A Reverse-Transcription Loop-Mediated Isothermal Amplification Technique to Detect Tomato Mottle Mosaic Virus, an Emerging Tobamovirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Isolates and Plant Materials
2.2. Total RNA Extraction
2.3. RT-PCR
2.4. RT-LAMP Primer Design
2.5. RT-LAMP
2.6. Direct Sampling of ToMMV from Infected Tomato Leaves
3. Results
3.1. RT-LAMP Primer Design and Selection
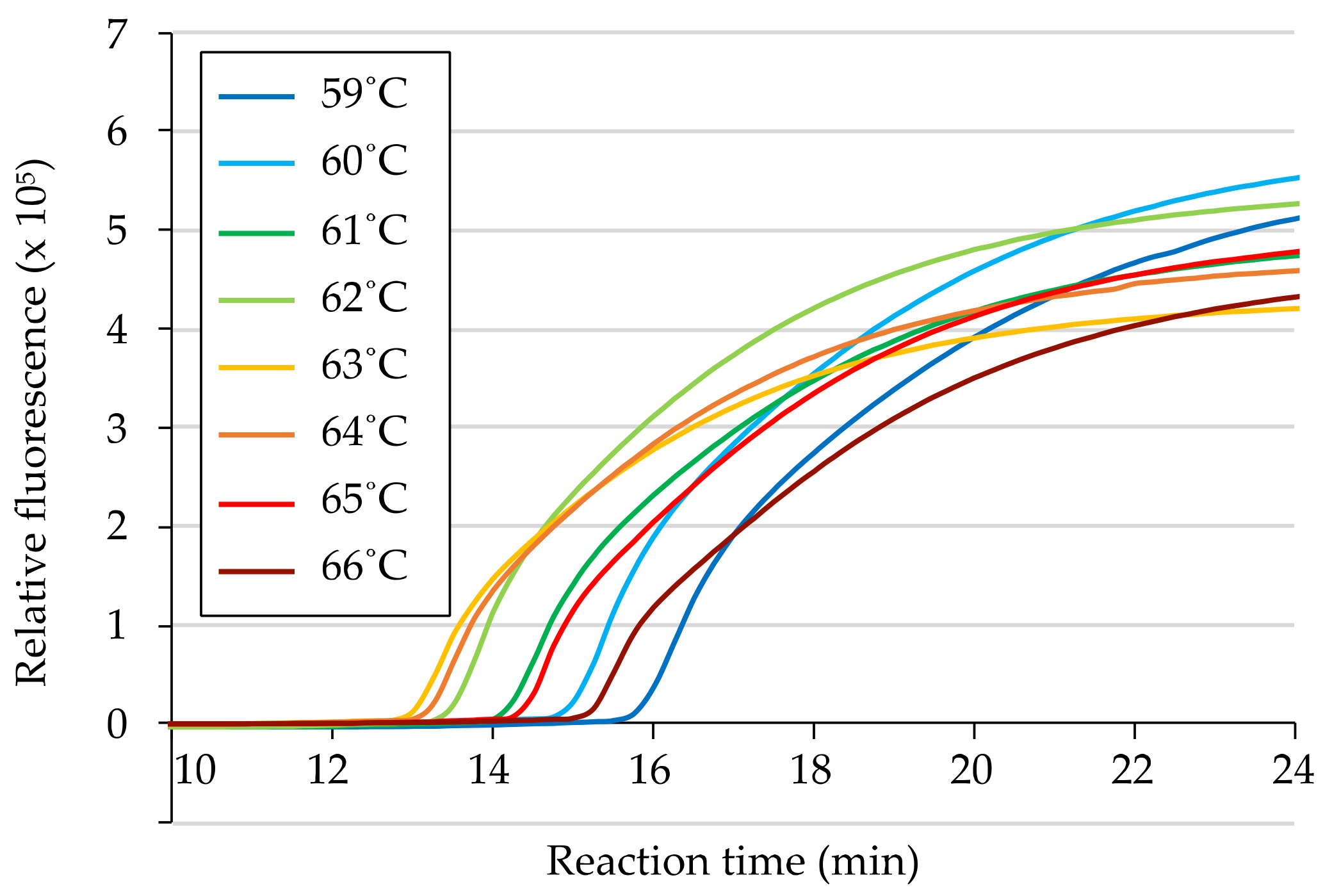
3.2. Determination of Reaction Conditions
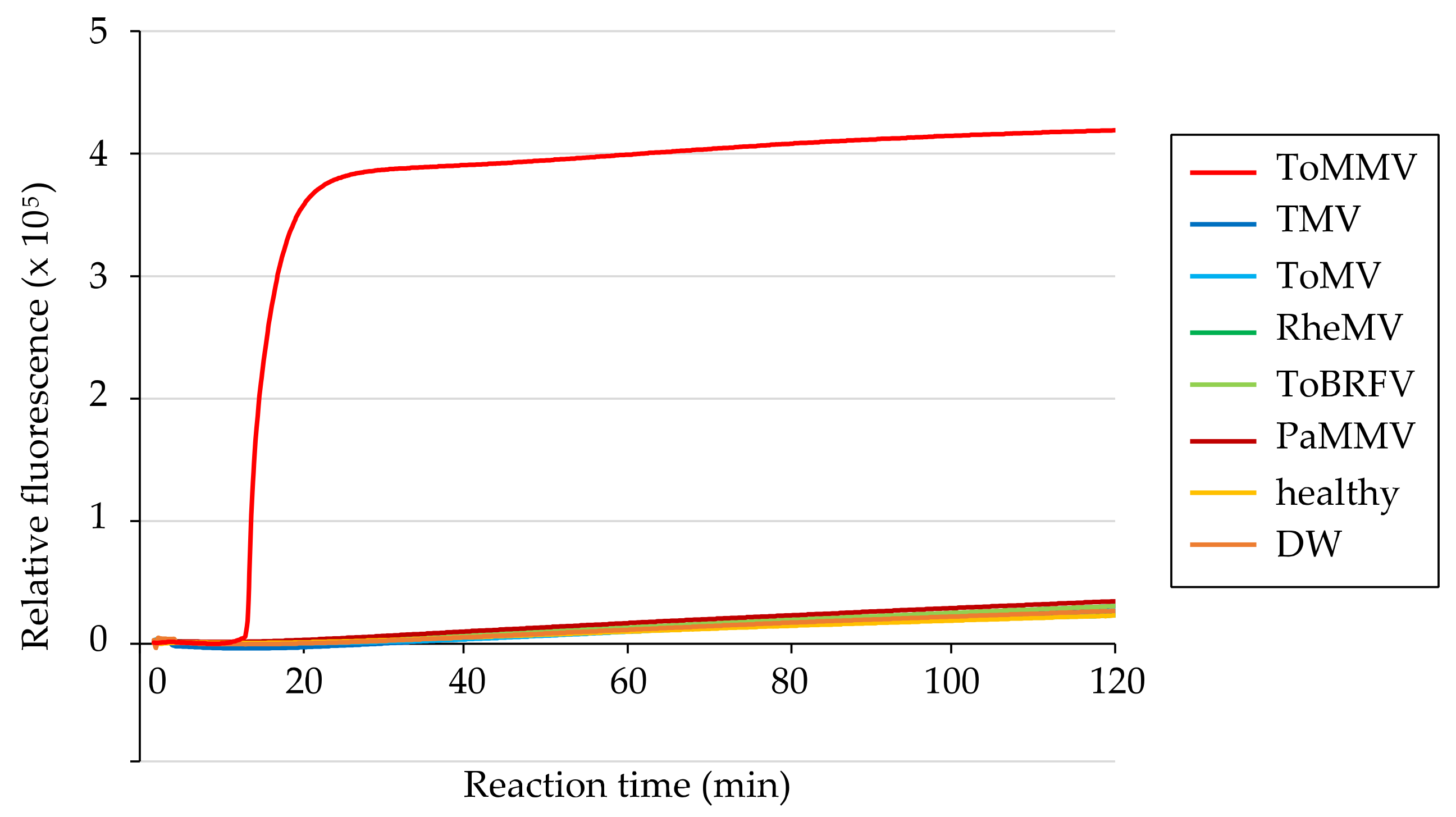
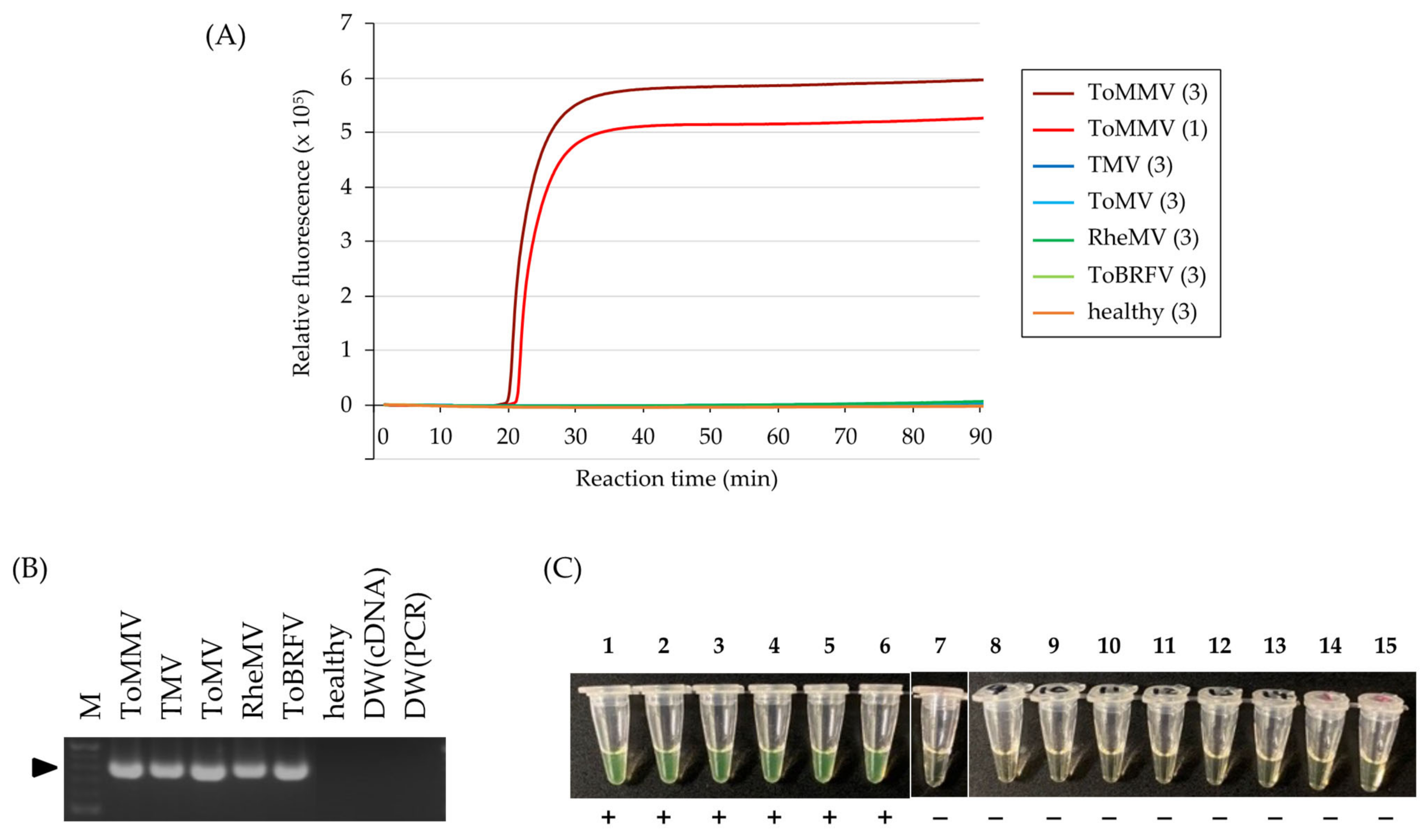
3.3. Specificity of ToMMV Detection by RT-LAMP
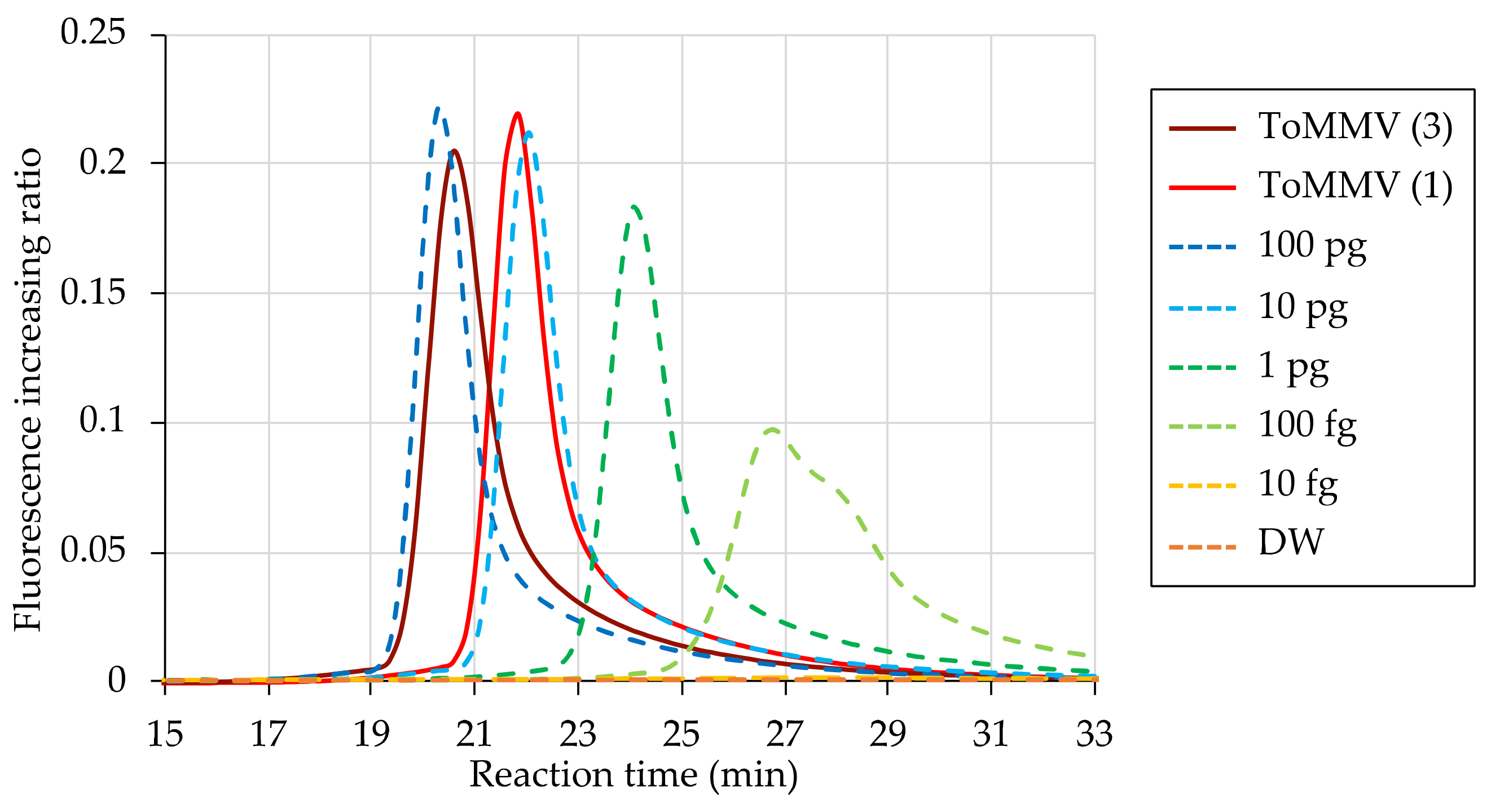
3.4. Relative Sensitivity of ToMMV Detection by RT-LAMP
3.5. Evaluation of the Direct Sampling Method from Infected Tomato Leaves
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging Infectious Diseases of Plants: Pathogen Pollution, Climate Change and Agrotechnology Drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Rivarez, M.P.S.; Vučurović, A.; Mehle, N.; Ravnikar, M.; Kutnjak, D. Global Advances in Tomato Virome Research: Current Status and the Impact of High-Throughput Sequencing. Front. Microbiol. 2021, 12, 671925. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Fukuta, S.; Anai, N.; Kato, M.; Yoshimura, Y.; Fukaya, M.; Yabe, K.; Oya, T.; Kanbe, M. Simplification of Template DNA Preparation for the Loop-Mediated Isothermal Amplification (LAMP) Method to Detect Tomato Yellow Leaf Curl. Virus. Annu. Rep. Kansai Plant Prot. Soc. 2005, 47, 37–41. [Google Scholar] [CrossRef][Green Version]
- Okada, K.; Kusakari, S.; Kawaratani, M.; Negoro, J.; Ohki, S.T.; Osaki, T. Tobacco Mosaic Virus Is Transmissible from Tomato to Tomato by Pollinating Bumblebees. J. Gen. Plant Pathol. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The Bumblebee Bombus Terrestris Carries a Primary Inoculum of Tomato Brown Rugose Fruit Virus Contributing to Disease Spread in Tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Smith, E. Seed Transmission of Tobamoviruses: Aspects of Global Disease Distribution. Adv. Seed Biol. 2017, 12, 233–260. [Google Scholar] [CrossRef]
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato Brown Rugose Fruit Virus: Seed Transmission Rate and Efficacy of Different Seed Disinfection Treatments. Plants 2020, 9, 1615. [Google Scholar] [CrossRef]
- Lanter, J.M.; McGuire, J.M.; Goode, M.J. Persistence of Tomato Mosaic Virus in Tomato Debris and Soil under Field Conditions. Plant Dis. 1982, 66, 552–555. [Google Scholar] [CrossRef]
- Boben, J.; Kramberger, P.; Petrovič, N.; Cankar, K.; Peterka, M.; Štrancar, A.; Ravnikar, M. Detection and Quantification of Tomato Mosaic Virus in Irrigation Waters. Eur. J. Plant Pathol. 2007, 118, 59–71. [Google Scholar] [CrossRef]
- Li, R.; Gao, S.; Fei, Z.; Ling, K.-S. Complete Genome Sequence of a New Tobamovirus Naturally Infecting Tomatoes in Mexico. Genome Announc. 2013, 1, e00794-13. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A New Tobamovirus Infecting Tomato Crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 22 February 2023).
- Sui, X.; Zheng, Y.; Li, R.; Padmanabhan, C.; Tian, T.; Groth-Helms, D.; Keinath, A.P.; Fei, Z.; Wu, Z.; Ling, K.-S. Molecular and Biological Characterization of Tomato Mottle Mosaic Virus and Development of RT-PCR Detection. Plant Dis. 2017, 101, 704–711. [Google Scholar] [CrossRef]
- Zhan, B.; Cao, N.; Wang, K.; Zhou, X. Detection and Characterization of an Isolate of Tomato Mottle Mosaic Virus Infecting Tomato in China. J. Integr. Agric. 2018, 17, 1207–1212. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, C.L.; Xiang, D.; Li, R.H.; Liu, Y.; Li, F. First Report of Tomato Mottle Mosaic Virus Infection of Pepper in China. Plant Dis. 2014, 98, 1447. [Google Scholar] [CrossRef]
- Webster, C.G.; Rosskopf, E.N.; Lucas, L.; Mellinger, H.C.; Adkins, S. First Report of Tomato Mottle Mosaic Virus Infecting Tomato in the United States. Plant Health Prog. 2014, 15, 151–152. [Google Scholar] [CrossRef]
- Turina, M.; Geraats, B.P.J.; Ciuffo, M. First Report of Tomato Mottle Mosaic Virus in Tomato Crops in Israel. New Dis. Rep. 2016, 33, 1. [Google Scholar] [CrossRef]
- Ambrós, S.; Martínez, F.; Ivars, P.; Hernández, C.; de la Iglesia, F.; Elena, S.F. Molecular and Biological Characterization of an Isolate of Tomato Mottle Mosaic Virus (ToMMV) Infecting Tomato and Other Experimental Hosts in Eastern Spain. Eur. J. Plant Pathol. 2017, 149, 261–268. [Google Scholar] [CrossRef]
- Nagai, A.; Duarte, L.M.L.; Chaves, A.L.R.; Alexandre, M.A.V.; Ramos-González, P.L.; Chabi-Jesus, C.; Harakava, R.; dos Santos, D.Y.A.C. First Complete Genome Sequence of an Isolate of Tomato Mottle Mosaic Virus Infecting Plants of Solanum Lycopersicum in South America. Genome Announc. 2018, 6, e00427-18. [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int/reporting/article-6930 (accessed on 22 February 2023).
