Corticosteroid Dosing Level, Incidence and Profile of Bacterial Blood Stream Infections in Hospitalized COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Ethical Approval
2.3. Statistical Analysis
3. Results
3.1. Overview of Patient Characteristics, Corticosteroid Dosing Level, and Bacteremia
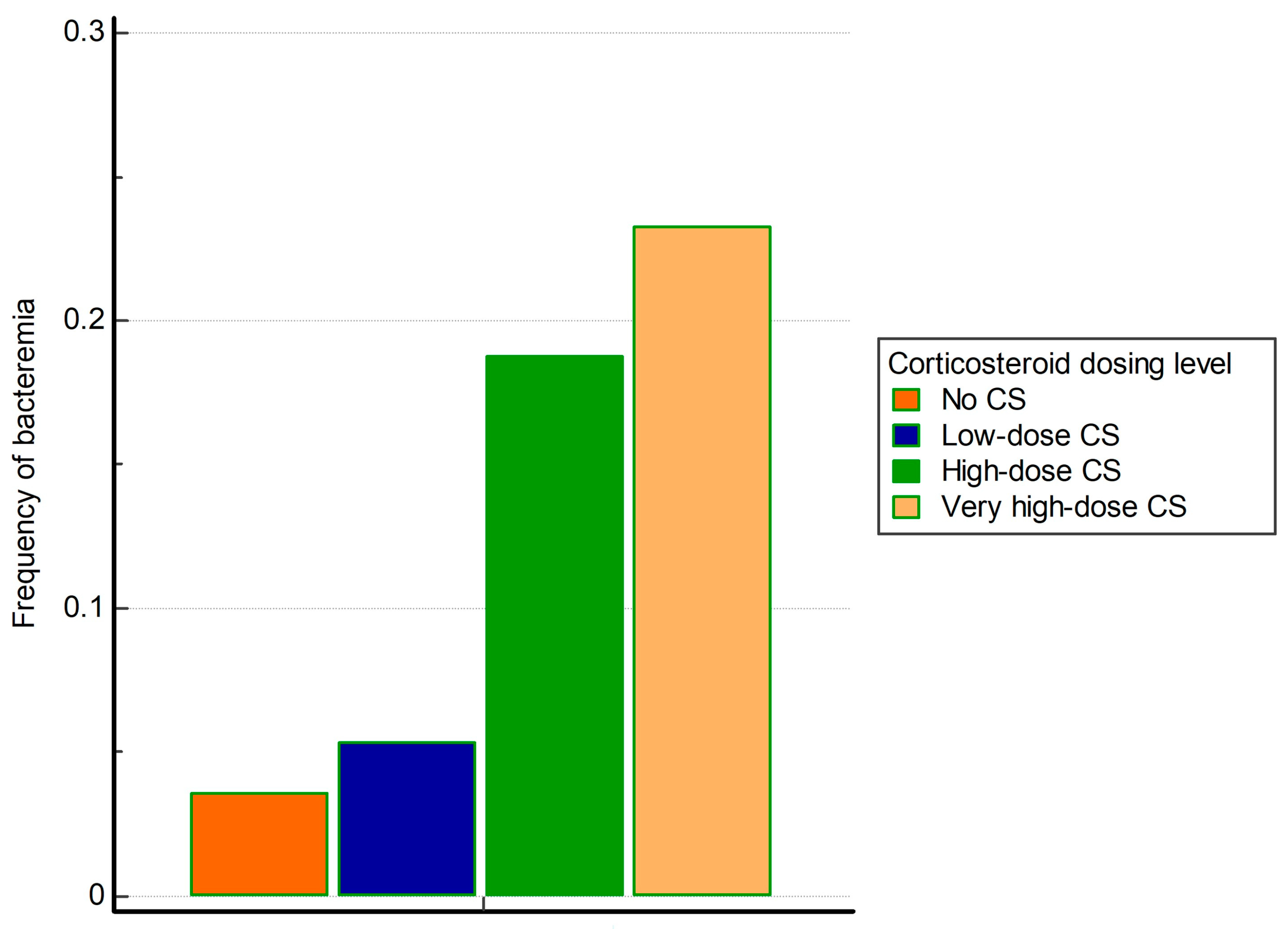
3.2. The Association of Corticosteroid Dosing Level with the Occurrence of Bacteremia
3.3. Profile of Bacterial Blood Stream Infections Regarding the Corticosteroid Dosing Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M.; et al. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef]
- WHO. Therapeutics and COVID-19: Living Guideline, 13 January 2023; 2023 (WHO/2019-nCoV/Therapeutics/2023.1). Lic Ence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Youssef, J.; Novosad, S.A.; Winthrop, K.L. Infection Risk and Safety of Corticosteroid Use. Rheum. Dis. Clin. N. Am. 2016, 42, 157–176. [Google Scholar] [CrossRef]
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit. Care Med. 2021, 49, e31. [Google Scholar] [CrossRef]
- Afzal, A.; Gutierrez, V.P.; Gomez, E.; Mon, A.M.; Sarmiento, C.M.; Khalid, A.; Polishchuk, S.; Al-Khateeb, M.; Yankulova, B.; Yusuf, M.; et al. Bloodstream Infections in Hospitalized Patients before and during the COVID-19 Surge in a Community Hospital in the South Bronx. Int. J. Infect. Dis. 2022, 116, 43–46. [Google Scholar] [CrossRef]
- Mormeneo Bayo, S.; Palacián Ruíz, M.P.; Moreno Hijazo, M.; Villuendas Usón, M.C. Bacteremia during COVID-19 Pandemic in a Tertiary Hospital in Spain. Enferm. Infecc. Microbiol. Clin. 2022, 40, 183–186. [Google Scholar] [CrossRef]
- Cauhapé, V.; Lamy, B.; Lotte, R.; Touitou, I.; Boyer, L.; Contenti, J.; Parisot, F.; Ruimy, R.; Carles, M.; Courjon, J. Lesson from the COVID-19 Pandemic Lockdown: A Major Change of Hospital-Diagnosed Bacteremia Epidemiology. Infect. Dis. Now 2023, 53, 104709. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, K.; Kamata, H.; Chubachi, S.; Namkoong, H.; Tanaka, H.; Lee, H.; Otake, S.; Fukushima, T.; Kusumoto, T.; Morita, A.; et al. Diagnostic Significance of Secondary Bacteremia in Patients with COVID-19. J. Infect. Chemother. 2023, 29, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.J.; Shiau, S.; Brunetti, L.; Xie, Y.; Solanki, K.; Khalid, S.; Mohayya, S.; Au, P.H.; Pham, C.; Uprety, P.; et al. Risk Factors and Outcomes of Hospitalized Patients with Severe Coronavirus Disease 2019 (COVID-19) and Secondary Bloodstream Infections: A Multicenter Case-Control Study. Clin. Infect. Dis. 2021, 72, e995–e1003. [Google Scholar] [CrossRef] [PubMed]
