Abstract
The efficacy of silver nanoparticles (AgNPs) was tested in vitro against three different fish viruses, causing significant economic damage in aquaculture. These viruses were the spring viraemia of carp virus (SVCV), European catfish virus (ECV), and Ictalurid herpesvirus 2 (IcHV-2). The safe concentration of AgNPs that did not cause cytotoxic effects in EPC cells proved to be 25 ng/mL. This dose of AgNPs decreased significantly (5–330×) the viral load of all three viruses in three different types of treatments (virus pre-treatment, cell pre-treatment, and cell post-treatment with the AgNPs). In a higher concentration, the AgNPs proved to be efficient against ECV and IcHV-2 even in a delayed post-cell-treatment experiment (AgNP treatment was applied 24 h after the virus inoculation). These first in vitro results against three devastating fish viruses are encouraging to continue the study of the applicability of AgNPs in aquaculture in the future.
1. Introduction
Nanoparticles are naturally occurring or engineered particles with a diameter ranging from 1 to 100 nm [1]. Extensive research on the applications of different nanoparticle types in the agricultural and veterinary sector has been conducted [2]. The applications vary from early diagnosis [3] and immunostimulant agents [4,5,6] to antimicrobial therapy [7,8,9].
Metallic nanoparticles have shown promising antiviral activities against different human and animal viruses, for example, both silver (AgNPs) and gold nanoparticles (AuNPs) acted as antiviral agents against human immunodeficiency virus by inhibition of the virus entry into the cells [10,11,12]. AgNPs exhibited antiviral activities against respiratory syncytial virus [13,14], hepatitis B virus [15], monkeypox virus [16], vaccinia virus [17], human parainfluenza virus [18], and herpes simplex virus [18,19]. AgNPs, silver-chitosan, iron oxide, and zinc oxide nanoparticles exhibited antiviral activity against H1N1 Influenza virus [20,21,22,23]. Copper iodide nanoparticles release reactive oxygen species (ROS), resulting in oxidation of the feline calicivirus capsid protein [24]. Copper–silver and copper–zinc nanoparticles have shown antiviral actions when tested against a bacteriophage as a model for DNA viruses [25].
The use of AgNPs in aquaculture has been reported in the last two decades [26]. Some of these investigations have focused on the use of AgNPs in water treatment, e.g., using nanoparticles in filter systems improves their efficacy [27,28]. While the rest of the publications have described the use of AgNPs in aquatic disease control, the majority of these experiments have focused on bacterial infections [29,30,31]. The main antimicrobial mechanisms of action of AgNPs include the release of Ag+ ions, ROS, adhesion to the cell membrane, interaction with microbial nucleic acids, and interference with the microbial cell signaling [32,33]. Ag+ ions could interact with thiol groups and were capable of deactivation of succinate dehydrogenase to exert their bactericidal action [34]. In spite of the several theories on AgNP’s mechanism of action in the literature, the exact molecular mechanisms of action have still not been clearly elucidated and need further investigations [34]. Up to now, very few studies have been conducted on the interaction between AgNPs and viruses in aquaculture. There is only one aquatic virus, namely the white spot syndrome virus affecting shrimp farming, against which AgNPs have been tested [35,36]. Currently, AgNPs are still not approved by the FDA as a certifiable cure. However, several products that contain AgNPs are commercially available such as Sovereign Silver and Argentyn 23, which are manufactured by Natural Immunogenics Corp. (NIC) [37].
For the present study, three different virus species isolated from fish were used. One of them is the spring viraemia of carp virus (SVCV), which has a negative-strand RNA genome belonging to the family Rhabdoviridae [38], and can cause significant losses, exceeding 50% mortality, particularly in yearling common carp (Cyprinus carpio) [39,40]. The other two chosen viruses have a dsDNA genome; however, they even belong to different taxonomic realms. The European catfish virus (ECV) is a ranavirus classified under the family Iridoviridae (realm Varidnaviria) [41], and mass mortality events are known due to this virus in populations of different catfish species (Ameiurus melas, A. nebulosus, Silurus glanis) [42,43,44,45,46,47,48,49]. The third virus is a herpesvirus (family Alloherpesviridae, realm Duplodnaviria) [50], namely the Ictalurid herpesvirus 2 (IcHV-2). The latter virus can also cause devastating losses in black and brown bullhead (A. melas, A. nebulosus) populations [51]. Under experimental conditions, both ECV and IcHV-2 can cause more than 90% mortality in juvenile fish stock [43,48,51].
