The Characterization and Pathogenicity of a Recombinant Porcine Epidemic Diarrhea Virus Variant ECQ1
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection and PEDV Detection
2.3. Amplification of the Complete S Gene and Whole Genome Sequence of PEDV
2.4. Sequence Alignment and Phylogenetic Analyses
2.5. Genome Recombination Event Analysis
2.6. Viral Isolation, Purification and Propagation
2.7. Growth Curve Assay
2.8. Immunofluorescence Assay
2.9. Animal Experiment
2.10. Statistical Analysis
3. Results
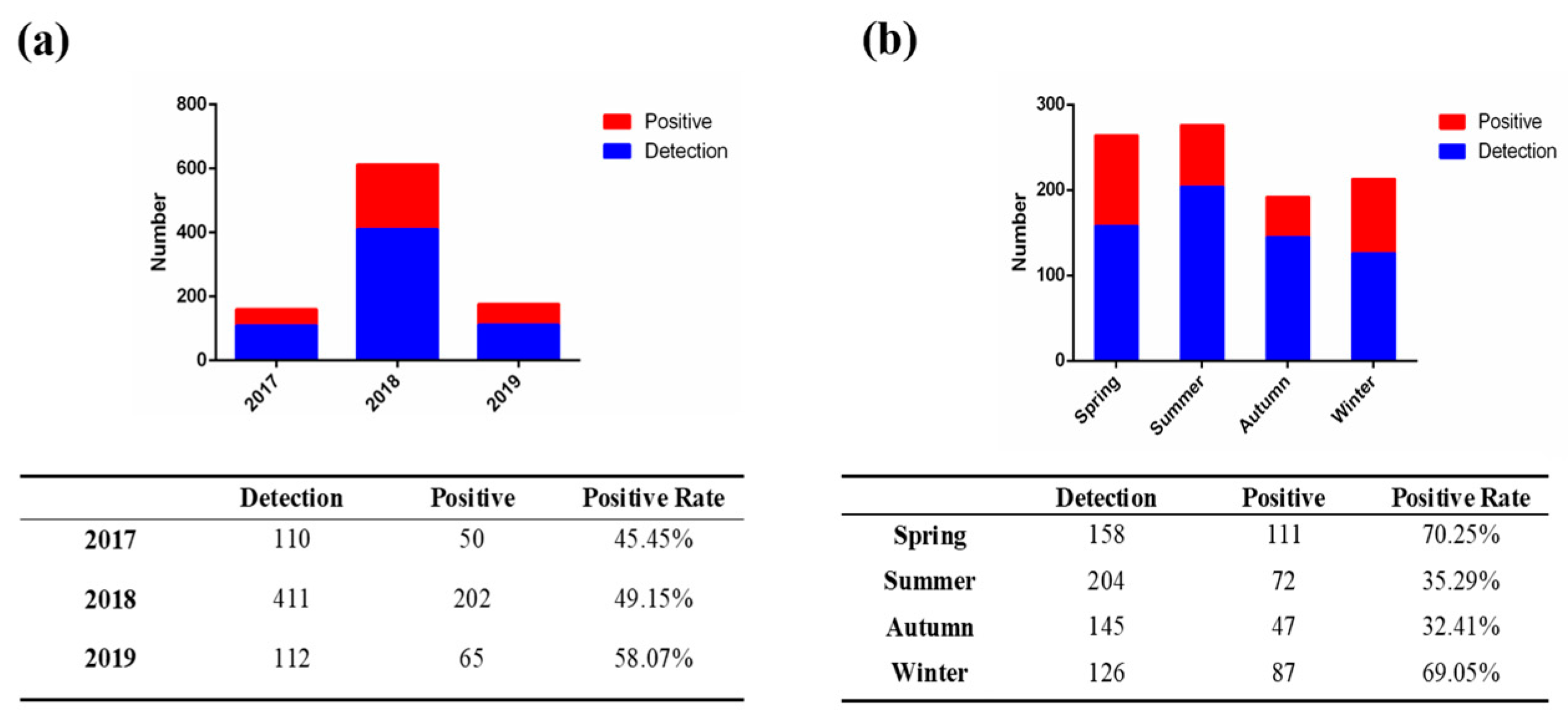
3.1. Epidemiological Investigation of PEDV in China from 2017 to 2019
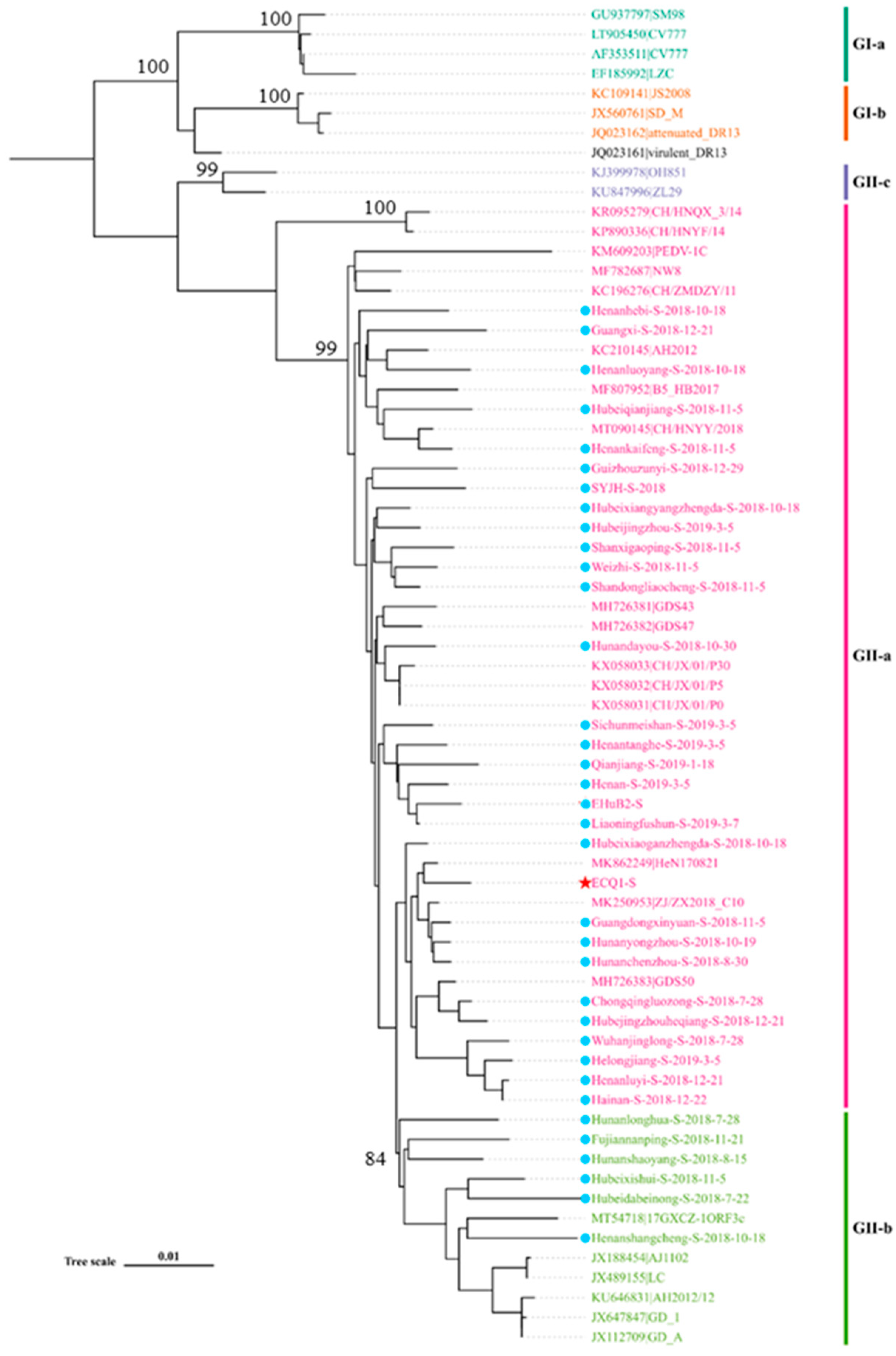
3.2. Phylogenetic Analysis of Full-Length S Genes of PEDV
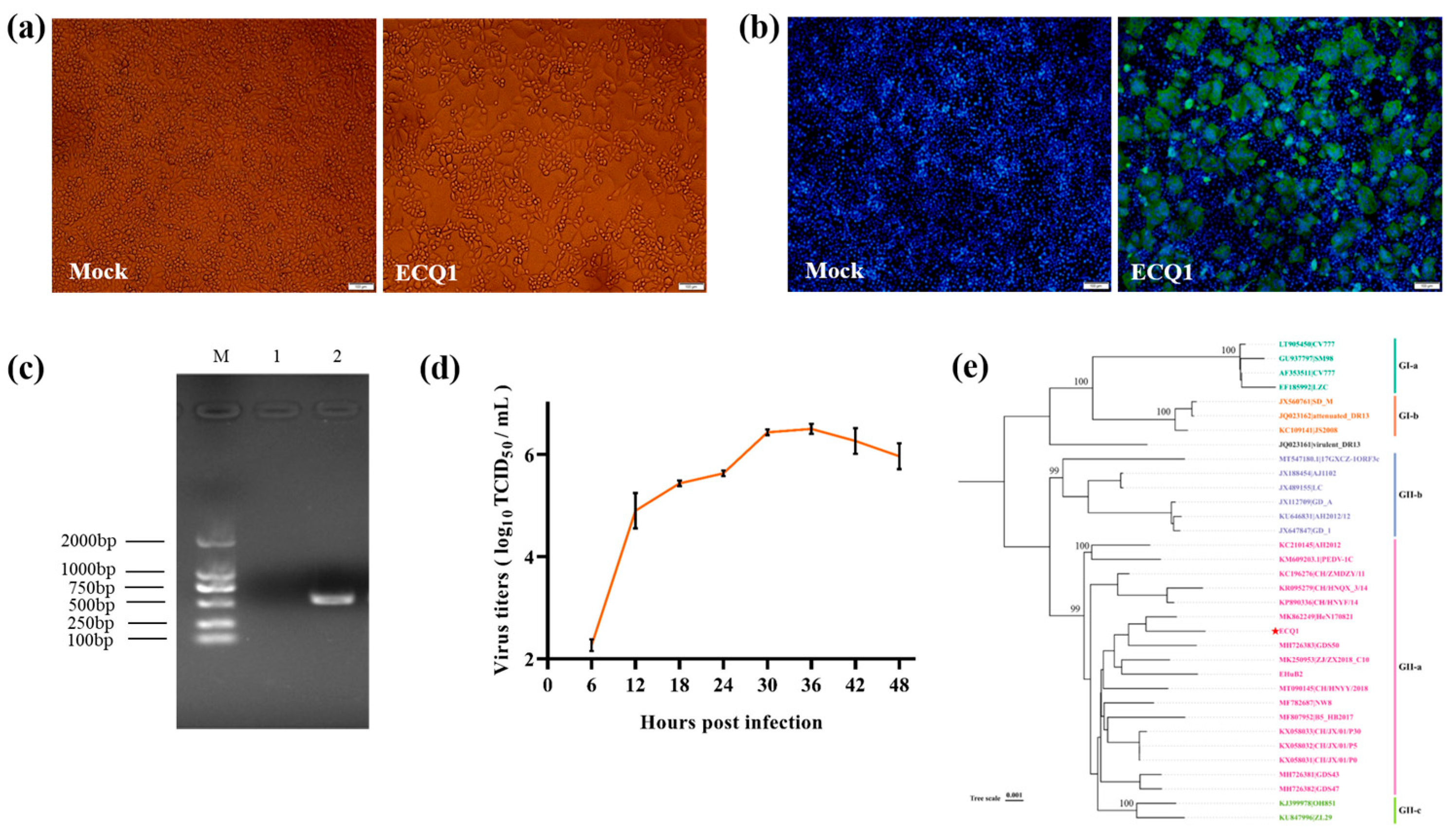
3.3. Isolation, Identification and Full-Length Genome Sequence and Phylogenetic Analyses of PEDV Strain ECQ1
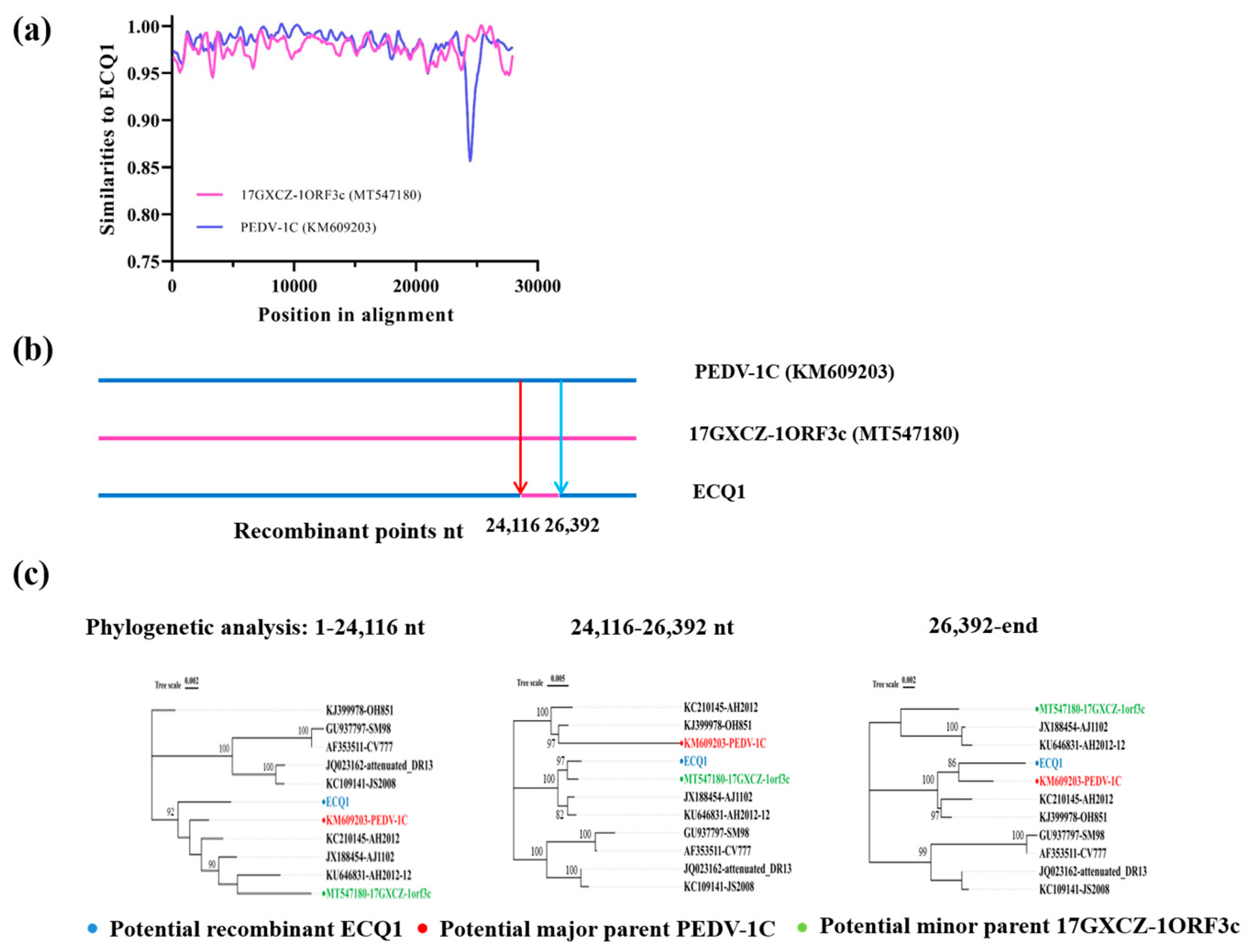
3.4. Recombination Analysis of PEDV Strain ECQ1
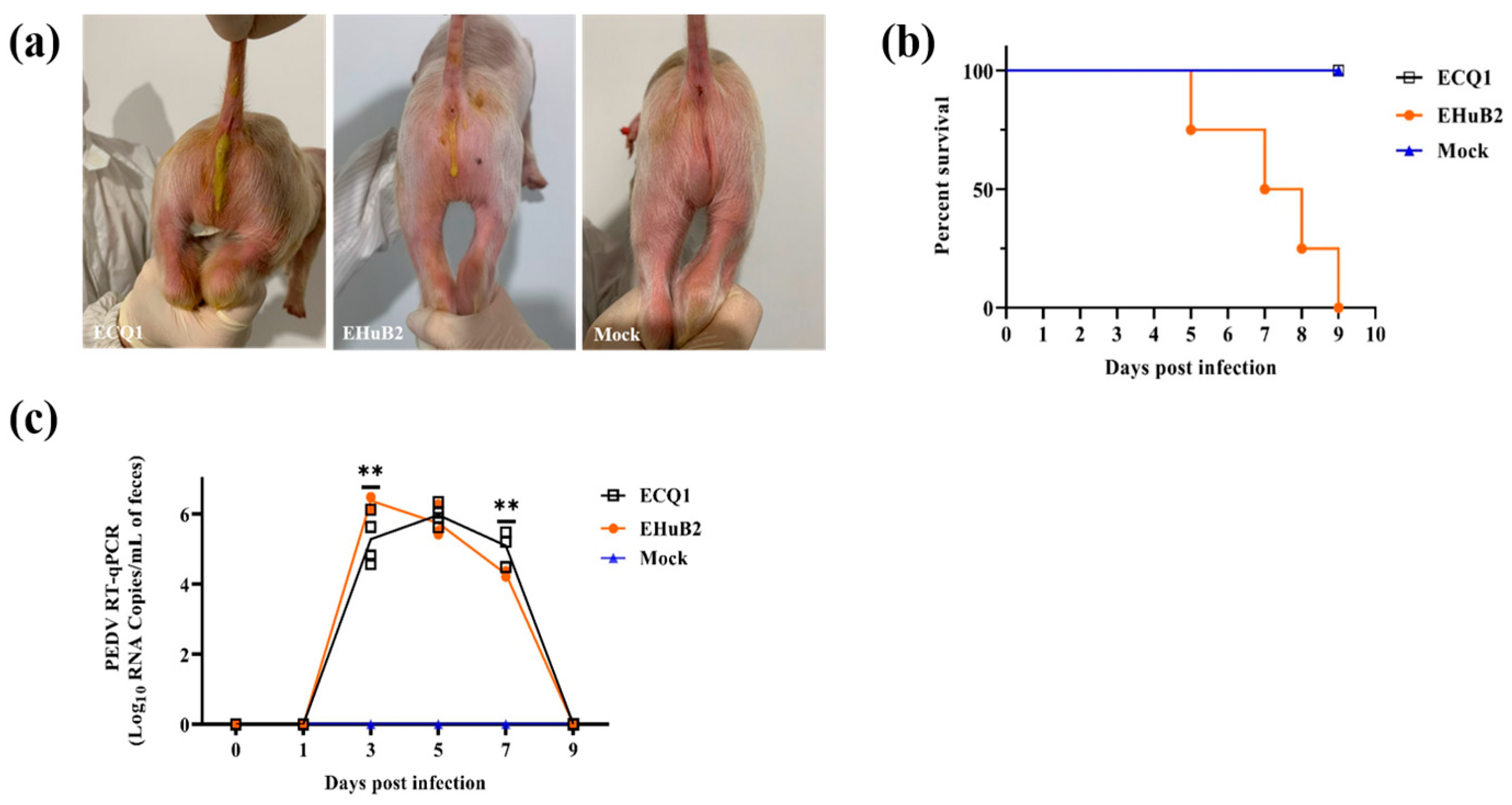
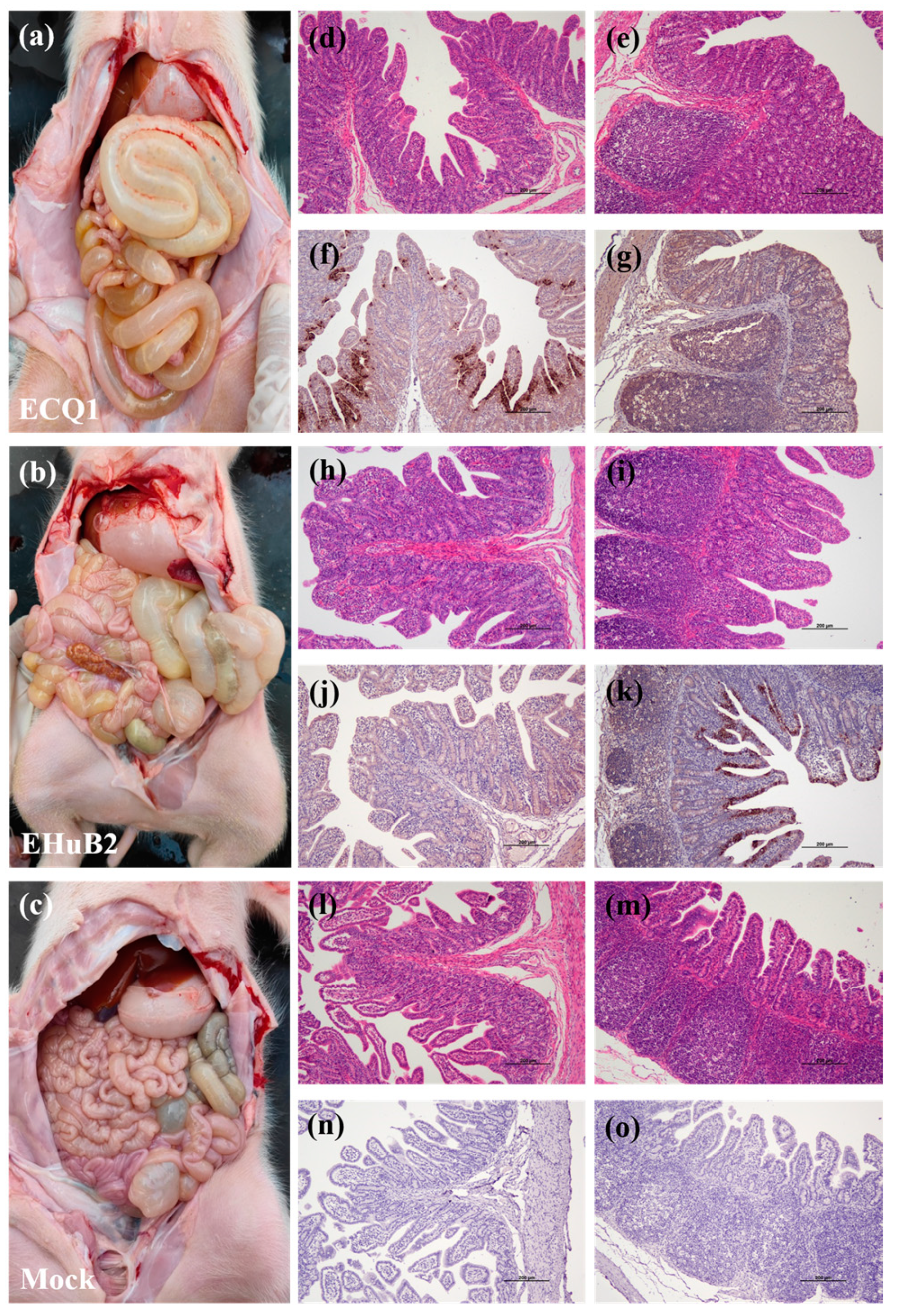
3.5. Pathogenicity of ECQ1 in Piglets
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sueyoshi, M.; Tsuda, T.; Yamazaki, K.; Yoshida, K.; Nakazawa, M.; Sato, K.; Minami, T.; Iwashita, K.; Watanabe, M.; Suzuki, Y.; et al. An immunohistochemical investigation of porcine epidemic diarrhoea. J. Comp. Pathol. 1995, 113, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Have, P.; Moving, V.; Svansson, V.; Uttenthal, A.; Bloch, B. Coronavirus infection in mink (Mustela vison). Serological evidence of infection with a coronavirus related to transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Vet. Microbiol. 