Seroepidemiology of Human Parvovirus B19 Infection among the Population of Vojvodina, Serbia, over a 16-Year Period (2008–2023)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Population and Data Collection
2.3. Laboratory Method
2.4. Statistical Analyses
2.5. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Arvia, R.; Bua, G.; Margheri, F.; Gallinella, G. Parvovirus B19: Insights and implication for pathogenesis, prevention and therapy. Asp. Mol. Med. 2023, 1, 100007. [Google Scholar] [CrossRef]
- Yu, M.W.; Alter, H.J.; Virata-Theimer, M.L.A.; Geng, Y.; Ma, L.; Schechterly, C.A.; Colvin, C.A.; Luban, N.L.C. Parvovirus B19 infection transmitted by transfusion of red blood cells confirmed by molecular analysis of linked donor and recipient samples. Transfusion 2010, 50, 1712–1721. [Google Scholar] [CrossRef]
- Young, N.S.; Brown, K.E. Parvovirus B19. N. Engl. J. Med. 2004, 350, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Servey, J.T.; Reamy, B.V.; Hodge, J. Clinical presentations of parvovirus B19 infection. Am. Fam. Physician 2007, 75, 373–376. [Google Scholar]
- Barah, F.; Whiteside, S.; Batista, S.; Morris, J. Neurological aspects of human parvovirus B19 infection: A systematic review. Rev. Med. Virol. 2014, 24, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Slavov, S.N.; Kashima, S.; Pinto, A.C.S.; Covas, D.T. Human parvovirus B19: General considerations and impact on patients with sickle-cell disease and thalassemia and on blood transfusions. FEMS Immunol. Med. Microbiol. 2011, 62, 247–262. [Google Scholar] [CrossRef]
- Mrzljak, A.; Kardum-Skelin, I.; Cvrlje, V.C.; Kanizaj, T.F.; Sustercić, D.; Gustin, D.; Kocman, B. Parvovirus B 19 (PVB19) induced pure red cell aplasia (PRCA) in immunocompromised patient after liver transplantation. Coll. Antropol. 2010, 34, 271–274. [Google Scholar]
- Mrzljak, A.; Tabain, I.; Premac, H.; Bogdanic, M.; Barbic, L.; Savic, V.; Stevanovic, V.; Jelic, A.; Mikulic, D.; Vilibic-Cavlek, T. The Role of Emerging and Neglected Viruses in the Etiology of Hepatitis. Curr. Infect. Dis. Rep. 2019, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Tabain, I.; Kolaric, B.; Mihulja, K.; Blazevic, L.; Bogdanic, M.; Navolan, D.; Beader, N.; Mrzljak, A. Parvovirus B19 in Croatia: A Large-Scale Seroprevalence Study. Medicina 2021, 57, 1279. [Google Scholar] [CrossRef]
- Dijkmans, A.C.; de Jong, E.P.; Dijkmans, B.A.C.; Lopriore, E.; Vossen, A.; Walther, F.J.; Oepkes, D. Parvovirus B19 in pregnancy. Curr. Opin. Obstet. Gynecol. 2012, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Enders, M.; Weidner, A.; Zoellner, I.; Searle, K.; Enders, G. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: Prospective evaluation of 1018 cases. Prenat. Diagn. 2004, 24, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Fairley, C.K.; Cohen, B.J.; Seng, C. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. BJOG Int. J. Obstet. Gynaecol. 1998, 105, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Nunoue, T.; Kusuhara, K.; Hara, T. Human fetal infection with parvovirus B19: Maternal infection time in gestation, viral persistence and fetal prognosis. Pediatr. Infect. Dis. J. 2002, 21, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, G.E.; Benedetti, S.; Alby, C.; Benzina, N.; Ville, Y. Cause of fetal demise in first-trimester parvovirus infection: Anemia, placentitis or myocarditis? Ultrasound Obstet. Gynecol. 2014, 44, 618–619. [Google Scholar] [CrossRef]
- Lamont, R.; Sobel, J.; Vaisbuch, E.; Kusanovic, J.; Mazaki-Tovi, S.; Kim, S.; Uldbjerg, N.; Romero, R. Parvovirus B19 infection in human pregnancy. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Mor, O.; Ofir, I.; Pavel, R.; Bassal, R.; Kra-Oz, Z.; Cohen, D.; Shohat, T.; Mendelson, E. Parvovirus B19V infection in Israel: Prevalence and occurrence of acute infection between 2008 and 2013. Epidemiol. Infect. 2016, 144, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Röhrer, C.; Gärtner, B.; Sauerbrei, A.; Böhm, S.; Hottenträger, B.; Raab, U.; Thierfelder, W.; Wutzler, P.; Modrow, S. Seroprevalence of parvovirus B19 in the German population. Epidemiol. Infect. 2008, 136, 1564–1575. [Google Scholar] [CrossRef]