- Giannitsioti, E.; Louka, C.; Mamali, V.; Kousouli, E.; Velentza, L.; Papadouli, V.; Loizos, G.; Mavroudis, P.; Kranidiotis, G.; Rekleiti, N.; et al. Bloodstream Infections in a COVID-19 Non-ICU Department: Microbial Epidemiology, Resistance Profiles and Comparative Analysis of Risk Factors and Patients’ Outcome. Microorganisms 2022, 10, 1314. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.; Erickson, D.; Manca, C.; Lozy, T.; Shashkina, E.; Kordalewska, M.; Mediavilla, J.R.; Chen, L.; Rojtman, A.; Kreiswirth, B.N. The Impact of COVID on Bacterial Sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.; Munch, M.W.; Myatra, S.N.; Vijayaraghavan, B.K.T.; Cronhjort, M.; Wahlin, R.R.; Jakob, S.M.; Cioccari, L.; Kjær, M.-B.N.; Vesterlund, G.K.; et al. Dexamethasone 12 Mg versus 6 Mg for Patients with COVID-19 and Severe Hypoxaemia: A Pre-Planned, Secondary Bayesian Analysis of the COVID STEROID 2 Trial. Intensive Care Med. 2022, 48, 45–55. [Google Scholar] [CrossRef]
- Kumar, G.; Patel, D.; Hererra, M.; Jefferies, D.; Sakhuja, A.; Meersman, M.; Dalton, D.; Nanchal, R.; Guddati, A.K. Do High-Dose Corticosteroids Improve Outcomes in Hospitalized COVID-19 Patients? J. Med. Virol. 2022, 94, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.J.; Ng, K.T.; Xin, C.E.; Atan, R.; Yunos, N.M.; Hasan, M.S. High-Dose versus Low-Dose Corticosteroids in COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3576–3586. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, Y.S.; Lee, S.-I.; Moon, Y.Y.; Hwang, B.S.; Baek, A.-R.; Kim, W.-Y.; Lee, B.Y.; Seong, G.M.; Baek, M.S. Corticosteroid Outcome May Be Dependent of Duration of Use in Severe COVID-19. Korean J. Intern. Med. 2023, 38, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.U.; Min, J.K.; Lee, S.H.; Park, S.H.; Cho, C.S.; Kim, H.Y. Causes of Death in Korean Patients with Systemic Lupus Erythematosus: A Single Center Retrospective Study. Clin. Exp. Rheumatol. 1999, 17, 539–545. [Google Scholar]
- Hakki, M.; Limaye, A.P.; Kim, H.W.; Kirby, K.A.; Corey, L.; Boeckh, M. Invasive Pseudomonas Aeruginosa Infections: High Rate of Recurrence and Mortality after Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2007, 39, 687–693. [Google Scholar] [CrossRef]
- Abedin, S.; McKenna, E.; Chhabra, S.; Pasquini, M.; Shah, N.N.; Jerkins, J.; Bairn, A.; Runaas, L.; Longo, W.; Drobyski, W.; et al. Efficacy, Toxicity, and Infectious Complications in Ruxolitinib-Treated Patients with Corticosteroid-Refractory Graft-versus-Host Disease after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1689–1694. [Google Scholar] [CrossRef]