There is no specific practical cure or effective therapy for the above-listed viral diseases. Usually, biosecurity and preventive measures are applied to fish farms in order to reduce the risk of viral infections [52]. For example, in the mid-1990s when black bullhead farming in Italy was devastated by IcHV-2, fish have been reared in farms where the water temperature could be kept below the optimal temperature for the replication of IcHV-2 (24 °C) [53]. Nevertheless, against the SVCV, several promising vaccines have been developed [54,55,56], none of which are available commercially.
To the best of our knowledge, this is the first report of the use of nanoparticles against finfish viruses.
2. Materials and Methods
2.1. Cells and Viruses
EPC (Epithelioma Papulosum Cyprini, ATCC CRL-2872) cells were grown in EMEM medium (Lonza, Visp, Switzerland) supplemented with 10% fetal bovine serum (FBS) (Biosera, Cholet, France), 1% HEPES buffer (1 M) (Biosera, Cholet, France), and 1% penicillin-streptomycin (Lonza, Switzerland) at 25 °C. The ECV strain (14612/2012) used in this study was isolated from brown bullhead in Hungary [49], while the IcHV-2 strain was kindly provided by Prof. Giuseppe Bovo [51], with both viruses being propagated in the EPC cell line at 25 °C, whilst the SVCV strain (ME/2020) isolated previously in our lab was propagated at 20 °C in the same cell line. The tissue culture infectious dose (TCID 50/mL) was calculated by the Reed and Muench method.
2.2. AgNP Synthesis
The synthesis of AgNPs was conducted by the chemical reduction method [57]. Silver nitrate (AgNO3) was subjected to reduction reaction with the aid of sodium citrate and sodium borohydride as reducing agents. Polyvinylpyrrolidone (PVP) was added to enhance the stability of silver nanoparticles and prevent their aggregation. The synthesized AgNPs were stored in the refrigerator at 4 ºC and covered with aluminum foil to protect from exposure to light.
2.3. AgNP Characterization
The synthesized AgNPs were diluted 1:10 with deionized water and imaged using transmission electron microscopy (TEM). Dynamic light scattering (DLS) was applied to measure the size distribution and zeta potential of silver nanoparticles.
2.4. Cytotoxicity Assay
MTT Cell Viability Assay Kit (Invitrogen, Carlsbad, CA, USA) was used to determine the cytotoxic effect of AgNPs on the EPC cells. Cells were seeded in 96-well plates (2 × 104 cell/well) and incubated for 24 h at 25 °C; then, AgNPs were added in the following final concentrations: 12.5, 25, 50, and 100 ng/mL (in quadruplicates). For blanks, wells without cells were handled in the same manner as wells containing cells. Cytotoxicity was calculated as a per cent of the control.
2.5. In Vitro Assays for Silver Nanoparticles and Virus Interaction Studies
EPC cells were seeded in 24-well plates (2 × 105 cells/well) and incubated overnight at 25 °C. Viruses (ECV and IcHV-2) at MOI of 0.01, with SVCV at MOI of 0.001, were used in the following experiments: virus pre-treatment assay, and cell pre- and post-treatment assays. AgNP was used in the following concentrations with the ECV: 12.5, 25, 50, and 100 ng/mL. Later, when the assays were carried out by the other two viruses, the 12.5 ng/mL concentration AgNP treatment was dismissed. Plates after the treatment were incubated for 48 h at 20 °C (SVCV) or at 25 °C (ECV and IcHV-2). Then, the plates were frozen at −20 °C and then proceeded with nucleic acid extraction and qPCR. All assays were performed in duplicates.
2.6. Virus Pre-Treatment Assay
Viruses in EMEM containing 2% FBS were incubated with different concentrations of AgNP for 1 h at 20 °C or at 25 °C depending on the virus. The virus-AgNP mixture was added to the EPC cells and incubated for 1 h; then, cells were rinsed with phosphate-buffered saline (PBS) twice, and medium with 2% FBS was added.
2.7. Cell Pre-Treatment Assay
EPC cells were treated with AgNP and incubated for 1 h at 20 °C or at 25 °C. The wells were then washed with PBS twice to remove free AgNPs in medium, topped up with EMEM containing 2% FBS with the virus, incubated for 1 h, and then rinsed twice with PBS. Then, medium (2% FBS) was added.