- Gillespie, S.M.; Cartter, M.L.; Asch, S.; Rokos, J.B.; Gary, G.W.; Tsou, C.J.; Hall, D.B.; Anderson, L.J.; Hurwitz, E.S. Occupational risk of human parvovirus B19 infection for school and day-care personnel during an outbreak of erythema infectiosum. JAMA 1990, 263, 2061–2065. [Google Scholar] [CrossRef]
- Maple, P.A.C.; Hedman, L.; Dhanilall, P.; Kantola, K.; Nurmi, V.; Söderlund-Venermo, M.; Brown, K.E.; Hedman, K. Identification of Past and Recent Parvovirus B19 Infection in Immunocompetent Individuals by Quantitative PCR and Enzyme Immunoassays: A Dual-Laboratory Study. J. Clin. Microbiol. 2014, 52, 947–956. [Google Scholar] [CrossRef]
- Kelly, H.A.; Siebert, D.; Hammond, R.; Leydon, J.; Kiely, P.; Maskill, W. The age-specific prevalence of human parvovirus immunity in Victoria, Australia compared with other parts of the world. Epidemiol. Infect. 2000, 124, 449–457. [Google Scholar] [CrossRef]
- Reinheimer, C.; Allwinn, R.; Doerr, H.W.; Wittek, M. Seroepidemiology of parvovirus B19 in the Frankfurt am Main area, Germany: Evaluation of risk factors. Infection 2010, 38, 381–385. [Google Scholar] [CrossRef] [PubMed]
- van Rijckevorsel, G.G.C.; Sonder, G.J.B.; Schim van der Loeff, M.F.; van den Hoek, J.A.R. Population-based study on the seroprevalence of parvovirus B19 in Amsterdam. J. Med. Virol. 2009, 81, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Göral, Ş.; Yenïcesu, I.; Bozdayi, G.; Çamurdan, A.D.; Koçak, A.A. Parvovirus B19 seroprevalence in Turkish blood donors. Turk. J. Med. Sci. 2018, 48, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Ziyaeyan, M.; Rasouli, M.; Alborzi, A. The seroprevalence of parvovirus B19 infection among to-be-married girls, pregnant women, and their neonates in Shiraz, Iran. Jpn. J. Infect. Dis. 2005, 58, 95–97. [Google Scholar] [PubMed]
- Mossong, J.; Hens, N.; Friederichs, V.; Davidkin, I.; Broman, M.; Litwinska, B.; Siennicka, J.; Trzcinska, A.; Van Damme, P.; Beutels, P.; et al. Parvovirus B19 infection in five European countries: Seroepidemiology, force of infection and maternal risk of infection. Epidemiol. Infect. 2008, 136, 1059–1068. [Google Scholar] [CrossRef]
- Ihara, T.; Furusyo, N.; Hayashi, T.; Toyoda, K.; Murata, M.; Hayashi, J. A population-based epidemiological survey of human parvovirus B19 infection: A project of the Kyushu and Okinawa Population Study (KOPS). Arch. Virol. 2013, 158, 2465–2472. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, C.; Pan, F.; Hong, L.; Luo, X.; Hu, S.; Xu, J.; Chen, Z. Epidemiologic study of human parvovirus B19 infection in East China. J. Med. Virol. 2016, 88, 1113–1119. [Google Scholar] [CrossRef]
- Maksheed, M.; Pacsa, A.; Essa, S.S.; Ahmed, M.A.; Monem, R.A.; Surkouh, M. The prevalence of antibody to human parvovirus B19 in pregnant women in Kuwait. Acta Trop. 1999, 73, 225–229. [Google Scholar] [CrossRef]
- Abiodun, I.; Opaleye, O.O.; Ojurongbe, O.; Fagbami, A.H. Seroprevalence of parvovirus B19 IgG and IgM antibodies among pregnant women in Oyo State, Nigeria. J. Infect. Dev. Ctries. 2013, 7, 946–950. [Google Scholar] [CrossRef][Green Version]
- Bernstein, D.I.; Sahly, H.M.E.; Keitel, W.A.; Wolff, M.; Simone, G.; Segawa, C.; Wong, S.; Shelly, D.; Young, N.S.; Dempsey, W. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine 2011, 29, 7357–7363. [Google Scholar] [CrossRef]
- Ballou, W.R.; Reed, J.L.; Noble, W.; Young, N.S.; Koenig, S. Safety and Immunogenicity of a Recombinant Parvovirus B19 Vaccine Formulated with MF59C.1. J. Infect. Dis. 2003, 187, 675–678. [Google Scholar] [CrossRef]
- Reno, M.L.; Cox, C.R.; Powell, E.A. Parvovirus B19: A Clinical and Diagnostic Review. Clin. Microbiol. Newsl. 2022, 44, 107–114. [Google Scholar] [CrossRef]
- Statistical Office of the Republic of Serbia the Census of Population, Households and Dwellings. Available online: https://www.stat.gov.rs/en-US/ (accessed on 2 January 2024).
