A Novel, Precise and High-Throughput Technology for Viroid Detection in Cannabis (MFDetectTM)
Abstract
1. Introduction
2. Material and Methods
2.1. Development of the MFDetectTM Method
2.2. Validation of MFDetectTM by RT-qPCR
2.3. Identifying a Preferred Tissue Type for Early Viroid Detection during Development of the Plants
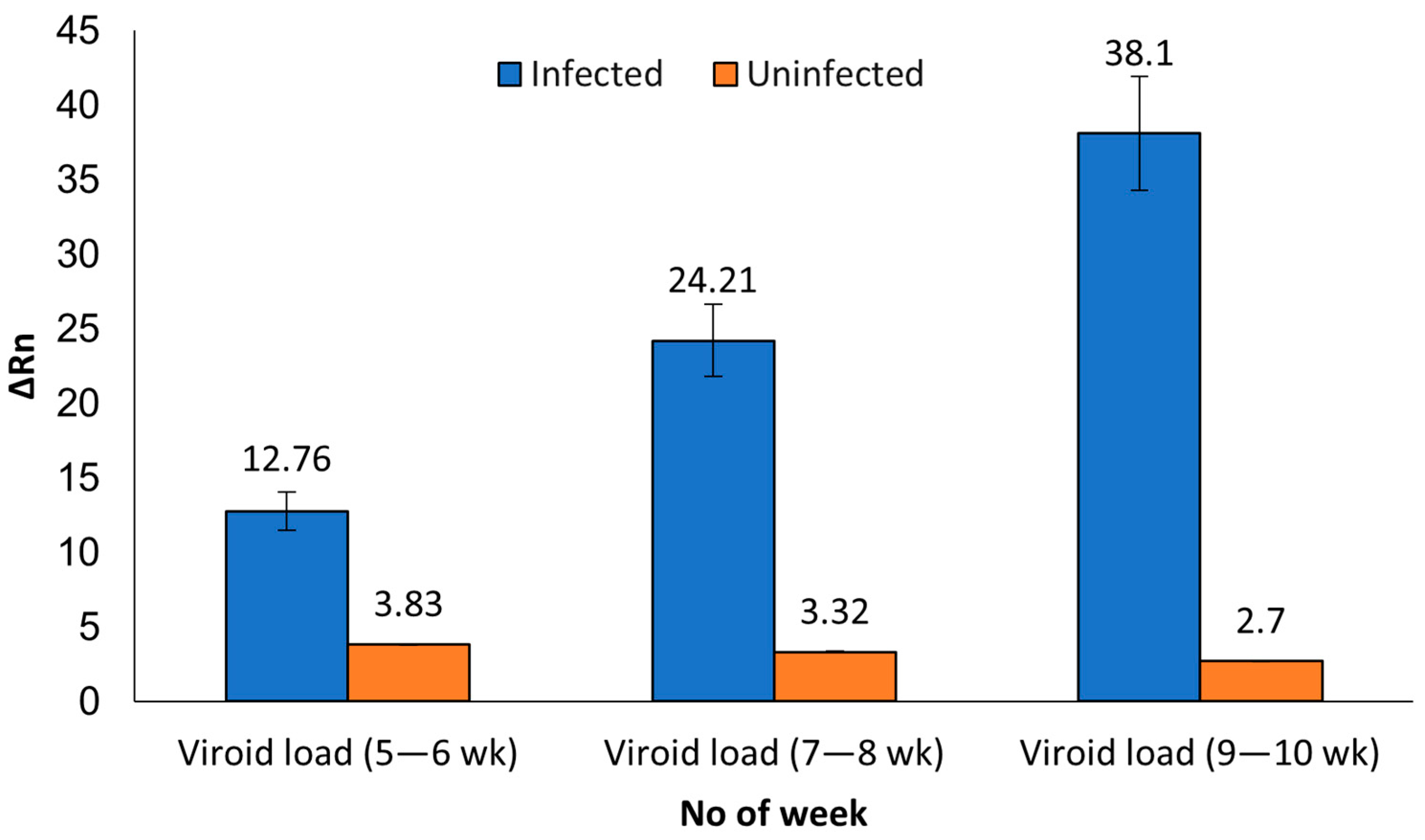
3. Results
3.1. Development and Optimization of the MFDetectTM Assay
3.2. Comparison of MFDetectTM and RT-qPCR
3.3. Identifying a Preferred Tissue Type for Early Viroid Detection during Plant Development
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karche, T.; Singh, M.R. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 2. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; van Eeden, T.; Beswa, D. Cannabis sativa Cannabinoids as Functional Ingredients in Snack Foods—Historical and Developmental Aspects. Plants 2022, 11, 3330. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2023, 62, 108074. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, I.; Pellino, M.; Rigault, P.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.E.; Alcorn, J.; Laprairie, R.B.; McKay, J.K.; et al. The Genomics of Cannabis and Its Close Relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Jones, A.M. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef]
- Pallas, V.; Navarro, A.; Flores, R. Isolation of a viroid-like RNA from hop different from hop stunt viroid. J. Gen. Virol. 1987, 68, 3201–3205. [Google Scholar] [CrossRef]
- Faggioli, F.; Durán-Vila, N.; Tsagris, M.; Pallás, V. Geographical Distribution of Viroids in Europe. In Viroids and Satellites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 473–484. [Google Scholar]
- Bektaş, A.; Hardwick, K.; Waterman, K.; Kristof, J. Occurrence of hop latent viroid in Cannabis sativa with symptoms of cannabis stunting disease in California. Plant Dis. 2019, 103, 2699. [Google Scholar] [CrossRef]
- Warren, J.; Mercado, J.; Grace, D. Occurrence of hop latent viroid causing disease in Cannabis sativa in California. Plant Dis. 2019, 103, 2699. [Google Scholar] [CrossRef]
- Adkar-Purushothama, C.R.; Sano, T.; Perreault, J.-P. Hop Latent Viroid: A Hidden Threat to the Cannabis Industry. Viruses 2023, 15, 681. [Google Scholar] [CrossRef]
- Diener, T.O. Potato spindle tuber “virus”: IV. A replicating, low molecular weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]
- Semancik, J. Considerations for the introduction of viroids for economic advantage. In Viroids; CSIRO Publishing: Collingwood, ON, Canada, 2003; pp. 357–362. [Google Scholar]
- Flores, R.; Hernández, C.; Alba, A.E.M.D.; Daròs, J.-A.; Serio, F.D. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P. Hop latent viroid: An emerging threat to cannabis industry. J. Plant Prot. Res. 2021, 61, 1–9. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Hay, F.S.; Barbara, D.J.; Eastwell, K.C.; Wilson, C.R. Viruses and viroids infecting hop: Significance, epidemiology, and management. Plant Dis. 2008, 92, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Puchta, H.; Ramm, K.; Sänger, H.L. The molecular structure of hop latent viroid (HLV), a new viroid occurring worldwide in hops. Nucleic Acids Res. 1988, 16, 4197–4216. [Google Scholar] [CrossRef] [PubMed]