2.8. Cell Post-Treatment Assay
EPC cells were infected with the three viruses and incubated for 1 h at 20 °C or at 25 °C. The wells were washed with PBS twice to remove extracellular viruses, topped up with EMEM containing 2% FBS with AgNPs, and incubated for 1 h at 20 °C or at 25 °C. Then, the wells were rinsed twice and medium was added. Additionally, delayed cell post-treatment was carried out with the ECV and IcHV-2. In these assays, the AgNP treatment was applied 24 h after the virus infection.
2.9. Quantitative PCRs
Viral DNA and RNA were extracted by Viral Nucleic Acid Extraction Kit II (Geneaid, New Taipei City, Taiwan) according to the instructions of the manufacturer. Extracted DNA was stored at −20 °C, while the RNA was stored at −80 °C.
Quantitative real-time PCRs (qPCRs) for determining the relative amount of the viral DNA or RNA in the wells were carried out in a Bio-Rad® Real-Time PCR System instrument (Bio-Rad, Hercules, CA, USA). SensiFASTTM SYBR Hi-ROX Kit and SensiFAST™ SYBR® Hi-ROX One-Step Kit (Bioline, London, UK) were used. The reaction mixture contained 10 µL of 2× SensiFast mix, 7.4 µL of distilled water, 0.8 µL of each primer (Table 1), and 1 µL of the target DNA in a final volume of 20 µL (in RT-PCR, 6.8 µL of water was used and it contained 0.2 µL of reverse transcriptase enzyme and 0.4 µL of RNase inhibitor). The program consisted of an initial denaturing at 95 °C for 3 min, followed by 40 cycles of 95 °C for 5 s, and 65 °C for 30 s. All qPCRs were performed in duplicates. The beta-actin gene was used as an internal standard. The results were analyzed by the Bio-Rad CFX Maestro software (Bio-Rad).

Table 1.
Primers used in this study.
2.10. Statistical Analysis
Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test with the R Commander (version i386 4.1.1.) software. p-values of 0.05 or below were considered statistically significant.
3. Results
3.1. AgNP Characterisation
TEM microphotographs of AgNPs revealed the spherical shape of the particles appearing as electron-dense particles with a mean diameter of 10.2 ± 1.6 nm (Figure 1).
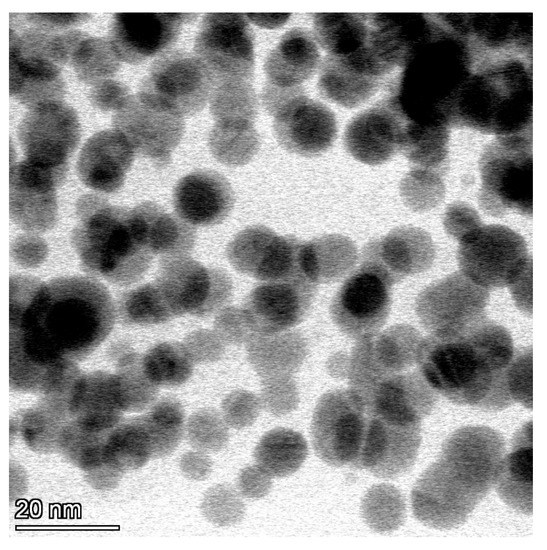
Figure 1.
Microphotograph of silver nanoparticles with transmission electron microscopy showing the spherical shape of the particles appearing as electron-dense particles with a mean diameter of 10.2 ± 1.6 nm (scale bar = 20 nm).
Moreover, DLS analysis showed the size distribution by number with a mean diameter of 22.4 ± 5.3 nm (Figure 2A). In addition, the charge of AgNPs was found to be negative (−19.2 ± 2.9 mV) (Figure 2B).
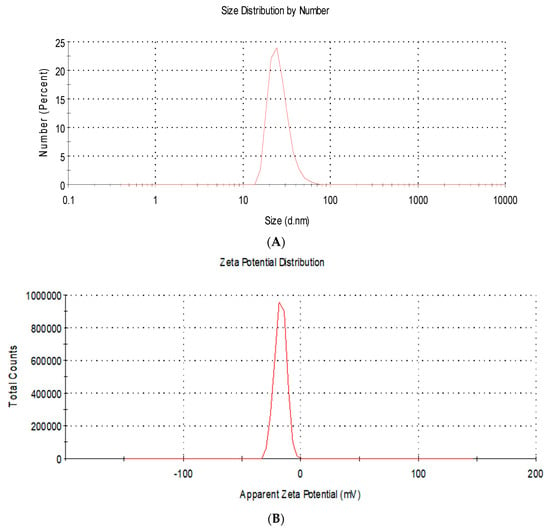
Figure 2.