1992, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Debouck, P.; Pensaert, M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980, 41, 219–223. [Google Scholar] [PubMed]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Park, B. Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 2012, 44, 167–175. [Google Scholar] [CrossRef]
- Carvajal, A.; Lanza, I.; Diego, R.; Rubio, P.; Carmenes, P. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J. Vet. Diagn. Investig. 1995, 7, 60–64. [Google Scholar] [CrossRef]
- Martelli, P.; Lavazza, A.; Nigrelli, A.D.; Merialdi, G.; Alborali, L.G.; Pensaert, M.B. Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Vet. Rec. 2008, 162, 307–310. [Google Scholar] [CrossRef]
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef]
- Song, D.S.; Yang, J.S.; Oh, J.S.; Han, J.H.; Park, B.K. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF3. Vaccine 2003, 21, 1833–1842. [Google Scholar] [CrossRef]
- Kweon, C.H.; Kwon, B.J.; Jung, T.S.; Kee, Y.J.; Hur, D.H.; Hwang, E.K.; Rhee, J.C.; An, S.H. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J. Vet. 1993, 33, 249–254. [Google Scholar]
- Puranaveja, S.; Poolperm, P.; Lertwatcharasarakul, P.; Kesdaengsakonwut, S.; Boonsoongnern, A.; Urairong, K.; Kitikoon, P.; Choojai, P.; Kedkovid, R.; Teankum, K.; et al. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Infect. Dis. 2009, 15, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Okada, K.; Ohshima, K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi 1983, 45, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.F.; Jin, Y.L.; Xing, G.; Qv, L.L.; Huang, Y.W.; Zhou, J.Y. Evidence of recombinant strains of porcine epidemic diarrhea virus, United States, 2013. Emerg. Infect. Dis. 2014, 20, 1735–1738. [Google Scholar] [CrossRef]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef]