- Lee, R.; Cho, S.-Y.; Lee, D.-G.; Choi, H.; Park, S.; Cho, B.-S.; Kim, Y.-J.; Kim, H.-J. Infections of Venetoclax-Based Chemotherapy in Acute Myeloid Leukemia: Rationale for Proper Antimicrobial Prophylaxis. Cancers 2021, 13, 6285. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Yang, K.-Y.; Chan, M.-C.; Kao, K.-C.; Wang, H.-C.; Perng, W.-C.; Wu, C.-L.; Liang, S.-J.; Fang, W.-F.; Tsai, J.-R.; et al. Impact of Corticosteroid Treatment on Clinical Outcomes of Influenza-Associated ARDS: A Nationwide Multicenter Study. Ann. Intensive Care 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream Infections in Critically Ill Patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef] [PubMed]
- Massart, N.; Maxime, V.; Fillatre, P.; Razazi, K.; Ferré, A.; Moine, P.; Legay, F.; Voiriot, G.; Amara, M.; Santi, F.; et al. Characteristics and Prognosis of Bloodstream Infection in Patients with COVID-19 Admitted in the ICU: An Ancillary Study of the COVID-ICU Study. Ann. Intensive Care 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit. Care Med. 2017, 45, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Widdifield, J.; Bernatsky, S.; Paterson, J.M.; Gunraj, N.; Thorne, J.C.; Pope, J.; Cividino, A.; Bombardier, C. Serious Infections in a Population-Based Cohort of 86,039 Seniors with Rheumatoid Arthritis. Arthritis Care Res. 2013, 65, 353–361. [Google Scholar] [CrossRef]
- Abelenda-Alonso, G.; Rombauts, A.; Gudiol, C.; Oriol, I.; Simonetti, A.; Coloma, A.; Rodríguez-Molinero, A.; Izquierdo, E.; Díaz-Brito, V.; Sanmartí, M.; et al. Immunomodulatory Therapy, Risk Factors and Outcomes of Hospital-Acquired Bloodstream Infection in Patients with Severe COVID-19 Pneumonia: A Spanish Case–Control Matched Multicentre Study (BACTCOVID). Clin. Microbiol. Infect. 2021, 27, 1685–1692. [Google Scholar] [CrossRef]
- Erbay, K.; Ozger, H.S.; Guzel Tunccan, O.; Gaygısız, Ü.; Buyukkoruk, M.; Sultanova, F.; Yıldız, M.; Boyacı Dündar, N.; Aydoğdu, M.; Bozdayi, G.; et al. Evaluation of Prevalance and Risk Factors for Bloodstream Infection in Severe Coronavirus Disease 2019 (COVID-19) Patients. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e30. [Google Scholar] [CrossRef]
- Santos, C.V.; Fukushima, E.A.; Zhao, W.; Sharma, M.; Youssef, D.; Spzunar, S.; Levine, M.; Saravolatz, L.; Bhargava, A. Incidence of Bloodstream Infections in Patients with COVID-19: A Retrospective Cohort Study of Risk Factors and Outcomes. Germs 2022, 12, 253–261. [Google Scholar] [CrossRef]
- Bonazzetti, C.; Rinaldi, M.; Giacomelli, A.; Colombo, R.; Ottolina, D.; Rimoldi, S.G.; Pagani, C.; Morena, V.; Ridolfo, A.L.; Vatamanu, O.; et al. Risk Factors Associated with Bacteremia in COVID-19 Patients Admitted to Intensive Care Unit: A Retrospective Multicenter Cohort Study. Infection 2023, 51, 129–136. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. Available online: https://jamanetwork.com/journals/jama/fullarticle/2770277 (accessed on 10 October 2023).