Dynamic light scattering analysis of silver nanoparticles showing (A) the size distribution by number with a mean diameter of 22.4 ± 5.3 nm; (B) the charge of AgNPs was found to be negative (−19.2 ± 2.9 mV).
3.2. Cytotoxicity Assays
The results of the MTT assay are shown in Table 2. If the difference between the control and counterpart at a certain concentration reached statistical significance, the concentration was considered to be harmful. There was no significant cytotoxic effect of AgNPs at the concentrations of 12.5 and 25 ng/mL.

Table 2.
Viability of EPC cells after one hour of exposure to different silver nanoparticle (AgNP) concentrations. * Statistically significant difference (p < 0.05).
3.3. In Vitro Assays
AgNPs at 12.5 ng/mL of concentration did not reduce the viral load of ECV. Due to this result and considering that the 25 ng/mL concentration was still not cytotoxic to the cells, the 12.5 ng/mL AgNP concentration was not used with the other two viruses. The viral load of each virus examined was significantly decreased by the higher concentrations of AgNPs (25, 50, and 100 ng/mL), except one case (100 ng/mL of NP in cell pre-treatment against IcHV-2, which proved not to be significant, due to the higher SEM value). Figure 3 shows the results of the in vitro assays. A reduction in viral loads was observed in all types of treatment assays (virus pre-treatment, and cell pre- and post-treatment) (Figure 4). The 25 ng/mL concentration was not cytotoxic to the EPC cells and proved to be efficient against all three viruses, in all types of assays. At this concentration, the rate of the reduction in the viral load ranged between 70 and 330 times in the case of ECV and between 10 and 54 times in the case of SVCV. It proved to be less efficient against IcHV-2 with the 5–17-times reduction.
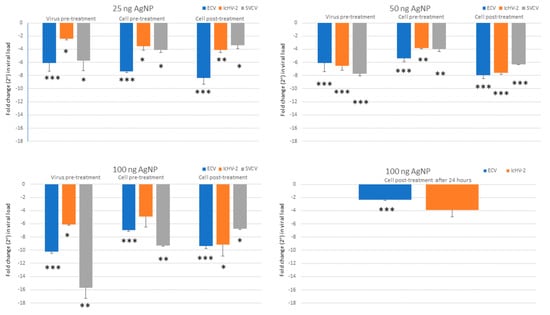
Figure 3.
Viral load measured by qPCR, normalized against beta-actin, and calculated as ΔΔCt values. The results are represented as a fold change relative to the mean of control (virus only, non-treated cells). Values are expressed as the mean ± SEM. One-way ANOVA and Tukey’s post hoc test applied. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. matched control.
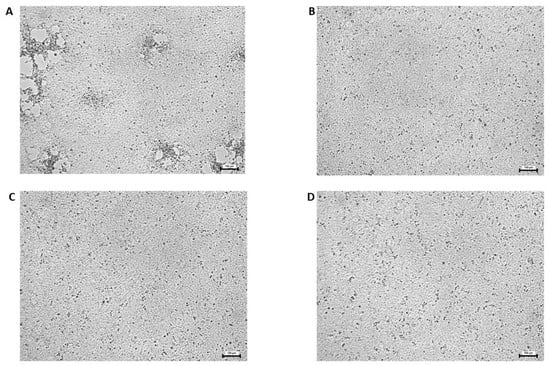
Figure 4.
The CPE caused by the ECV 48 h after the inoculation. (A) Control cells (virus only), (B) virus pre-treatment, (C) cell pre-treatment, and (D) cell post-treatment with 25 ng/mL of AgNP. 100× magnitude.
The cell post-treatment assays were the most effective against the ECV and SVCV; hence, with these viruses, delayed post-treatment assays (24 h) were carried out. A reduction in viral load was observed in both cases; however, the results proved to be significant only with the ECV.