- Fan, B.; Jiao, D.; Zhao, X.; Pang, F.; Xiao, Q.; Yu, Z.; Mao, A.; Guo, R.; Yuan, W.; Zhao, P.; et al. Characterization of Chinese Porcine Epidemic Diarrhea Virus with Novel Insertions and Deletions in Genome. Sci. Rep. 2017, 7, 44209. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Guo, J.H.; Fang, L.R.; Ye, X.; Chen, J.Y.; Xu, S.E.; Zhu, X.Y.; Miao, Y.M.; Wang, D.; Xiao, S.B. Evolutionary and genotypic analyses of global porcine epidemic diarrhea virus strains. Transbound. Emerg. Dis. 2019, 66, 111–118. [Google Scholar] [CrossRef]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef]
- Song, D.; Moon, H.; Kang, B. Porcine epidemic diarrhea: A review of current epidemiology and available vaccines. Clin. Exp. Vaccine Res. 2015, 4, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, G.W.; Stasko, J.; Thomas, J.T.; Stensland, W.R.; Pillatzki, A.E.; Gauger, P.C.; Schwartz, K.J.; Madson, D.; Yoon, K.J.; et al. Isolation and Characterization of Porcine Epidemic Diarrhea Viruses Associated with the 2013 Disease Outbreak among Swine in the United States. J. Clin. Microbiol. 2014, 52, 234–243. [Google Scholar] [CrossRef]
- Chang, S.H.; Bae, J.L.; Kang, T.J.; Kim, J.; Chung, G.H.; Lim, C.W.; Laude, H.; Yang, M.S.; Jang, Y.S. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol. Cells 2002, 14, 295–299. [Google Scholar] [PubMed]
- Gong, L.; Lin, Y.; Qin, J.; Li, Q.; Xue, C.; Cao, Y. Neutralizing antibodies against porcine epidemic diarrhea virus block virus attachment and internalization. Virol. J. 2018, 15, 133. [Google Scholar] [CrossRef]