- The COVID STEROID 2 Trial Group Effect of 12 Mg, vs. 6 Mg of Dexamethasone on the Number of Days Alive without Life Support in Adults with COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA 2021, 326, 1807–1817. [CrossRef]
- Bouadma, L.; Mekontso-Dessap, A.; Burdet, C.; Merdji, H.; Poissy, J.; Dupuis, C.; Guitton, C.; Schwebel, C.; Cohen, Y.; Bruel, C.; et al. High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients with Severe COVID-19 Acute Hypoxemic Respiratory Failure: The COVIDICUS Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 906–916. [Google Scholar] [CrossRef]
- Frattari, A.; Polilli, E.; Rapacchiale, G.; Coladonato, S.; Ianniruberto, S.; Mazzotta, E.; Patarchi, A.; Battilana, M.; Ciulli, R.; Moretta, A.; et al. Predictors of Bacteremia and Death, Including Immune Status, in a Large Single-Center Cohort of Unvaccinated ICU Patients with COVID-19 Pneumonia. Eur. J. Med. Res. 2023, 28, 219. [Google Scholar] [CrossRef]
- Monreal, E.; Sainz de la Maza, S.; Natera-Villalba, E.; Beltran-Corbellini, A.; Rodriguez-Jorge, F.; Fernandez-Velasco, J.I.; Walo-Delgado, P.; Muriel, A.; Zamora, J.; Alonso-Canovas, A.; et al. High versus Standard Doses of Corticosteroids in Severe COVID-19: A Retrospective Cohort Study. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 761–769. [Google Scholar] [CrossRef]
- Taboada, M.; Rodríguez, N.; Varela, P.M.; Rodríguez, M.T.; Abelleira, R.; González, A.; Casal, A.; Peromingo, J.A.D.; Lama, A.; Domínguez, M.J.; et al. Effect of High versus Low Dose of Dexamethasone on Clinical Worsening in Patients Hospitalised with Moderate or Severe COVID-19 Pneumonia: An Open-Label, Randomised Clinical Trial. Eur. Respir. J. 2021, 324, 1307–1316. [Google Scholar] [CrossRef]
- Toroghi, N.; Abbasian, L.; Nourian, A.; Davoudi-Monfared, E.; Khalili, H.; Hasannezhad, M.; Ghiasvand, F.; Jafari, S.; Emadi-Kouchak, H.; Yekaninejad, M.S. Comparing Efficacy and Safety of Different Doses of Dexamethasone in the Treatment of COVID-19: A Three-Arm Randomized Clinical Trial. Pharmacol. Rep. 2022, 74, 229–240. [Google Scholar] [CrossRef]
- Rajni, E.; Garg, V.K.; Bacchani, D.; Sharma, R.; Vohra, R.; Mamoria, V.; Malhotra, H. Prevalence of Bloodstream Infections and Their Etiology in COVID-19 Patients Admitted in a Tertiary Care Hospital in Jaipur. Indian J. Crit. Care Med. 2021, 25, 369–373. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; de Mendoza, C.; de la Fuente, S.; Sánchez, E.; Martínez-Urbistondo, M.; Herráiz, J.; Gutiérrez, A.; Gutiérrez, Á.; Hernández, C.; Callejas, A.; et al. Bacterial Infections in Patients Hospitalized with COVID-19. Intern. Emerg. Med. 2022, 17, 431–438. [Google Scholar] [CrossRef]