4. Discussion
Infectious diseases caused by various pathogens such as bacteria, fungi, parasites, and viruses induce major risks to aquaculture. There are different antiparasitic and antibacterial treatments in the market for fish farms, but no direct antiviral therapy yet [1]. One of the most investigated nanoparticles in agriculture, veterinary medicine, and aquaculture is AgNPs [2]. Although the application of AgNPs in aquaculture has been reported in the past decades [26], the majority of these studies focused on the water treatment (filter system improvements) [27,28], or disease control of bacterial and protozoan infections [29,30,31]. To the best of our knowledge, this is the first report on the evaluation of the applicability of AgNPs against finfish viruses, which are known to cause devastating losses in aquaculture.
In this experiment, we studied the antiviral activity of synthesized AgNPs. To stabilize the newly formed nanoparticles, we used polyvinylpyrrolidone as a capping agent to prevent them from aggregation and agglomeration [30]. TEM photos revealed that AgNPs were electron-dense and spherically shaped particles. Their average particle size was calculated to be 10.2 ± 1.6 nm. As TEM provides information about the shape and the average size of the nanoparticles, DLS analysis is capable of showing the size distribution and particle charge. The size distribution by number showed a mean diameter of 22.4 ± 5.3 nm, which is higher than the mean calculated from TEM results due to the dispersant effect on DLS [60]. AgNPs exhibited a negative charge of −19.2 ± 2.9 mV, which reflects good stability of the synthesized particles. This negative charge is attributed to the interaction between the AgNP surface and PVP [61].
For the antiviral experiments, three very distantly related virus species were chosen intentionally. The SVCV is a –ssRNA virus causing significant economic losses in carp farming worldwide [38,39], while the other two viruses are dsDNS viruses belonging to different taxonomic realms. The IcHV-2 has been isolated from different bullhead species (Ictaluridae), while the ECV is known to cause losses in other catfish species as well (Siluridae) [42,43,44,45,46,47,48,49]. There is no effective therapy against the above-mentioned viruses. Hence, the efficacy of AgNPs was tested in vitro against these economically important fish viruses.
Firstly, the safe concentration of the AgNPs was determined in EPC cells, which proved to be 25 ng/mL. At this dose, the AgNPs decreased significantly the viral load of all three viruses in all different types of treatments (virus pre-treatment, cell pre-treatment, and cell post-treatment with the AgNPs). It proved to be the most efficient against the ECV (viral load was reduced by 70–330 times), and it was also very convincing against SVCV (10–54×). As for IcHV-2, the viral load was decreased by 5–17×, which proved to be a still significant reduction. A lower concentration of AgNPs did not prove to be efficient. Although the higher concentrations of AgNPs were harmful for the EPC cells, the in vitro experiments were also carried out in order to see whether the reductions were dose-dependent, but this was not clearly proven by the data. At a higher concentration, the AgNPs proved to be the most efficient against ECV and IcHV-2 in the cell post-treatment; hence, we tried it as a delayed post-cell-treatment experiment (AgNP treatment was applied 24 h after the virus inoculation). In these experiments, the significant reduction in the viral load of ECV is very promising. It might imply that the usage of AgNPs could be efficient therapy against ECV in vivo even after the observation of the first clinical signs of the disease.
The exact mechanism by which AgNPs execute its destroying effect on viruses is still unclear. The AgNPs might inhibit the viruses in different ways, for example, inhibition of the virus–host cell binding by preventing viral attachment or damaging the surface proteins (monkeypox virus, influenza virus, respiratory syncytial virus, herpes simplex virus, human immunodeficiency virus), inactivation of the virus prior to entry (Tacaribe virus), and interaction with the dsDNA and inhibition of viral replication (hepatitis B virus) [10,13,15,16,62,63,64,65,66]. AgNPs could bind to gp120 of HIV-1 virus, which prevents CD4-dependent virion binding and fusion [10], and they are capable of blocking the transmission of HIV-1 between infected and healthy cells [63]. In our study, we suggest that more than one mechanism occurred, since the inhibition by the AgNPs was successful in three different treatment types. The only feature common in these viruses besides that they infect finfish and cause high mortality is that the virions are enveloped. That might imply that during the virus pre-treatment, the AgNPs damage the envelope of the virions, which reduces its ability to enter into the host cells. However, in the case of the cell pre-treatment and cell post-treatment, intracellular antiviral activity must have occurred [15].
These first in vitro results against three devastating fish viruses are encouraging to continue in vivo studies and investigate the applicability and efficacy of AgNPs against viral diseases in aquaculture in the future.