- Maudarbaccus, F.; Lobin, K.; Vally, V.; Gungoosingh-Bunwaree, A.; Menzel, W. First Report of Tomato Mottle Mosaic Virus on Tomato in Mauritius. New Dis. Rep. 2021, 44, e12041. [Google Scholar] [CrossRef]
- Fowkes, A.R.; Botermans, M.; Frew, L.; de Koning, P.P.M.; Buxton-Kirk, A.; Westenberg, M.; Ward, R.; Schenk, M.F.; Webster, G.; Alraiss, K.; et al. First Report of Tomato Mottle Mosaic Virus in Solanum Lycopersicum Seeds in The Netherlands and Intercepted in Seed Imported from Asia. New Dis. Rep. 2022, 45, 12067. [Google Scholar] [CrossRef]
- Schoen, R.; de Koning, P.; Oplaat, C.; Roenhorst, A.; Westenberg, M.; van der Gaag, D.J.; Barnhoorn, R.; Koenraadt, H.; van Dooijeweert, W.; Lievers, R.; et al. Identification of Tomato Mottle Mosaic Virus in Historic Seed Accessions Originating from France, the Netherlands and Spain, Indicates a Wider Presence before Its First Description. Eur. J. Plant Pathol. 2023, 166, 485–489. [Google Scholar] [CrossRef]
- Pirovano, W.; Miozzi, L.; Boetzer, M.; Pantaleo, V. Bioinformatics Approaches for Viral Metagenomics in Plants Using Short RNAs: Model Case of Study and Application to a Cicer Arietinum Population. Front. Microbiol. 2015, 5, 790. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.L.; Chen, L.D.; Li, B.J.; Xie, X.W.; Shi, Y.X. First Report of a Mixed Infection of Tomato Mottle Mosaic Virus and Tobacco Mild Green Mosaic Virus on Eggplants in China. Plant Dis. 2018, 102, 2668. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, G.L.; Li, F. First Report of Pea as a Natural Host of Tomato Mottle Mosaic Virus in China. Plant Dis. 2022, 106, 775. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Hu, J.; Xiao, L.; Tan, G.; Lan, P.; Liu, Y.; Li, F. The Complete Genome Sequence, Occurrence and Host Range of Tomato Mottle Mosaic Virus Chinese Isolate. Virol. J. 2017, 14, 15. [Google Scholar] [CrossRef]
- Hall, T.J. Resistance at the TM-2 Locus in the Tomato to Tomato Mosaic Virus. Euphytica 1980, 29, 189–197. [Google Scholar] [CrossRef]
- Lanfermeijer, F.C.; Dijkhuis, J.; Sturre, M.J.G.; de Haan, P.; Hille, J. Cloning and Characterization of the Durable Tomato Mosaic Virus Resistance Gene Tm-22 from Lycopersicon Esculentum. Plant Mol. Biol. 2003, 52, 1039–1051. [Google Scholar] [CrossRef][Green Version]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef]
- Nagai, A.; Duarte, L.M.L.; Chaves, A.L.R.; Peres, L.E.P.; dos Santos, D.Y.A.C. Tomato Mottle Mosaic Virus in Brazil and Its Relationship with Tm-22 Gene. Eur. J. Plant Pathol. 2019, 155, 353–359. [Google Scholar] [CrossRef]
- Sarkes, A.; Fu, H.; Feindel, D.; Harding, M.; Feng, J. Development and Evaluation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for the Detection of Tomato Brown Rugose Fruit Virus (ToBRFV). PLoS ONE 2020, 15, e0230403. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.; Da Lio, D.; Panattoni, A.; Salemi, C.; Cappellini, G.; Bartolini, L.; Parrella, G. Rapid and Sensitive Detection of Tomato Brown Rugose Fruit Virus in Tomato and Pepper Seeds by Reverse Transcription Loop-Mediated Isothermal Amplification Assays (Real Time and Visual) and Comparison with RT-PCR End-Point and RT-QPCR Methods. Front. Microbiol. 2021, 12, 640932. [Google Scholar] [CrossRef] [PubMed]