- Amer, M.; Kamel, A.M.; Bawazeer, M.; Maghrabi, K.; Butt, A.; Dahhan, T.; Kseibi, E.; Khurshid, S.M.; Abujazar, M.; Alghunaim, R.; et al. Clinical Characteristics and Outcomes of Critically Ill Mechanically Ventilated COVID-19 Patients Receiving Interleukin-6 Receptor Antagonists and Corticosteroid Therapy: A Preliminary Report from a Multinational Registry. Eur. J. Med. Res. 2021, 26, 117. [Google Scholar] [CrossRef]
- DeVoe, C.; Segal, M.R.; Wang, L.; Stanley, K.; Madera, S.; Fan, J.; Schouest, J.; Graham-Ojo, R.; Nichols, A.; Prasad, P.A.; et al. Increased Rates of Secondary Bacterial Infections, Including Enterococcus Bacteremia, in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19). Infect. Control Hosp. Epidemiol. 2022, 43, 1416–1423. [Google Scholar] [CrossRef]
- Monaco, M.; Floridia, M.; Giuliano, M.; Palmieri, L.; Lo Noce, C.; Pantosti, A.; Palamara, A.T.; Brusaferro, S.; Onder, G. Hospital-Acquired Bloodstream Infections in Patients Deceased with COVID-19 in Italy (2020–2021). Front. Med. 2022, 9, 1041668. [Google Scholar] [CrossRef]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-Resistant Acinetobacter Baumannii Infections in COVID-19 Patients Hospitalized in Intensive Care Unit. Infection 2022, 50, 83–92. [Google Scholar] [CrossRef]
- Ballouz, T.; Aridi, J.; Afif, C.; Irani, J.; Lakis, C.; Nasreddine, R.; Azar, E. Risk Factors, Clinical Presentation, and Outcome of Acinetobacter Baumannii Bacteremia. Front. Cell. Infect. Microbiol. 2017, 7, 156. [Google Scholar] [CrossRef]
- Gaibani, P.; D’Amico, F.; Bartoletti, M.; Lombardo, D.; Rampelli, S.; Fornaro, G.; Coladonato, S.; Siniscalchi, A.; Re, M.C.; Viale, P.; et al. The Gut Microbiota of Critically Ill Patients with COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 670424. [Google Scholar] [CrossRef]
- Bernard-Raichon, L.; Venzon, M.; Klein, J.; Axelrad, J.E.; Zhang, C.; Sullivan, A.P.; Hussey, G.A.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut Microbiome Dysbiosis in Antibiotic-Treated COVID-19 Patients Is Associated with Microbial Translocation and Bacteremia. Nat. Commun. 2022, 13, 5926. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Dupper, A.C.; Malik, Y.; Gavioli, E.M.; Banga, J.; Berbel Caban, A.; Nadkarni, D.; Obla, A.; Vasa, C.V.; Mazo, D.; et al. Staphylococcus Aureus Bacteremia in Patients Infected with COVID-19: A Case Series. Open Forum Infect. Dis. 2020, 7, ofaa518. [Google Scholar] [CrossRef]
- Falces-Romero, I.; Bloise, I.; García-Rodríguez, J.; Cendejas-Bueno, E. SARS-CoV-2 Working Group Staphylococcus Aureus Bacteremia in Patients with SARS-CoV-2 Infection. Med. Clin. 2023, 160, 495–498. [Google Scholar] [CrossRef]
- Lucijanic, M.; Cikara, T.; Bistrovic, P.; Papic, I.; Ortner Hadziabdic, M.; Busic, N.; Lackovic, M.; Cesar, N.; Koscak, V.; Mitrovic, J.; et al. Remdesivir use in COVID-19 patients might predispose bacteremia, matched case-control analysis. J. Infect. 2022, 85, 174–211. [Google Scholar] [CrossRef]
