Ding, T.; Yu, Y.; Zuo, Z.; Hu, Y.; Zhong, Z.; Yu, S.; Deng, H.; et al. Antiviral Effect of Selenomethionine on Porcine Deltacoronavirus in Pig Kidney Epithelial Cells. Front. Microbiol. 2022, 13, 846747. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhi, X.; Sun, G.; Guo, W.; Huang, Y.; Sun, W.; Tian, X.; Zhao, F.; Hu, K. Sodium selenite suppresses hepatitis B virus transcription and replication in human hepatoma cell lines. J. Med. Virol. 2016, 88, 653–663. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- Gan, F.; Hu, Z.; Huang, Y.; Xue, H.; Huang, D.; Qian, G.; Hu, J.; Chen, X.; Wang, T.; Huang, K. Overexpression of pig selenoprotein S blocks OTA-induced promotion of PCV2 replication by inhibiting oxidative stress and p38 phosphorylation in PK15 cells. Oncotarget 2016, 7, 20469. [Google Scholar] [CrossRef] [PubMed]
- Morales-Nebreda, L.; Misharin, A.V.; Perlman, H.; Budinger, G.R. The heterogeneity of lung macrophages in the susceptibility to disease. Eur. Respir. Rev. 2015, 24, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Shor-Posner, G.; Lai, S.; Zhang, G.; Lai, H.; Fletcher, M.A.; Sauberlich, H.; Page, J.B. High Risk of HIV-Related Mortality Is Associated With Selenium Deficiency. JAIDS J. Acquir. Immune Defic. Syndr. 1997, 15, 370–374. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.; Bourne, C.; Byrne, J.; Sirisereewan, C.; Pecoraro, B.M.; Crisci, E. Differential Effects of Selenium Compounds on Mitochondrial Function in PRRSV-Infected Porcine Alveolar Macrophages. Viruses 2025, 17, 1303. https://doi.org/10.3390/v17101303
Williams A, Bourne C, Byrne J, Sirisereewan C, Pecoraro BM, Crisci E. Differential Effects of Selenium Compounds on Mitochondrial Function in PRRSV-Infected Porcine Alveolar Macrophages. Viruses. 2025; 17(10):1303. https://doi.org/10.3390/v17101303
Chicago/Turabian StyleWilliams, Abigail, Christina Bourne, John Byrne, Chaitawat Sirisereewan, Brittany M. Pecoraro, and Elisa Crisci. 2025. "Differential Effects of Selenium Compounds on Mitochondrial Function in PRRSV-Infected Porcine Alveolar Macrophages" Viruses 17, no. 10: 1303. https://doi.org/10.3390/v17101303
APA StyleWilliams, A., Bourne, C., Byrne, J., Sirisereewan, C., Pecoraro, B. M., & Crisci, E. (2025). Differential Effects of Selenium Compounds on Mitochondrial Function in PRRSV-Infected Porcine Alveolar Macrophages. Viruses, 17(10), 1303. https://doi.org/10.3390/v17101303