- Thavorasak, T.; Chulanetra, M.; Glab-Ampai, K.; Mahasongkram, K.; Sae-Lim, N.; Teeranitayatarn, K.; Songserm, T.; Yodsheewan, R.; Nilubol, D.; Chaicumpa, W.; et al. Enhancing epitope of PEDV spike protein. Front. Microbiol. 2022, 13, 933249. [Google Scholar] [CrossRef]
- Temeeyasen, G.; Srijangwad, A.; Tripipat, T.; Tipsombatboon, P.; Piriyapongsa, J.; Phoolcharoen, W.; Chuanasa, T.; Tantituvanont, A.; Nilubol, D. Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infect. Genet. Evol. 2014, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Thavorasak, T.; Chulanetra, M.; Glab-Ampai, K.; Teeranitayatarn, K.; Songserm, T.; Yodsheewan, R.; Sae-Lim, N.; Lekcharoensuk, P.; Sookrung, N.; Chaicumpa, W. Novel Neutralizing Epitope of PEDV S1 Protein Identified by IgM Monoclonal Antibody. Viruses 2022, 14, 125. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Holmes, E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011, 9, 617–626. [Google Scholar] [CrossRef]
- Makino, S.; Keck, J.G.; Stohlman, S.A.; Lai, M.M.C. High-Frequency Rna Recombination of Murine Coronaviruses. J. Virol. 1986, 57, 729–737. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Lam, H.C.; Zhang, Y.; Wang, L.Y.; Hesse, R.A.; Hause, B.M.; Vlasova, A.; Wang, Q.H.; Zhang, J.Q.; Nelson, M.I.; et al. Genomic and evolutionary inferences between American and global strains of porcine epidemic diarrhea virus. Prev. Vet. Med. 2016, 123, 175–184. [Google Scholar] [CrossRef]