- Caffrey, A.R.; Liao, J.X.; Lopes, V.V.; LaPlante, K.L.; Appaneal, H.J. Real-World Safety and Effectiveness of Remdesivir and Corticosteroids in Hospitalized Patients with COVID-19. COVID 2023, 3, 198–217. [Google Scholar] [CrossRef]
- Layland, J.; Carrick, D.; Lee, M.; Oldroyd, K.; Berry, C. Adenosine: Physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv. 2014, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Bistrovic, P.; Jordan, A.; Mihaljevic, I.; Bukvic, S.; Kovacevic, S.; Ranilovic, D.; Sakota, S.; Vlasac Glasnovic, J.; Delic-Brkljacic, D. Remdesivir use in severe and critical COVID-19 patients might be associated with lower incidence of arterial thrombotic events. Am. J. Emerg. Med. 2023, 70, 41–45. [Google Scholar] [CrossRef] [PubMed]
| Comorbidity | n = 1558 |
|---|---|
| Arterial hypertension | 966 (62%) |
| Diabetes mellitus | 454 (29.1%) |
| Hyperlipoproteinemia | 337 (21.6%) |
| Obesity | 600 (38.5%) |
| Active smoking | 96 (6.2%) |
| Chronic heart failure | 135 (8.7%) |
| Atrial fibrillation | 176 (11.3%) |
| Chronic kidney disease | 98 (6.3%) |
| Chronic liver disease | 29 (1.9%) |
| Chronic obstructive lung disease | 85 (5.5%) |
| Asthma | 62 (4%) |
| Active malignancy | 94 (6%) |
| Autoimmune/rheumatic disease | 64 (4.1%) |
| Dementia | 128 (8.2%) |
| Transplanted organ | 19 (1.2%) |
| OR with 95% CI | p Value | |
|---|---|---|
| No corticosteroids Low-dose High-dose Very high-dose | Reference category - OR 1.85, 95% CI (1.21–2.82) OR 1.96, 95% CI (1.12–3.44) | - 0.004 * 0.017 * |
| Age (years) | OR 1.02, 95% CI (0.99–1.04) | 0.123 |
| Male sex | OR 1.73, 95% CI (1.16–2.58) | 0.008 * |
| Charlson comorbidity index | OR 0.88, 95% CI (0.79–0.99) | 0.046 * |
| Mechanical ventilation | OR 18.9, 95% CI (11.9–30.03) | <0.001 * |
| BSI Profile | No CS (N = 167) | Low-Dose CS (N = 710) | High-Dose CS (N = 539) | Very High-Dose CS (N = 142) | p for Trend | p for Difference |
|---|---|---|---|---|---|---|
| Positive blood cultures | 6 (3.6%) | 38 (5.4%) | 101 (18.7%) | 33 (23.2%) | <0.001 * | <0.001 * |
| Gram-negative bacteria | 5 (3%) | 24 (3.4%) | 72 (13.4%) | 27 (19%) | <0.001 * | <0.001 * |
| Gram-positive bacteria | 4 (2.4%) | 16 (2.3%) | 53 (9.8%) | 18 (12%) | <0.001 * | <0.001 * |
| Both Gram-positive and -negative bacteria (polymicrobial) | 3 (1.8%) | 8 (1.1%) | 28 (5.2%) | 11 (7.7%) | <0.001 * | <0.001 * |
| Acinetobacter baumannii | 3 (1.8%) | 17 (2.4%) | 59 (10.9%) | 18 (12.7%) | <0.001 * | <0.001 * |
| Staphylococcus aureus | 0 (0%) | 3 (0.4%) | 24 (4.5%) | 4 (2.8%) | <0.001 * | <0.001 * |
| Enterococcus faecalis | 1 (0.6%) | 3 (0.4%) | 17 (3.2%) | 3 (2.1%) | 0.002 * | <0.001 * |