Author Contributions
Conceptualization, A.D., M.S. and M.E.-M.; Methodology, A.D. and M.S.; Validation, A.D. and M.E.-M.; Investigation, A.D. and M.S.; Resources, A.D., M.S. and M.E.-M.; Writing—Original Draft Preparation, A.D. and M.S.; Writing—Review and Editing, A.D., M.S. and M.E.-M.; Visualization, A.D.; Supervision, A.D. and M.E.-M.; Funding Acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the grant NKFI K140348 OTKA and by the Bolyai János Research Scholarship (A. Doszpoly).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaalan, M.; Saleh, M.; El-Mahdy, M.; El-Matbouli, M. Recent progress in applications of nanoparticles in fish medicine: A review. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Mission, E.G.; Fuaad, A.A.; Shaalan, M. Nanoparticle tools to improve and advance precision practices in the Agrifoods Sector towards sustainability-A review. J. Clean. Prod. 2021, 293, 126063. [Google Scholar] [CrossRef]
- Kobayashi, R.K.; Dhakal, S.; Nakazato, G. The Use of Nanoparticles in the Diagnosis and Therapy of Infectious Disease in Animals. Front. Vet. Sci. 2021, 8, 1602. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elala, N.M.; Shaalan, M.; Ali, S.E.; Younis, N.A. Immune responses and protective efficacy of diet supplementation with selenium nanoparticles against cadmium toxicity in Oreochromis niloticus. Aquac. Res. 2021, 52, 3677–3686. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Shahin, K.; Mahmoud, S.M.; Altohamy, D.E.; Husseiny, W.A.; Mansour, D.A.; Shalaby, S.I.; Gaballa, M.M.; Shaalan, M.; Alkafafy, M.; et al. Silica nanoparticles are novel aqueous additive mitigating heavy metals toxicity and improving the health of African catfish, Clarias gariepinus. Aquat. Toxicol. 2022, 249, 106238. [Google Scholar] [CrossRef]
- Saleh, M.; Essawy, E.; Shaalan, M.; Osman, S.; Ahmed, F.; El-Matbouli, M. Therapeutic Intervention with Dietary Chitosan Nanoparticles Alleviates Fish Pathological and Molecular Systemic Inflammatory Responses against Infections. Mar. Drugs 2022, 20, 425. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Edirisinghe, M. Nanocomposites: Suitable alternatives as antimicrobial agents. Nanotechnology 2018, 29, 282001. [Google Scholar] [CrossRef]
- Elgendy, M.Y.; Shaalan, M.; Abdelsalam, M.; Eissa, A.E.; El-Adawy, M.M.; Seida, A.A. Antibacterial activity of silver nanoparticles against antibiotic-resistant Aeromonas veronii infections in Nile tilapia, Oreochromis niloticus (L.), in vitro and in vivo assay. Aquac. Res. 2022, 53, 901–920. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Riddick, M.; García, A.J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 2022, 7, 39–54. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ganesan, S. Gold nanoparticles as an HIV entry inhibitor. Curr. HIV Res. 2012, 10, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Singh, A.K.; Vig, K.; Pillai, S.R.; Singh, S.R. Silver nanoparticles inhibit replication of respiratory syncytial virus. J. Biomed. Nanotechnol. 2008, 4, 149–158. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R.P. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef]
- Lu, L.; Sun, R.W.Y.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.; Che, C.M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar] [CrossRef]
- Trefry, J.C.; Wooley, D.P. Silver nanoparticles inhibit vaccinia virus infection by preventing viral entry through a macropinocytosis-dependent mechanism. J. Biomed. Nanotechnol. 2013, 9, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar]
- Akbarzadeh, A.; Kafshdooz, L.; Razban, Z.; Dastranj Tbrizi, A.; Rasoulpour, S.; Khalilov, R.; Kavetskyy, T.; Saghfi, S.; Nasibova, A.N.; Kaamyabi, S.; et al. An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 263–267. [Google Scholar] [CrossRef]
- Mehrbod, P.; Motamed, N.; Tabatabaeian, M.; Soleymani, E.R.; Amini, E.; Shahidi, M.; Kheyri, M.T. In Vitro Antiviral Effect of “Nanosilver” on Influenza Virus. DARU 2009, 17, 88–93. [Google Scholar]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.; Topno, R.K.; Madhukar, M.; Das, P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef]