- Chiginsky, J.; Langemeier, K.; MacWilliams, J.; Albrecht, T.; Cranshaw, W.; Fulladolsa, A.C.; Kapuscinski, M.; Stenglein, M.; Nachappa, P. First Insights Into the Virus and Viroid Communities in Hemp (Cannabis sativa). Front. Agron. 2021, 3, 778433. [Google Scholar] [CrossRef]
- Postman, J.D.; Hadidi, A. Elimination of apple scar skin viroid from pears by in vitro thermo-therapy and apical meristem culture. In Proceedings of the XVI International Symposium on Fruit Tree Virus Diseases, Rome, Italy, 27 June–2 July 1994; Volume 386, pp. 536–543. [Google Scholar] [CrossRef]
- Howell, W.E.; Burgess, J.; Mink, G.I.; Skrzeczkowski, L.J.; Zhang, Y.P. Elimination of apple fruit and bark deforming agents by heat therapy. In Proceedings of the XVII International Symposium Virus and Virus-Like Diseases of Temperate Fruit Crops, Bethesda, MD, USA, 23–27 June 1997; Volume 472, pp. 641–648. [Google Scholar] [CrossRef]
- Owens, R.A.; Diener, T. Sensitive and rapid diagnosis of potato spindle tuber viroid disease by nucleic acid hybridization. Science 1981, 213, 670–672. [Google Scholar] [CrossRef]
- Schumacher, J.; Meyer, N.; Riesner, D.; Weidemann, H.L. Diagnostic procedure for detection of viroids and viruses with circular RNAs by “return”-gel electrophoresis. J. Phytopathol. 1986, 115, 332–343. [Google Scholar] [CrossRef]
- Boonham, N.; Pérez, L.G.; Mendez, M.; Peralta, E.L.; Blockley, A.; Walsh, K.; Barker, I.; Mumford, R. Development of a real-time RT-PCR assay for the detection of Potato spindle tuber viroid. J. Virol. Methods 2004, 116, 139–146. [Google Scholar] [CrossRef]
- Chandelier, A.; Planchon, V.; Oger, R. Determination of cycle cut off in real-time PCR for the detection of regulated plant pathogens. EPPO Bull. 2010, 40, 52–58. [Google Scholar] [CrossRef]
- Mascia, T.; Santovito, E.; Gallitelli, D.; Cillo, F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol. Plant Pathol. 2010, 11, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, N.; Yanagisawa, H.; Fujiwara, Y.; Ohara, T. Detection of Potato spindle tuber viroid by reverse transcription-loop mediated isothermal amplification. Res. Bull. Plant Prot. Jpn. 2010, 46, 61–67. [Google Scholar] [CrossRef]
- Chambers, G.A.; Geering, A.D.; Holford, P.; Kehoe, M.A.; Vidalakis, G.; Donovan, N.J. A reverse transcription loop-mediated isothermal amplification assay for the detection of citrus exocortis viroid in Australian citrus. Australas. Plant Pathol. 2023, 52, 121–132. [Google Scholar] [CrossRef]
- Panno, S.; Matić, S.; Tiberini, A.; Caruso, A.G.; Bella, P.; Torta, L.; Stassi, R.; Davino, A.S. Loop Mediated Isothermal Amplification: Principles and Applications in Plant Virology. Plants 2020, 9, 461. [Google Scholar] [CrossRef]