- Wang, H.N.; Zhang, L.B.; Shang, Y.B.; Tan, R.R.; Ji, M.X.; Yue, X.L.; Wang, N.N.; Liu, J.; Wang, C.H.; Li, Y.G.; et al. Emergence and evolution of highly pathogenic porcine epidemic diarrhea virus by natural recombination of a low pathogenic vaccine isolate and a highly pathogenic strain in the spike gene. Virus Evol. 2020, 6, veaa049. [Google Scholar] [CrossRef] [PubMed]
- Dong, N. Isolation, Identification and Serial Passaging Attenuation of Poricine Delta Coronavirus Strain CHN-HN-2014. Ph.D. Thesis, Huazhong Agricultural Univeristy, Wuhan, China, 2017. [Google Scholar]
- Song, D.P.; Huang, D.Y.; Peng, Q.; Huang, T.; Chen, Y.J.; Zhang, T.S.; Nie, X.W.; He, H.J.; Wang, P.; Liu, Q.L.; et al. Molecular Characterization and Phylogenetic Analysis of Porcine Epidemic Diarrhea Viruses Associated with Outbreaks of Severe Diarrhea in Piglets in Jiangxi, China 2013. PLoS ONE 2015, 10, e0120310. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef]
- Zeng, L. Proteome Analysis of PEDV-Infected Vero Cells and Attenution of PEDV by Serial Passage in Vero Cells. Ph.D. Thesis, Huazhong Agricultural Univeristy, Wuhan, China, 2016. [Google Scholar]
- Dong, N.; Fang, L.; Yang, H.; Liu, H.; Du, T.; Fang, P.; Wang, D.; Chen, H.; Xiao, S. Isolation, genomic characterization, and pathogenicity of a Chinese porcine deltacoronavirus strain CHN-HN-2014. Vet. Microbiol. 2016, 196, 98–106. [Google Scholar] [CrossRef]
- Holmes, E.C. The Evolution and Emergence of RNA Viruses; Oxford University Press: Oxford, UK; New York, NY, USA, 2009; 254p. [Google Scholar]
- Lee, D.K.; Park, C.K.; Kim, S.H.; Lee, C. Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea. Virus Res. 2010, 149, 175–182. [Google Scholar] [CrossRef]