- Bernabé-Orts, J.M.; Torre, C.; Méndez-López, E.; Hernando, Y.; Aranda, M.A. New Resources for the Specific and Sensitive Detection of the Emerging Tomato Brown Rugose Fruit Virus. Viruses 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Honda, A.; Iwata, A.; Ueda, S.; Hibi, T.; Ishihama, A. Isolation from Tobacco Mosaic Virus-Infected Tobacco of a Solubilized Template-Specific RNA-Dependent RNA Polymerase Containing a 126K/183K Protein Heterodimer. J. Virol. 1999, 73, 2633–2640. [Google Scholar] [CrossRef]
- Letschert, B.; Adam, G.; Lesemann, D.-E.; Willingmann, P.; Heinze, C. Detection and Differentiation of Serologically Cross-Reacting Tobamoviruses of Economical Importance by RT-PCR and RT-PCR-RFLP. J. Virol. Methods 2002, 106, 1–10. [Google Scholar] [CrossRef]
- Ishikawa, K.; Maejima, K.; Netsu, O.; Fukuoka, M.; Nijo, T.; Hashimoto, M.; Takata, D.; Yamaji, Y.; Namba, S. Rapid Detection of Fig Mosaic Virus Using Reverse Transcription Loop-Mediated Isothermal Amplification. J. Gen. Plant Pathol. 2015, 81, 382–389. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated Reaction by Loop-Mediated Isothermal Amplification Using Loop Primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Tu, L.; Wu, S.; Gao, D.; Liu, Y.; Zhu, Y.; Ji, Y. Synthesis and Characterization of a Full-Length Infectious CDNA Clone of Tomato Mottle Mosaic Virus. Viruses 2021, 13, 1050. [Google Scholar] [CrossRef]
- Tiberini, A.; Manglli, A.; Taglienti, A.; Vučurović, A.; Brodarič, J.; Ferretti, L.; Luigi, M.; Gentili, A.; Mehle, N. Development and Validation of a One-Step Reverse Transcription Real-Time PCR Assay for Simultaneous Detection and Identification of Tomato Mottle Mosaic Virus and Tomato Brown Rugose Fruit Virus. Plants 2022, 11, 489. [Google Scholar] [CrossRef]
- Gadkar, V.J.; Goldfarb, D.M.; Gantt, S.; Tilley, P.A.G. Real-Time Detection and Monitoring of Loop Mediated Amplification (LAMP) Reaction Using Self-Quenching and De-Quenching Fluorogenic Probes. Sci. Rep. 2018, 8, 5548. [Google Scholar] [CrossRef] [PubMed]
- Rolando, J.C.; Jue, E.; Barlow, J.T.; Ismagilov, R.F. Real-Time Kinetics and High-Resolution Melt Curves in Single-Molecule Digital LAMP to Differentiate and Study Specific and Non-Specific Amplification. Nucleic Acids Res. 2020, 48, e42. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Mashiko, T.; Wilisiani, F.; Hartono, S.; Nishigawa, H.; Natsuaki, T.; Neriya, Y. Development of a RT-LAMP Assay for Real-Time Detection of Criniviruses Infecting Tomato. J. Virol. Methods 2023, 312, 114662. [Google Scholar] [CrossRef]
- Samuel, G. The Movement of Tobacco Mosaic Virus within the Plant. Ann. Appl. Biol. 1934, 21, 90–111. [Google Scholar] [CrossRef]
- Vaisman, M.; Hak, H.; Arazi, T.; Spiegelman, Z. The Impact of Tobamovirus Infection on Root Development Involves Induction of Auxin Response Factor 10a in Tomato. Plant Cell Physiol. 2022, 63, 1980–1993. [Google Scholar] [CrossRef]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and Cellular Factors Involved in Phloem Transport of Plant Viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Kappagantu, M.; Collum, T.D.; Dardick, C.; Culver, J.N. Viral Hacks of the Plant Vasculature: The Role of Phloem Alterations in Systemic Virus Infection. Annu. Rev. Virol. 2020, 7, 72410. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, I.F. Tansley Review No. 27 The Control of Carbon Partitioning in Plants. New Phytol. 1990, 116, 341–381. [Google Scholar] [CrossRef]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon Source–Sink Relationship in Arabidopsis Thaliana: The Role of Sucrose Transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef]