- Bostan, H.; Nie, X.; Singh, R.P. An RT-PCR primer pair for the detection of Pospiviroid and its application in surveying ornamental plants for viroids. J. Virol. Methods 2004, 116, 189–193. [Google Scholar] [CrossRef]
- Tseng, Y.I.-W.; Wu, C.H.; Chnag, C.-J.; Chen, Y.H.; Jan, F.J. Universal primers for rapid detection of six pospiviroids in Solanaceae plants using one-step Reverse-Transcription PCR and Reverse-Transcription Loop-mediated Isothermal Amplification. Plant Dis. 2021, 105, 2867–2872. [Google Scholar] [CrossRef]
- Massart, S.; Candresse, T.; Gil, J.; Lacomme, C.; Predajna, L.; Ravnikar, M.; Reynard, J.-S.; Rumbou, A.; Saldarelli, P.; Skorić1, D.; et al. A framework for the evaluation of biosecurity, commercial, regulatory, and scientific impacts of plant viruses and viroids identified by NGS technologies. Front. Microbiol. 2017, 8, 45. [Google Scholar] [CrossRef]
- Singh, R.P. Seed transmission of Potato spindle tuber virus in Tomato and Potato. Am. Potato J. 1970, 47, 225–227. [Google Scholar] [CrossRef]
- Nachappa, P.; Fulladolsa, A.C.; Stenglein, M. Wild wild west: Emerging viruses and viroids of Hemp. Outlooks Pest Manag. 2020, 31, 175–179. [Google Scholar] [CrossRef]
- Punja, Z.K. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag. Sci. 2021, 77, 3857–3870. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Hataya, T.; Uyeda, I. A simple, rapid method of nucleic acid extraction without tissue homogenization for detecting viroids by hybridization and RT-PCR. J. Virol. Methods 1999, 77, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Luigi, M.; Faggioli, F. Development of quantitative real-time RT-PCR for the detection and quantification of Peach latent mosaic viroid. Eur. J. Plant Pathol. 2011, 130, 109–116. [Google Scholar] [CrossRef]
- Luigi, M.; Faggioli, F. Development of a quantitative real-time RT-PCR (RT-qPCR) for the detection of hop stunt viroid. Eur. J. Plant Pathol. 2013, 137, 231–235. [Google Scholar] [CrossRef]
- Malandraki, I.; Varveri, C.; Olmos, A.; Vassilakos, N. One-step multiplex quantitative RT-PCR for the simultaneous detection of viroids and phytoplasmas of pome fruit trees. J. Virol. Methods 2015, 213, 12–17. [Google Scholar] [CrossRef]
- Hagemann, M.H.; Born, U.; Sprich, E.; Seigner, L.; Oechsner, H.; Hülsemann, B.; Steinbrenner, J.N.; Wünsche, E.L. Fate of the hop latent viroid during ensiling of hop harvest residues. In Proceedings of the V International Humulus Symposium, Stuttgart Germany, 8–12 March 2021; Volume 1328, pp. 67–74. [Google Scholar] [CrossRef]
- Roslan, N.D.; Vadamalai, G.; Idris, A.S.; Ling, K.L.; Sundram, S. Comparison of real-time PCR, conventional PCR and RT-LAMP for the detection of Coconut Cadang-Cadang viroid variant in oil palm. J. Oil Palm Res. 2023, 35, 121–132. [Google Scholar] [CrossRef]
- Gardner, S.N.; Kuczmarski, T.A.; Vitalis, E.A.; Slezak, T.R. Limitations of TaqMan PCR for detecting divergent viral pathogens illustrated by hepatitis A, B, C, and E viruses and human immunodeficiency virus. J. Clin. Microbiol. 2003, 41, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Vitásková, E.; Černíková, L.; Křivda, V.; Jiřincová, H.; Sedlák, K.; Horníčková, J.; Havlíčková, M. Evaluation of TaqMan qPCR system integrating two identically labelled hydrolysis probes in single assay. Sci. Rep. 2017, 7, 41392. [Google Scholar] [CrossRef]