- Chen, Q.; Gauger, P.C.; Stafne, M.R.; Thomas, J.T.; Madson, D.M.; Huang, H.Y.; Zheng, Y.; Li, G.W.; Zhang, J.Q. Pathogenesis comparison between the United States porcine epidemic diarrhoea virus prototype and S-INDEL-variant strains in conventional neonatal piglets. J. Gen. Virol. 2016, 97, 1107–1121. [Google Scholar] [CrossRef]
- Boniotti, M.B.; Papetti, A.; Lavazza, A.; Alborali, G.; Sozzi, E.; Chiapponi, C.; Faccini, S.; Bonilauri, P.; Cordioli, P.; Marthaler, D. Porcine Epidemic Diarrhea Virus and Discovery of a Recombinant Swine Enteric Coronavirus, Italy. Emerg. Infect. Dis. 2016, 22, 83–87. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Marthaler, D.; Wang, Q.; Culhane, M.R.; Rossow, K.D.; Rovira, A.; Collins, J.; Saif, L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg. Infect. Dis. 2014, 20, 1620–1628. [Google Scholar] [CrossRef]
- Li, R.F.; Qiao, S.L.; Yang, Y.Y.; Guo, J.Q.; Xie, S.; Zhou, E.M.; Zhang, G.P. Genome sequencing and analysis of a novel recombinant porcine epidemic diarrhea virus strain from Henan, China. Virus Genes 2016, 52, 91–98. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, M.; Zhan, J.; Liu, Q.Y.; Fang, L.L.; Zhao, C.Y.; Jiang, P.; Li, Y.F.; Bai, J. Pathogenicity and immunogenicity of a new strain of porcine epidemic diarrhea virus containing a novel deletion in the N gene. Vet. Microbiol. 2020, 240, 108511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Huang, J.; Yao, Y.; Zhao, W.; Zhang, Y.; Qing, J.; Ren, J.; Yan, Z.; Wang, Z.; et al. Isolation and oral immunogenicity assessment of porcine epidemic diarrhea virus NH-TA2020 strain: One of the predominant strains circulating in China from 2017 to 2021. Virol. Sin. 2022, 37, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shibahara, T.; Yamaguchi, R.; Nakade, K.; Yamamoto, T.; Miyazaki, A.; Ohashi, S. Pig epidemic diarrhoea virus S gene variant with a large deletion non-lethal to colostrum-deprived newborn piglets. J. Gen. Virol. 