- Matharu, R.K.; Cheong, Y.K.; Ren, G.; Edirisinghe, M.; Ciric, L. Exploiting the antiviral potential of intermetallic nanoparticles. Emergent Mater. 2021, 5, 1251–1260. [Google Scholar] [CrossRef]
- De Silva, C.; Nawawi, N.M.; Abd Karim, M.M.; Abd Gani, S.; Masarudin, M.J.; Gunasekaran, B.; Ahmad, S.A. The Mechanistic Action of Biosynthesised Silver Nanoparticles and Its Application in Aquaculture and Livestock Industries. Animals 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Sarkheil, M.; Sourinejad, I.; Mirbakhsh, M.; Kordestani, D.; Johari, S. Application of silver nanoparticles immobilized on TEPA-Den-SiO2 as water filter media for bacterial disinfection in culture of Penaeid shrimp larvae. Aquac. Eng. 2016, 74, 17–29. [Google Scholar] [CrossRef]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Film 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Ramasamy, P.; Chen, J. Antibacterial activity of silver nanoparticles (AgNps) synthesized by tea leaf extracts against pathogenic Vibrio harveyi and its protective efficacy on juvenile Feneropenaeus indicus. Lett. Appl. Microbiol. 2010, 50, 352–356. [Google Scholar] [CrossRef]
- Shaalan, M.; Sellyei, B.; El-Matbouli, M.; Székely, C. Efficacy of silver nanoparticles to control flavobacteriosis caused by Flavobacterium johnsoniae in common carp Cyprinus carpio. Dis. Aquat. Organ. 2020, 137, 175–183. [Google Scholar] [CrossRef]
- Pelgrift, R.; Friedman, A. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; De Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Ochoa-Meza, A.; Alvarez-Sanchez, A.; Romo-Quinonez, C.; Barraza, A.; Magallon-Barajas, F.; Chavez-Sanchez, A.; Garcia-Ramos, J.; Toledano-Magana, Y.; Bogdanchikova, N.; Pestryakov, A.; et al. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol. 2019, 84, 1083–1089. [Google Scholar] [CrossRef]
- Romo-Quinonez, C.; Alvarez-Sanchez, A.; Alvarez-Ruiz, P.; Chavez-Sanchez, M.; Bogdanchikova, N.; Pestryakov, A.; Mejia-Ruiz, C. Evaluation of a new Argovit as an antiviral agent included in feed to protect the shrimp Litopenaeus vannamei against White Spot Syndrome Virus infection. PeerJ 2020, 8, e8446. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Hemmes, P.; Mitchen, K. Characterization of the Silver Nanoparticles in the Sovereign Silver® and Argentyn 23®, Bio-Active Silver Hydrosol™ Products. Int. J. Nanomed. 2022, 17, 983–986. [Google Scholar] [CrossRef]
- Walker, P.; Freitas-Astua, J.; Bejerman, N.; Blasdell, K.; Breyta, R.; Dietzgen, R.; Fooks, A.; Kondo, H.; Kurath, G.; Kuzmin, I.; et al. ICTV Virus Taxonomy Profile: Rhabdoviridae 2022. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef] [PubMed]
- Fijan, N.; Petrinec, Z.; Sulimanovic, D.; Zwillenberg, L. Isolation of the viral causative agent from the acute form of infectious dropsy of carp. Vet. Arch. 1971, 41, 125–138. [Google Scholar]
- Wolf, K. Fish Viruses and Fish Viral Diseases; Comstock Publishing Associates; Cornell University Press: Ithaca, NY, USA, 1988. [Google Scholar]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. ICTV Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef]
- Ahne, W.; Schlotfeldt, H.; Thomsen, I. Fish Viruses—Isolation of an icosahedral cytoplasmic deoxyribovirus from sheatfish (Silurus glanis). Zent. Vet. B 1989, 36, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Leimbach, S.; Schütze, H.; Bergmann, S. Susceptibility of European Sheatfish Silurus Glanis to a Panel of Ranaviruses. J. Appl. Ichthyol 2014, 30, 93–101. [Google Scholar] [CrossRef]
- Pozet, F.; Morand, M.; Moussa, A.; Torhy, C.; Dekinkelin, P. Isolation and preliminary characterization of a pathogenic icosahedral deoxyribovirus from the catfish Ictalurus melas. Dis. Aquat. Org. 1992, 14, 35–42. [Google Scholar] [CrossRef]