- National Centers for Environmental Information Meteorological Versus Astronomical Seasons. Available online: https://www.ncei.noaa.gov/news/meteorological-versus-astronomical-seasons (accessed on 28 December 2023).
- Siennicka, J.; Stefanoff, P.; Trzcińska, A.; Rosińska, M.; Litwińska, B. Seroprevalence study of parvovirus B19 in Poland. Przegl. Epidemiol. 2006, 60, 571–580. [Google Scholar] [PubMed]
- Cohen, B.J.; Buckley, M.M. The prevalence of antibody to human parvovirus B 19 in England and Wales. J. Med. Microbiol. 1988, 25, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, V.; Jerant-Patic, V.; Hrnjakovic-Cvjetkovic, I.; Vukmanovic-Papuga, M.; Radovanov-Tadic, J.; Kovacevic, G. The frequency of human parvovirus B19 infections in Vojvodina. Med. Pregl. 2007, 60, 575–579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broliden, K.; Tolfvenstam, T.; Norbeck, O. Clinical aspects of parvovirus B19 infection. J. Intern. Med. 2006, 260, 285–304. [Google Scholar] [CrossRef]
- Aktaş, O.; Aydin, H.; Uslu, H. Serological prevalence of human parvovirus B19 in diseases or disorders related to different human body systems. Turk. J. Med. Sci. 2016, 46, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Nicolay, N.; Cotter, S. Clinical and epidemiological aspects of parvovirus B19 infections in Ireland, January 1996–June 2008. Eurosurveillance 2009, 14, 19249. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Ramadas, P.; Rajendran, P.; Madhavan, P.; Alex, A.; Jayaschandran, V.; Humayun, S.; Ali, N.; Sachdeva, M.; Flecha, A.; et al. Effects of Parvovirus B19 Infection in Renal Transplant Recipients: A Retrospective Review of Three Cases. Int. J. Angiol. 2014, 24, 087–092. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Chen, R.; Zhao, Y.; Wang, H.; Ma, X.; Li, D.; Liu, Q.; Chen, X.; He, L.; et al. Parvovirus B19 infection in kidney transplant recipients: A prospective study in a teaching hospital in Shanghai, China. Transpl. Immunol. 2022, 74, 101667. [Google Scholar] [CrossRef]
- Schmidt, I.; Blümel, J.; Seitz, H.; Willkommen, H.; Löwer, J. Parvovirus B19 DNA in plasma pools and plasma derivatives. Vox Sang. 2001, 81, 228–235. [Google Scholar] [CrossRef]