- Motoki, G.; Eiichi, H.; Atsuo, O.; Akio, N.; Ken-Ichi, H. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques 2009, 46, 167–172. [Google Scholar]
- Ali, Ç.; Ali, F.M.; Orkun, E.; Mehmet, Z.Y.; Göksel, Ö.; Vahdettin, Ç. The use of colorimetric loop-mediated isothermal amplification assay for naked-eye detection of bean common mosaic virus. Physiol. Mol. Plant Pathol. 2023, 125, 102017. [Google Scholar] [CrossRef]
- Alhamid, G.; Tombuloglu, H.; Al-Suhaimi, E. Development of loop-mediated isothermal amplification (LAMP) assays using five primers reduces the false-positive rate in COVID-19 diagnosis. Sci. Rep. 2023, 13, 5066. [Google Scholar] [CrossRef] [PubMed]
- Tangkanchanapas, P.; Höfte, M.; De Jonghe, K. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) designed forfast and sensitive on-site detection of Pepper chat fruit viroid (PCFVd). J. Virol. Methods 2018, 259, 81–91. [Google Scholar] [CrossRef]
- Warghane, A.; Misra, P.; Bhose, S.; Biswas, K.K.; Sharma, A.K.; Reddy, M.K.; Ghosh, D.K. Development of a simple and rapid reverse transcription-loop mediated isothermal amplification (RT-LAMP) assay for sensitive detection of Citrus tristeza virus. J. Virol. Methods 2017, 250, 6–10. [Google Scholar] [CrossRef]
- Zhao, L.M.; Li, G.; Gao, Y.; Zhu, Y.R.; Liu, J.; Zhu, X.P. Reverse transcription loop-mediated isothermal amplification assay for detecting tomato chlorosis virus. J. Virol. Methods 2015, 213, 93–97. [Google Scholar] [CrossRef]
- Kokane, A.D.; Kokane, S.B.; Warghane, A.J.; Gubyad, M.G.; Sharma, A.K.; Reddy, M.K.; Ghosh, D.K. A Rapid and Sensitive Reverse Transcription-Loop-Mediated Isothermal Amplification (RT-LAMP) Assay for the Detection of Indian Citrus Ringspot Virus. Plant Dis. 2021, 105, 1346–1355. [Google Scholar] [CrossRef]
- Barbosa, C.J.; Pina, J.A.; Pérez-Panadés, J.; Bernad, L.; Serra, P.; Navarro, L.; Duran-Vila, N. Mechanical transmission of citrus viroids. Plant Dis. 2005, 89, 749–754. [Google Scholar] [CrossRef]
- Mahaffee, W.F.; Pethybridge, S.J.; Gent, D.H. Compendium of Hop Diseases and Pests; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2009. [Google Scholar]
- Nabeshima, T.; Doi, M.; Hosokawa, M. Comparative analysis of Chrysanthemum stunt viroid accumulation and movement in two Chrysanthemum (Chrysanthemum morifolium) cultivars with differential susceptibility to the viroid infection. Front. Plant Sci. 2017, 8, 1940. [Google Scholar] [CrossRef]