- Tettey, C.K.; Yan, Z.; Ma, H.; Zhao, M.; Geng, C.; Tian, Y.; Li, X. Tomato Mottle Mosaic Virus: Characterization, Resistance Gene Effectiveness, and Quintuplex RT-PCR Detection System. J. Integr. Agric. 2022, 21, 2641–2651. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G. TOBACCO MOSAIC VIRUS: A Model System for Plant Biology. Annu. Rev. Phytopathol. 2004, 42, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Dall, D.J.; Lovelock, D.A.; Penrose, L.D.J.; Constable, F.E. Prevalences of Tobamovirus Contamination in Seed Lots of Tomato and Capsicum. Viruses 2023, 15, 883. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequence (5′–3′) | Length (nt) | Position * | Reference |
|---|---|---|---|---|
| ToMMV_2-3_FOP | ACTAATGAAAGAAAAGGGCGG | 21 | 5585–5605 (F3) | This study |
| ToMMV_2-3_BOP | ATATTTATTAATTCTACAGGGTCG | 24 | 5798–5775 (B3) | This study |
| ToMMV_2-3_FIP (F1c ** + F2) | CGTCTCGGTATCATCTTCAATCAAA | 47 | 5689–5665 (F1c) | This study |
| TCTAATTTCCGTAAGAAACAAG | 5606–5627 (F2) | |||
| ToMMV_2-3_BIP (B1c ** + B2) | CAGTCGCGGGATCTGATTCG | 40 | 5691–5710 (B1c) | This study |
| ATGCTGATGATAAAAACACG | 5770–5751 (B2) | |||
| ToMMV_2_LF | TCACTAACTCCATAACTCTCCTGGT | 25 | 5652–5628 (LF) | This study |
| ToMMV_2_LB | ATGTCTTACGCTATTACTTCTCCGT | 25 | 5719–5743 (LB) | This study |
| Tob-Uni1 | ATTTAAGTGGASGGAAAAVCACT | 21 | 6285–6263 | [38] |
| Tob-Uni2 | GTYGTTGATGAGTTCRTGGA | 18 | 5486–5505 | |
| ToMMV-F | CGACCCTGTAGAATTAATAAATATT | 25 | 5775–5799 | [15] |
| ToMMV-R | CACTCTGCGAGTGGCATCCAAT | 22 | 6063–6042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, K.; Miyazaki, A.; Suzuki, T.; Yamamoto, T.; Kitazawa, Y.; Maejima, K.; Namba, S.; Yamaji, Y. A Reverse-Transcription Loop-Mediated Isothermal Amplification Technique to Detect Tomato Mottle Mosaic Virus, an Emerging Tobamovirus. Viruses 2023, 15, 1688. https://doi.org/10.3390/v15081688
Kimura K, Miyazaki A, Suzuki T, Yamamoto T, Kitazawa Y, Maejima K, Namba S, Yamaji Y. A Reverse-Transcription Loop-Mediated Isothermal Amplification Technique to Detect Tomato Mottle Mosaic Virus, an Emerging Tobamovirus. Viruses. 2023; 15(8):1688. https://doi.org/10.3390/v15081688
Chicago/Turabian StyleKimura, Kan, Akio Miyazaki, Takumi Suzuki, Toya Yamamoto, Yugo Kitazawa, Kensaku Maejima, Shigetou Namba, and Yasuyuki Yamaji. 2023. "A Reverse-Transcription Loop-Mediated Isothermal Amplification Technique to Detect Tomato Mottle Mosaic Virus, an Emerging Tobamovirus" Viruses 15, no. 8: 1688. https://doi.org/10.3390/v15081688
APA StyleKimura, K., Miyazaki, A., Suzuki, T., Yamamoto, T., Kitazawa, Y., Maejima, K., Namba, S., & Yamaji, Y. (2023). A Reverse-Transcription Loop-Mediated Isothermal Amplification Technique to Detect Tomato Mottle Mosaic Virus, an Emerging Tobamovirus. Viruses, 15(8), 1688. https://doi.org/10.3390/v15081688