- Kleinman, S.H.; Glynn, S.A.; Lee, T.-H.; Tobler, L.H.; Schlumpf, K.S.; Todd, D.S.; Qiao, H.; Yu, M.W.; Busch, M.P. A linked donor-recipient study to evaluate parvovirus B19 transmission by blood component transfusion. Blood 2009, 114, 3677–3683. [Google Scholar] [CrossRef]
- Heegaard, E.D.; Laub Petersen, B. Parvovirus B19 transmitted by bone marrow. Br. J. Haematol. 2000, 111, 659–661. [Google Scholar] [CrossRef]
- Eid, A.J.; Chen, S.F. Human Parvovirus B19 in Solid Organ Transplantation. Am. J. Transplant. 2013, 13, 201–205. [Google Scholar] [CrossRef]
- Eid, A.J.; Brown, R.A.; Patel, R.; Razonable, R.R. Parvovirus B19 Infection after Transplantation: A Review of 98 Cases. Clin. Infect. Dis. 2006, 43, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D. Clinical Manifestations of Human Parvovirus B19 in Adults. Arch. Intern. Med. 1989, 149, 1153. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L. Parvovirus-associated arthritis. Curr. Opin. Rheumatol. 2000, 12, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.E.; Storch, G.A.; Lee, C.K.; Ward, K.E.; Danon, S.; Simon, C.M.; Delaney, J.W.; Tong, A.; Canter, C.E. High Frequency of Detection by PCR of Viral Nucleic Acid in the Blood of Infants Presenting with Clinical Myocarditis. Pediatr. Cardiol. 2016, 37, 399–404. [Google Scholar] [CrossRef]
- Gilbert, N.L.; Gyorkos, T.W.; Béliveau, C.; Rahme, E.; Muecke, C.; Soto, J.C. Seroprevalence of parvovirus B19 infection in daycare educators. Epidemiol. Infect. 2005, 133, 299–304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bouraddane, M.; Warda, K.; Zouhair, S. Parvovirus B19 and Pregnant Women: A Bibliographic Review. Open J. Obstet. Gynecol. 2021, 11, 1543–1564. [Google Scholar] [CrossRef]
- de Jong, E.P.; de Haan, T.R.; Kroes, A.C.M.; Beersma, M.F.C.; Oepkes, D.; Walther, F.J. Parvovirus B19 infection in pregnancy. J. Clin. Virol. 2006, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]



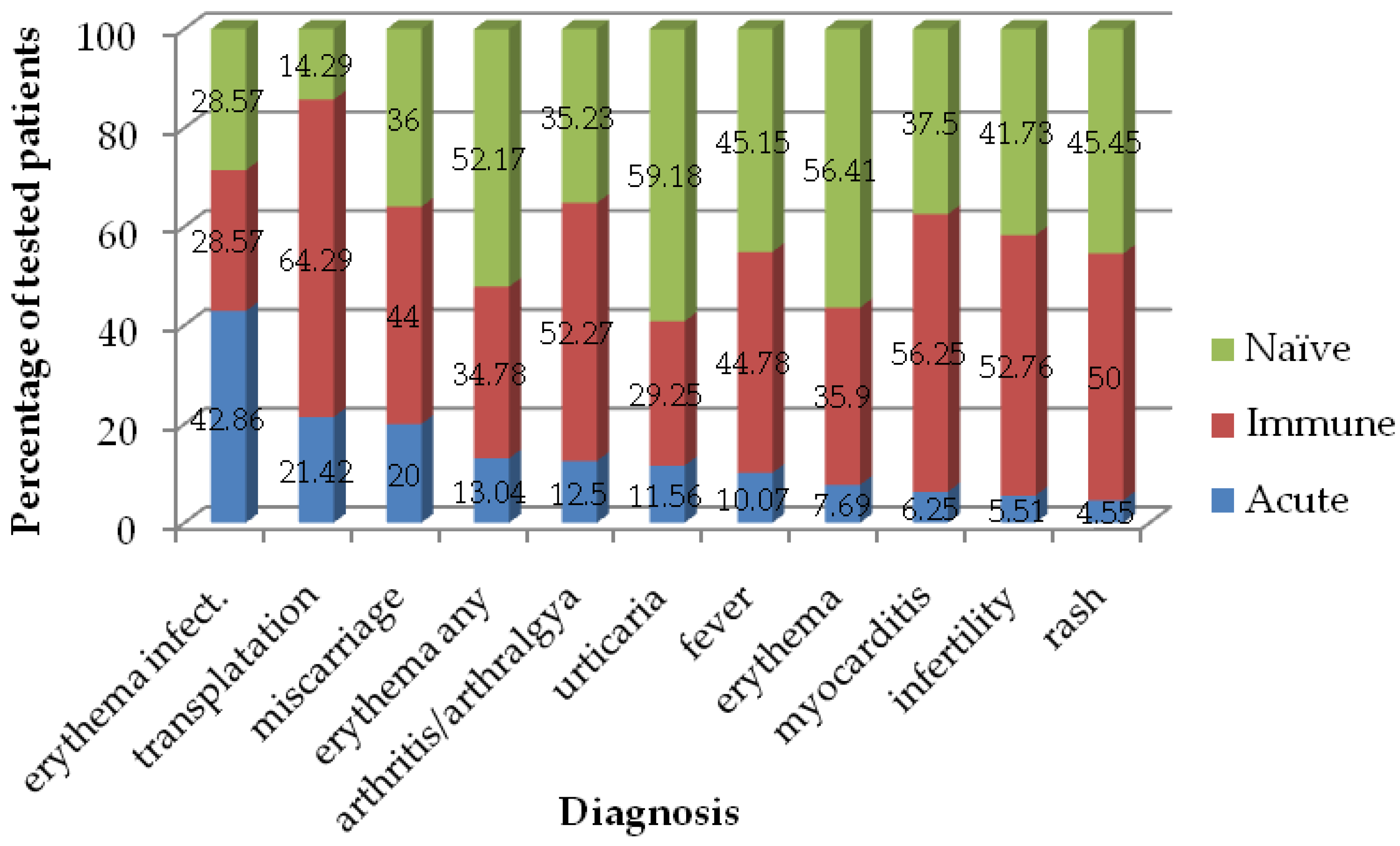
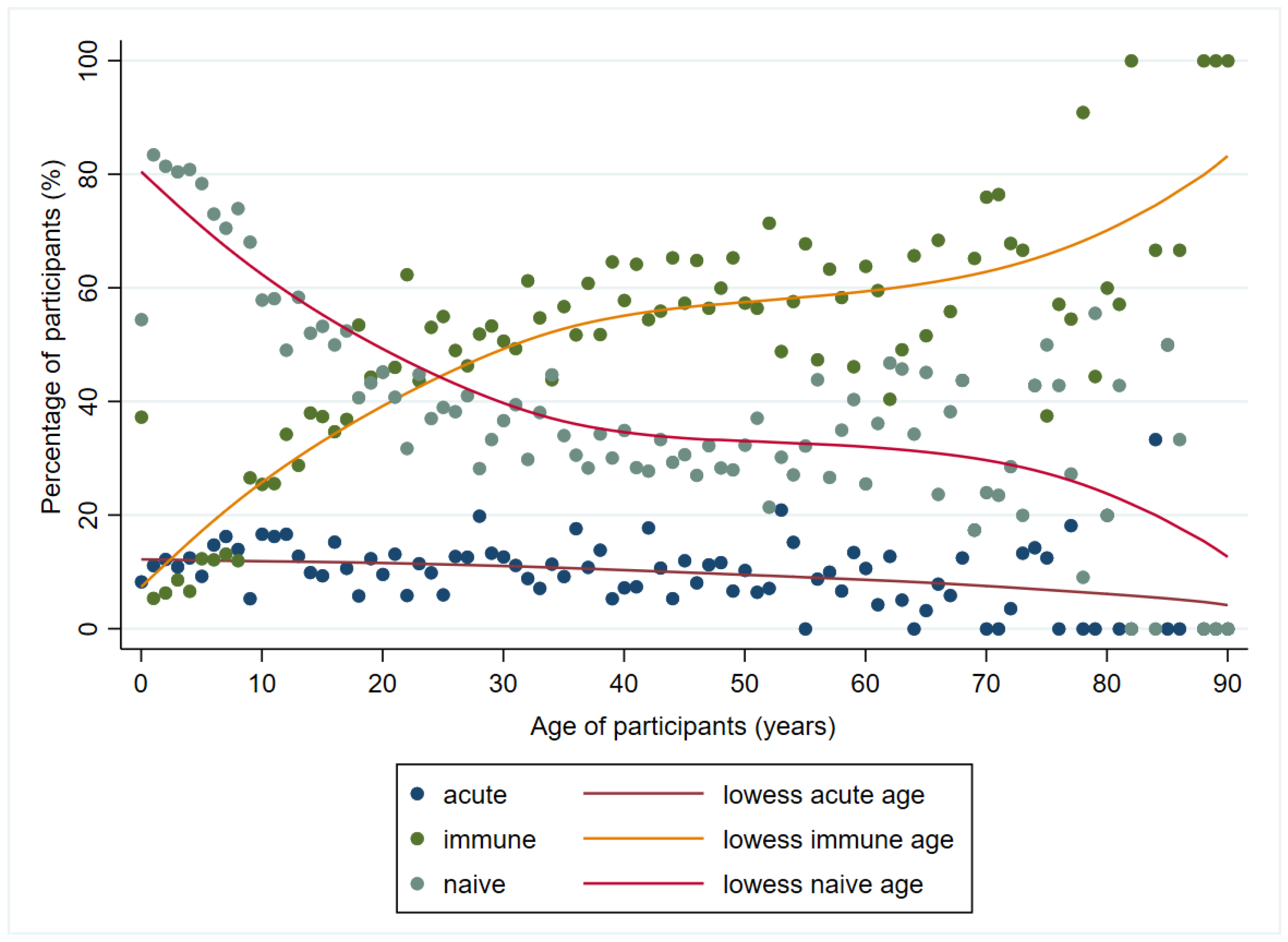
| IgM | Total Samples Tested, n | Total IgM Positive Samples | Ambulatory Patients’ Samples | Hospitalized Patients’ Samples | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | Tested | IgM Positive, n | % (95% CI) | Tested | IgM Positive, n | % (95% CI) | |||
| Total | 8692 | 753 | 8.66 (8.08–9.27) | 4200 | 389 | 9.26 (8.40–10.18) | 4492 | 364 | 8.10 (7.32–8.94) | 0.055 |