| Enterococcus faecium | 1 (0.6%) | 6 (0.8%) | 7 (1.3%) | 1 (0.7%) | 0.585 | 0.783 |
| Coagulase negative Staphylococcus | 1 (0.6%) | 9 (1.3%) | 19 (3.5%) | 8 (5.6%) | <0.001 * | 0.001 * |
| Klebsiella pneumoniae | 2 (1.2%) | 7 (1%) | 10 (1.9%) | 4 (2.8%) | 0.097 | 0.314 |
| Pseudomonas aeruginosa | 2 (1.2%) | 2 (0.3%) | 3 (0.6%) | 2 (1.4%) | 0.613 | 0.271 |
| Corynebacterium species | 0 (0%) | 0 (0%) | 3 (0.6%) | 3 (2.1%) | <0.001 * | 0.002 * |
| Escherichia coli | 1 (0.6%) | 2 (0.3%) | 5 (0.9%) | 3 (2.1%) | 0.042 * | 0.101 |
| Klebsiella aerogenes | 0 (0%) | 0 (0%) | 2 (0.4%) | 0 (0%) | 0.306 | 0.286 |
| Proteus mirabilis | 3 (1.8%) | 0 (0%) | 1 (0.2%) | 0 (0%) | 0.021 * | <0.001 * |
| Stenotrophomonas maltophilia | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.7%) | 0.048 * | 0.019 * |
| Enterobacter cloacae | 1 (0.6%) | 0 (0%) | 2 (0.4%) | 0 (0%) | 0.849 | 0.265 |
| Staphylococcus haemolyticus | 0 (0%) | 0 (0%) | 2 (0.4%) | 0 (0%) | 0.306 | 0.286 |
| Serratia marcescens | 0 (0%) | 0 (0%) | 1 (0.2%) | 1 (0.7%) | 0.056 | 0.176 |
| Providencia stuartii | 0 (0%) | 0 (0%) | 2 (0.4%) | 0 (0%) | 0.306 | 0.286 |
| Beta hemolytic Streptococcus | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.7%) | 0.048 * | 0.019 * |
| Streptococcus pneumoniae | 0 (0%) | 1 (0.1%) | 0 (0%) | 0 (0%) | 0.598 | 0.754 |
| Bacteroides species | 0 (0%) | 0 (0%) | 1 (0.2%) | 0 (0%) | 0.469 | 0.595 |
| Haemophilus parainfluenzae | 0 (0%) | 0 (0%) | 1 (0.2%) | 0 (0%) | 0.469 | 0.595 |
| Providencia rettgeri | 0 (0%) | 0 (0%) | 1 (0.2%) | 0 (0%) | 0.469 | 0.595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papic, I.; Bistrovic, P.; Cikara, T.; Busic, N.; Keres, T.; Ortner Hadziabdic, M.; Lucijanic, M. Corticosteroid Dosing Level, Incidence and Profile of Bacterial Blood Stream Infections in Hospitalized COVID-19 Patients. Viruses 2024, 16, 86. https://doi.org/10.3390/v16010086
Papic I, Bistrovic P, Cikara T, Busic N, Keres T, Ortner Hadziabdic M, Lucijanic M. Corticosteroid Dosing Level, Incidence and Profile of Bacterial Blood Stream Infections in Hospitalized COVID-19 Patients. Viruses. 2024; 16(1):86. https://doi.org/10.3390/v16010086
Chicago/Turabian StylePapic, Ivan, Petra Bistrovic, Tomislav Cikara, Nikolina Busic, Tatjana Keres, Maja Ortner Hadziabdic, and Marko Lucijanic. 2024. "Corticosteroid Dosing Level, Incidence and Profile of Bacterial Blood Stream Infections in Hospitalized COVID-19 Patients" Viruses 16, no. 1: 86. https://doi.org/10.3390/v16010086
APA StylePapic, I., Bistrovic, P., Cikara, T., Busic, N., Keres, T., Ortner Hadziabdic, M., & Lucijanic, M. (2024). Corticosteroid Dosing Level, Incidence and Profile of Bacterial Blood Stream Infections in Hospitalized COVID-19 Patients. Viruses, 16(1), 86. https://doi.org/10.3390/v16010086