- Palukaitis, P. Potato spindle tuber viroid: Investigation of the long-distance, intra-plant transport route. Virology 1987, 158, 239–241. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, M.L.; Shen, T.L. A mutual titer-enhancing relationship and similar localization patterns between Citrus exocortis viroid and Hop stunt viroid co-infecting two citrus cultivars. Virol. J. 2015, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Antignus, Y.; Lachman, O.; Pearlsman, M. Spread of Tomato apical stunt viroid (TASVd) in greenhouse tomato crops is associated with seed transmission and bumble bee activity. Plant Dis. 2007, 91, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in Phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qi, Y.; Xun, Y.; Owens, R.; Ding, B. Movement of potato spindle tuber viroid reveals regulatory points of phloem-mediated RNA traffic. Plant Physiol. 2002, 130, 138–146. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Sequence 5′–3′ |
|---|---|
| F3_HLVD_G2 | TAAGCTCGGCGCTCAAGA |
| B3_HLVD_G2 | CCCCTCTGGGGAATACACTA |
| FIP_HLVD_G2 | GTTCGCGTCCTGCGTGGAACCCGGGTAGTTTCCAACTCC |
| BIP_HLVD_G2 | GCACGAACTGGCGCTCGATCGTATGGTGGCAAGGGCTC |
| LF_HLVD_G2 | TCCTTCTTCACACCAGCC |
| LB_HLVD_G2 | CTCGCTCGAGTAGGTTTCC |
| Tissue Type | Initial Screening (5–6 wk) MFDetectTM | Avg ΔRn * | Set 2 (7–8 wk) MFDetectTM | Avg ΔRn ± SE | Set 2 (7–8 wk) TaqMan RT-qPCR | Avg CT ± SE | Set 3 (9–10 wk) MFDetectTM | Avg ΔRn ± SE | Set 3 (9–10 wk) TaqMan RT-qPCR | Avg CT ± SE |
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | 44 | 12.8 ± 0.97 | 44 | 24.2 ± 1.2 | 43 | 21.9 ± 0.35 | 44 | 38.1 ± 2.6 | 44 | 19.7 ± 0.5 |
| Petiole | nc | nc | 43 | 27.1 ± 2.0 | 43 | 21.3 ± 0.5 | 43 | 26.1 ± 2.2 | 43 | 21.8 ± 0.3 |
| Root | nc | nc | 41 | 23.8 ± 1.2 | 44 | 21.5 ± 0.3 | 43 | 19.7 ± 1.6 | 44 | 23.0 ± 0.3 |
| Total | 44 | 128 | 130 | 130 | 131 |
| Plant Type | Tissue | Set 1 (5–6 wk) MFDetectTM | Set 2 (7–8 wk) MFDetectTM | Set 3 (9–10 wk) MFDetectTM | MFDetectTM Consistency (Set1, Set2 and Set 3) |
|---|---|---|---|---|---|
| Infected | Leaf | 44 | 44 | 44 | 99.9% |
| Uninfected | Leaf | 6 | 5 | 5 | 83.3% |
| Inconsistent | Leaf | 0 | 1 | 1 | - |
| Total | 50 | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez i Marti, A.; Parungao, M.; Hollin, J.; Selimotic, B.; Farrar, G.; Seyler, T.; Anand, A.; Ahmad, R. A Novel, Precise and High-Throughput Technology for Viroid Detection in Cannabis (MFDetectTM). Viruses 2023, 15, 1487. https://doi.org/10.3390/v15071487
Fernandez i Marti A, Parungao M, Hollin J, Selimotic B, Farrar G, Seyler T, Anand A, Ahmad R. A Novel, Precise and High-Throughput Technology for Viroid Detection in Cannabis (MFDetectTM). Viruses. 2023; 15(7):1487. https://doi.org/10.3390/v15071487
Chicago/Turabian StyleFernandez i Marti, Angel, Marcus Parungao, Jonathan Hollin, Berin Selimotic, Graham Farrar, Tristan Seyler, Ajith Anand, and Riaz Ahmad. 2023. "A Novel, Precise and High-Throughput Technology for Viroid Detection in Cannabis (MFDetectTM)" Viruses 15, no. 7: 1487. https://doi.org/10.3390/v15071487
APA StyleFernandez i Marti, A., Parungao, M., Hollin, J., Selimotic, B., Farrar, G., Seyler, T., Anand, A., & Ahmad, R. (2023). A Novel, Precise and High-Throughput Technology for Viroid Detection in Cannabis (MFDetectTM). Viruses, 15(7), 1487. https://doi.org/10.3390/v15071487