| Sex, n (%) | ||||||||||
| male | 3477 | 242 | 6.96 (6.14–7.86) | 1297 | 93 | 7.17 (5.83–8.71) | 2180 | 149 | 6.83 (5.81–7.98) | 0.707 |
| female | 5215 | 511 | 9.80 (9.00–10.64) | 2903 | 296 | 10.20 (9.12–11.35) | 2312 | 215 | 9.30 (8.15–10.56) | 0.279 |
| Age category, n (%) | ||||||||||
| <1 | 238 | 14 | 5.88 (3.25–9.67) | 31 | 1 | 3.23 (0.08–16.70) | 207 | 13 | 6.28 (3.39–10.50) | 0.500 |
| 1–4 | 1015 | 92 | 9.06 (7.37–11.00) | 204 | 25 | 12.25 (8.09–17.56) | 811 | 67 | 8.26 (6.46–10.37) | 0.076 |
| 5–9 | 721 | 72 | 9.99 (7.89–12.41) | 213 | 24 | 11.27 (7.35–16.30) | 508 | 48 | 9.45 (7.05–12.33) | 0.457 |
| 10–19 | 1340 | 141 | 10.52 (8.93–12.29) | 529 | 57 | 10.78 (8.26–13.73) | 811 | 84 | 10.36 (8.35–12.66) | 0.808 |
| 20–39 | 2850 | 265 | 9.30 (8.26–10.42) | 1919 | 195 | 10.16 (8.85–11.60) | 931 | 70 | 7.52 (5.91–9.40) | 0.023 |
| 40–59 | 1710 | 130 | 7.60 (6.39–8.96) | 964 | 73 | 7.57 (5.98–9.43) | 746 | 57 | 7.64 (5.84–9.79) | 0.958 |
| ≥60 | 818 | 39 | 4.77 (3.41–6.46) | 340 | 14 | 4.12 (2.27–6.81) | 478 | 25 | 5.23 (3.41–7.62) | 0.462 |
| IgG | Total Samples Tested, n | Total IgG Positive Samples | Ambulatory Patients’ Samples | Hospitalized Patients’ Samples | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | Tested | IgG Positive, n | % (95% CI) | Tested | IgG Positive, n | % (95% CI) | |||
| Total | 7325 | 3529 | 48.18 (47.03–49.33) | 3995 | 2195 | 54.94 (53.39–56.49) | 3330 | 1334 | 40.06 (38.39–41.75) | <0.001 |
| Sex, n (%) | ||||||||||
| male | 2892 | 1229 | 42.50 (40.69–44.32) | 1219 | 591 | 48.48 (45.64–51.33) | 1673 | 638 | 38.14 (35.80–40.51) | <0.001 |
| female | 4433 | 2300 | 51.88 (50.41–53.36) | 2776 | 1604 | 57.78 (55.92–59.63) | 1657 | 696 | 42.00 (39.61–44.42) | <0.001 |
| Age category, n (%) | ||||||||||
| <1 | 183 | 76 | 41.53 (34.31–49.03) | 25 | 8 | 32.00 (14.95–53.50) | 158 | 68 | 43.04 (35.20–51.14) | 0.298 |
| 1–4 | 866 | 79 | 9.12 (7.29–11.24) | 185 | 19 | 10.27 (6.30–15.57) | 681 | 60 | 8.81 (6.79–11.19) | 0.541 |
| 5–9 | 640 | 126 | 19.69 (16.67–22.98) | 197 | 45 | 22.84 (17.18–29.35) | 443 | 81 | 18.28 (14.79–22.20) | 0.181 |
| 10–19 | 1174 | 505 | 43.02 (40.16–45.90) | 492 | 239 | 48.58 (44.08–53.09) | 682 | 266 | 39.00 (35.32–42.78) | 0.001 |