2016, 97, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Z.; Li, Y.; Gao, S.; Xiao, S. Porcine epidemic diarrhea virus: Molecular mechanisms of attenuation and vaccines. Microb. Pathog. 2020, 149, 104553. [Google Scholar] [CrossRef]
- Deng, X.; van Geelen, A.; Buckley, A.C.; O’Brien, A.; Pillatzki, A.; Lager, K.M.; Faaberg, K.S.; Baker, S.C. Coronavirus Endoribonuclease Activity in Porcine Epidemic Diarrhea Virus Suppresses Type I and Type III Interferon Responses. J. Virol. 2019, 93, e02000–e02018. [Google Scholar] [CrossRef]
- Hou, Y.; Ke, H.; Kim, J.; Yoo, D.; Su, Y.; Boley, P.; Chepngeno, J.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Engineering a Live Attenuated Porcine Epidemic Diarrhea Virus Vaccine Candidate via Inactivation of the Viral 2′-O-Methyltransferase and the Endocytosis Signal of the Spike Protein. J. Virol. 2019, 93, e00406–e00419. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Li, W.; van Kuppeveld, F.J.M.; He, Q.; Rottier, P.J.M.; Bosch, B.J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016, 226, 117–127. [Google Scholar] [CrossRef]
- Hulswit, R.J.; de Haan, C.A.; Bosch, B.J. Coronavirus Spike Protein and Tropism Changes. Adv. Virus Res. 2016, 96, 29–57. [Google Scholar]
| DPI | Strain | Clinical Observation | Fecal Consistency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | Normal | Mild Diarrhea | Water Diarrhea | ||
| 0 | ECQ1 | √ | 5/5 | 0/5 | 0/5 | |||||||
| EHuB2 | √ | 5/5 | 0/5 | 0/5 | ||||||||
| 1 | ECQ1 | √ | 2/5 | 3/5 | 0/5 | |||||||