- Bigarre, L.; Cabon, J.; Baud, M.; Pozet, F.; Castric, J. Ranaviruses associated with high mortalities in catfish in France. Bull. Eur. Assoc. Fish Pathol. 2008, 28, 163–168. [Google Scholar]
- Fehér, E.; Doszpoly, A.; Horváth, B.; Marton, S.; Forró, B.; Farkas, S.L.; Bányai, K.; Juhász, T. Whole genome sequencing and phylogenetic characterization of brown bullhead (Ameiurus nebulosus) origin ranavirus strains from independent disease outbreaks. Infect. Genet. Evol. 2016, 45, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Borzym, E.; Karpińska, T.A.; Reichert, M. Outbreak of ranavirus infection in sheatfish, Silurus glanis (L.), in Poland. Pol. J. Vet. Sci. 2015, 18, 607–611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abonyi, F.; Varga, A.; Sellyei, B.; Eszterbauer, E.; Doszpoly, A. Juvenile Wels Catfish (Silurus glanis) Display Age-Related Mortality to European Catfish Virus (ECV) under Experimental Conditions. Viruses 2022, 14, 1832. [Google Scholar] [CrossRef]
- Juhasz, T.; Woynarovichne, L.; Csaba, G.; Farkas, L.; Dan, A. Isolation of ranavirus causing mass mortality in brown bullheads (Ameiurus nebulosus) in Hungary. Magy. Allatorv. Lapja 2013, 135, 763–768. [Google Scholar]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Alborali, L.; Bovo, G.; Lavazza, A.; Capellaro, H.; Guadagnini, P.F. Isolation of a herpesvirus in breeding catfish (Ictalurus melas). Bull. Eur. Assoc. Fish Pathol. 1996, 16, 134–137. [Google Scholar]
- Mugimba, K.K.; Byarugaba, D.K.; Mutoloki, S.; Evensen, Ø.; Munang’andu, H.M. Challenges and solutions to viral diseases of finfish in marine aquaculture. Pathogens 2021, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, A.; Mordenti, O.; Stocchi, L.; Melotti, P. Comparison of growth performance of ‘Common Catfish Ameiurus melas, Rafinesque1820’, reared in pond and in recirculating aquaculture system. J. Aquacult. Res. Dev. 2014, 5, 218. [Google Scholar]
- Emmenegger, E.J.; Kurath, G. DNA vaccine protects ornamental koi (Cyprinus carpio koi) against North American spring viremia of carp virus. Vaccine 2008, 26, 6415–6421. [Google Scholar] [CrossRef] [PubMed]
- Fijan, N.; Petrinec, Z.; Stancl, Z. Vaccination of carp against spring viraemia: Comparison of intraperitoneal and peroral application of live virus to fish kept in ponds. Bull. Off. Int. Des Epizoot. 1977, 87, 441–442. [Google Scholar]
- Embregts, C.W.E.; Rigaudeau, D.; Veselý, T.; Pokorová, D.; Lorenzen, N.; Petit, J.; Houel, A.; Dauber, M.; Schütze, H.; Boudinot, P.; et al. Intramuscular DNA Vaccination of Juvenile Carp against Spring Viremia of Carp Virus Induces Full Protection and Establishes a Virus-Specific B and T Cell Response. Front. Immunol. 2017, 8, 1340. [Google Scholar] [CrossRef]
- Salem, H.M.; Ismael, E.; Shaalan, M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021, 16, 6783. [Google Scholar] [CrossRef]
- Yue, Z.; Teng, Y.; Liang, C.; Xie, X.; Xu, B.; Zhu, L.; Lei, Z.; He, J.; Liu, Z.; Jiang, Y.; et al. Development of a sensitive and quantitative assay for spring viremia of carp virus based on real-time RT-PCR. J. Virol. Methods 2008, 152, 43–48. [Google Scholar] [CrossRef]
- Goodwin, A.E.; Marecaux, E. Validation of a qPCR assay for the detection of Ictalurid herpesvirus-2 (IcHV-2) in fish tissues and cell culture supernatants. J. Fish Dis. 2010, 33, 341–346. [Google Scholar] [CrossRef]
- Souza, T.G.; Ciminelli, V.S.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 6–11. [Google Scholar] [CrossRef]
- Farouk, M.M.; El-Molla, A.; Salib, F.A.; Soliman, Y.A.; Shaalan, M. The role of silver nanoparticles in a treatment approach for multidrug-resistant Salmonella species isolates. Int. J. Nanomed. 2020, 23, 6993–7011. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ixtepan-Turrent, L.; Garza-Treviño, E.N.; Rodriguez-Padilla, C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J. Nanobiotechnol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 19–27. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).