| 20–39 | 2468 | 1461 | 59.20 (57.23–61.14) | 1847 | 1097 | 59.39 (57.11–61.64) | 621 | 364 | 58.62 (54.63–62.52) | 0.733 |
| 40–59 | 1399 | 916 | 65.48 (62.92–67.97) | 922 | 590 | 63.99 (60.80–67.10) | 477 | 326 | 68.34 (63.96–72.50) | 0.105 |
| ≥60 | 595 | 366 | 61.51 (57.47–65.44) | 327 | 197 | 60.24 (54.71–65.59) | 268 | 169 | 63.06 (56.98–68.85) | 0.483 |
| IgM Seropositive, n (%) | IgM Seronegative, n (%) | p-Value | ||
|---|---|---|---|---|
| Males | children and adolescents | 147 (8.62) | 1558 (91.38) | 0.001 |
| adults | 90 (6.28) | 1342 (93.72) | ||
| elderly | 5 (2.58) | 189 (97.42) | ||
| total | 242 (7.27) | 3089 (92.73) | NA | |
| Females | children and adolescents | 172 (12.14) | 1245 (87.86) | 0.008 |
| adults | 321 (9.91) | 2919 (90.09) | ||
| elderly | 18 (6.57) | 256 (93.43) | ||
| total | 511 (10.36) | 4420 (89.64) | NA | |
| Total | children and adolescents | 319 (10.22) | 2803 (89.78) | 0.001 |
| adults | 411 (8.80) | 4261 (91.20) | ||
| elderly | 23 (4.91) | 445 (95.09) | ||
| total | 753 (9.11) | 7509 (90.89) | NA | |
| IgG Seropositive, n (%) | IgG Seronegative, n (%) | p-value | ||
| Males | children and adolescents # | 347 (24.79) | 1053 (75.21) | <0.001 |
| adults | 738 (62.86) | 436 (37.14) | ||
| elderly | 98 (71.01) | 40 (28.99) | ||
| total | 1183 (43.62) | 1529 (56.38) | NA | |
| Females | children and adolescents # | 363 (29.71) | 859 (70.29) | <0.001 |
| adults | 1781 (62.58) | 1065 (37.42) | ||
| elderly | 126 (64.62) | 69 (35.38) | ||
| total | 2270 (53.25) | 1993 (46.75) | NA | |
| Total | children and adolescents # | 710 (27.08) | 1912 (72.92) | <0.001 |
| adults | 2519 (62.66) | 1501 (37.34) | ||
| elderly | 224 (67.27) | 109 (32.73) | ||
| total | 3453 (49.51) | 3522 (50.49) | NA |
| Serological Results | n | % | 95% Confidence Interval |
|---|---|---|---|
| IgM+/IgG− | 247 | 3.66 | 3.23–4.14 |
| IgM+/IgG+ | 366 | 5.43 | 4.90–5.99 |
| IgM−/IgG+ | 2942 | 43.63 | 42.44–44.82 |
| IgM−/IgG− | 3188 | 47.28 | 46.08–48.48 |
| Total | 6743 | 100 | 100 |
| Total, n (%) | Acute, n (%) | Immune, n (%) | Naive, n (%) | p-Value | ||
|---|---|---|---|---|---|---|
| Males | children and adolescents | 1438 (100) | 147 (10.22) | 314 (21.84) | 977 (67.94) | <0.001 |
| adults | 1152 (100) | 90 (7.81) | 666 (57.81) | 396 (34.38) | ||