| EHuB2 | √ | 5/5 | 0/5 | 0/5 | ||||||||
| 2 * | ECQ1 | √ | 2/4 | 2/4 | 0/4 | |||||||
| EHuB2 | √ | 2/4 | 2/4 | 0/4 | ||||||||
| 3 | ECQ1 | √ | 2/4 | 2/4 | 0/4 | |||||||
| EHuB2 | √ | 1/4 | 3/4 | 0/4 | ||||||||
| 4 | ECQ1 | √ | 3/4 | 1/4 | 0/4 | |||||||
| EHuB2 | √ | 1/4 | 3/4 | 0/4 | ||||||||
| 5 | ECQ1 | √ | 4/4 | 0/4 | 0/4 | |||||||
| EHuB2 | √ | 0/3 | 1/3 | 2/3 | ||||||||
| 6 | ECQ1 | √ | 4/4 | 0/4 | 0/4 | |||||||
| EHuB2 | √ | 0/3 | 0/3 | 3/3 | ||||||||
| 7 | ECQ1 | √ | 4/4 | 0/4 | 0/4 | |||||||
| EHuB2 | √ | 0/2 | 0/2 | 2/2 | ||||||||
| 8 | ECQ1 | √ | 4/4 | 0/4 | 0/4 | |||||||
| EHuB2 | √ | 0/1 | 0/1 | 1/1 | ||||||||
| 9 | ECQ1 | √ | 4/4 | 0/4 | 0/4 | |||||||
| EHuB2 | √ | 0 | 0 | 0 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, X.; Guo, J.; Fang, P.; Ma, J.; Li, M.; Fang, L. The Characterization and Pathogenicity of a Recombinant Porcine Epidemic Diarrhea Virus Variant ECQ1. Viruses 2023, 15, 1492. https://doi.org/10.3390/v15071492
Mei X, Guo J, Fang P, Ma J, Li M, Fang L. The Characterization and Pathogenicity of a Recombinant Porcine Epidemic Diarrhea Virus Variant ECQ1. Viruses. 2023; 15(7):1492. https://doi.org/10.3390/v15071492
Chicago/Turabian StyleMei, Xiaowei, Jiahui Guo, Puxian Fang, Jun Ma, Mingxiang Li, and Liurong Fang. 2023. "The Characterization and Pathogenicity of a Recombinant Porcine Epidemic Diarrhea Virus Variant ECQ1" Viruses 15, no. 7: 1492. https://doi.org/10.3390/v15071492
APA StyleMei, X., Guo, J., Fang, P., Ma, J., Li, M., & Fang, L. (2023). The Characterization and Pathogenicity of a Recombinant Porcine Epidemic Diarrhea Virus Variant ECQ1. Viruses, 15(7), 1492. https://doi.org/10.3390/v15071492