| elderly | 134 (100) | 5 (3.73) | 90 (67.16) | 39 (29.10) | ||
| total | 2724 (100) | 242 (8.88) | 1070 (39.28) | 1412 (51.84) | NA | |
| Females | children and adolescents | 1232 (100) | 172 (13.96) | 283 (22.97) | 777 (63.07) | <0.001 |
| adults | 2730 (100) | 321 (11.76) | 1474 (53.99) | 935 (34.25) | ||
| elderly | 197 (100) | 18 (9.14) | 115 (58.38) | 64 (32.49) | ||
| total | 4159 (100) | 511 (12.29) | 1872 (45.01) | 1776 (42.70) | NA | |
| Total | children and adolescents | 2670 (100) | 319 (11.95) | 597 (22.36) | 1754 (65.69) | <0.001 |
| adults | 3882 (100) | 411 (10.59) | 2140 (55.13) | 1331 (34.29) | ||
| elderly | 331 (100) | 23 (6.95) | 205 (61.93) | 103 (31.12) | ||
| total | 6883 (100) | 753 (10.94) | 2942 (42.74) | 3188 (46.32) | NA |
| Acute | Immune | Naïve | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Category | n (%) | OR (95% CI) | p-Value | n (%) | OR (95% CI) | p-Value | n (%) | OR (95% CI) | p-Value | n (%) |
| 14–25 | 2 (3.70) | ref. | 25 (46.30) | ref. | 27 (50.00) | ref. | 54 (100) | |||
| 26–35 | 28 (10.73) | 3.12 (0.72–13.53) | 0.128 | 140 (53.64) | 1.34 (0.75–2.42) | 0.326 | 93 (35.63) | 0.55 (0.31–1.00) | 0.05 | 261 (100) |
| 36–45 | 12 (12.50) | 3.71 (0.80–17.26) | 0.094 | 53 (55.21) | 1.43 (0.73–2.79) | 0.295 | 31 (32.29) | 0.48 (0.24–0.94) | 0.034 | 96 (100) |
| Total | 42 (10.22) | NA | NA | 218 (53.04) | NA | NA | 151 (36.74) | NA | NA | 411 (100) |
| Acute | Immune | Naïve | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Category | n (%) | OR (95% CI) | p-Value | n (%) | OR (95% CI) | p-Value | n (%) | OR (95% CI) | p-Value | n (%) |
| 14–25 | 69 (11.90) | ref. | 255 (43.97) | ref. | 256 (44.14) | ref. | 580 (100) | |||
| 26–35 | 88 (12.81) | 1.09 (0.78–1.52) | 0.623 | 361 (52.55) | 1.41 (1.13–1.76) | 0.002 | 238 (34.64) | 0.67 (0.53–0.84) | 0.001 | 687 (100) |
| 36–45 | 87 (13.18) | 1.12 (0.80–1.58) | 0.496 | 369 (55.91) | 1.62 (1.29–2.02) | <0.001 | 204 (30.91) | 0.57 (0.45–0.71) | <0.001 | 660 (100) |
| Total | 244 (12.66) | NA | NA | 985 (51.12) | NA | NA | 698 (36.22) | NA | NA | 1927 (100) |
| OR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| male | ref. | ref. | ref. | - | - | - |
| female | 1.44 | 1.22–1.69 | <0.001 | - | - | - |
| Women | ||||||
| generative age non-pregnant | ref. | ref. | ref. | ref. | ref. | ref. |
| pregnant women | 0.81 | 0.57–1.14 | 0.229 | 0.81 | 0.57–1.14 | 0.223 |
| Age, cont. (one-year increase) | 0.99 | 0.99–0.99 | 0.001 | - | - | - |
| Age category | ||||||
| children and adolescents | ref. | ref. | ref. | ref. | ref. | ref. |
| adults | 0.87 | 0.75–1.02 | 0.086 | 0.99 | 0.75–1.34 | 0.995 |
| elderly | 0.55 | 0.35–0.85 | 0.008 | 0.85 | 0.43–1.66 | 0.631 |
| School-age category | ||||||
| <1 | ref. | ref. | ref. | ref. | ref. | ref. |
| 1–3 | 1.43 | 0.79–2.60 | 0.239 | 1.52 | 0.82–2.80 | 0.181 |
| 4–6 | 1.49 | 0.80–2.78 | 0.209 | 1.7 | 0.83–3.48 | 0.148 |
| 7–14 | 1.74 | 0.97–3.10 | 0.062 | 2.3 | 0.89–5.94 | 0.084 |
| 15–19 | 1.39 | 0.76–2.55 | 0.288 | 2.16 | 0.56–8.33 | 0.264 |
| Season when testing was performed | ||||||
| spring | ref. | ref. | ref. | ref. | ref. | ref. |
| summer | 0.94 | 0.77–1.15 | 0.532 | 0.94 | 0.77–1.15 | 0.568 |
| autumn | 0.61 | 0.49–0.76 | <0.001 | 0.61 | 0.49–0.76 | <0.001 |
| winter | 0.82 | 0.66–1.01 | 0.057 | 0.81 | 0.66–1.01 | 0.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković, V.; Patić, A.; Ristić, M.; Kovačević, G.; Hrnjaković Cvjetković, I.; Petrović, V. Seroepidemiology of Human Parvovirus B19 Infection among the Population of Vojvodina, Serbia, over a 16-Year Period (2008–2023). Viruses 2024, 16, 180. https://doi.org/10.3390/v16020180
Vuković V, Patić A, Ristić M, Kovačević G, Hrnjaković Cvjetković I, Petrović V. Seroepidemiology of Human Parvovirus B19 Infection among the Population of Vojvodina, Serbia, over a 16-Year Period (2008–2023). Viruses. 2024; 16(2):180. https://doi.org/10.3390/v16020180
Chicago/Turabian StyleVuković, Vladimir, Aleksandra Patić, Mioljub Ristić, Gordana Kovačević, Ivana Hrnjaković Cvjetković, and Vladimir Petrović. 2024. "Seroepidemiology of Human Parvovirus B19 Infection among the Population of Vojvodina, Serbia, over a 16-Year Period (2008–2023)" Viruses 16, no. 2: 180. https://doi.org/10.3390/v16020180
APA StyleVuković, V., Patić, A., Ristić, M., Kovačević, G., Hrnjaković Cvjetković, I., & Petrović, V. (2024). Seroepidemiology of Human Parvovirus B19 Infection among the Population of Vojvodina, Serbia, over a 16-Year Period (2008–2023). Viruses, 16(2), 180. https://doi.org/10.3390/v16020180







