Mass-Spectrometric Evaluation of the African Swine Fever Virus-Induced Host Shutoff Using Dynamic Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Isolation and Cultivation of Monocyte-Derived Macrophages
2.3. PrV Growth Kinetics
2.4. SDS PAGE, Immunoblotting and Immunofluorescence Analysis
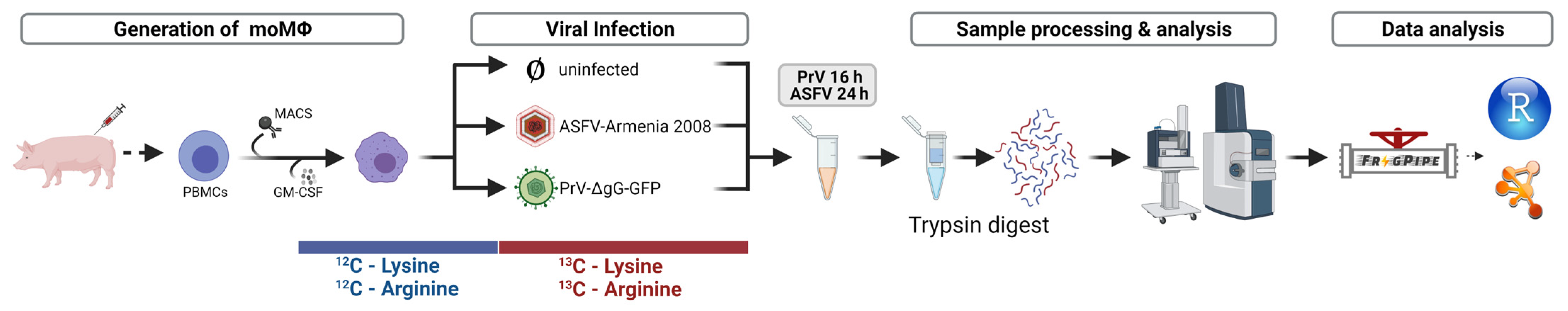
2.5. Generation of SILAC Samples for MS Analysis
2.6. MS-Analysis of SILAC Samples
2.7. Data Analysis
3. Results
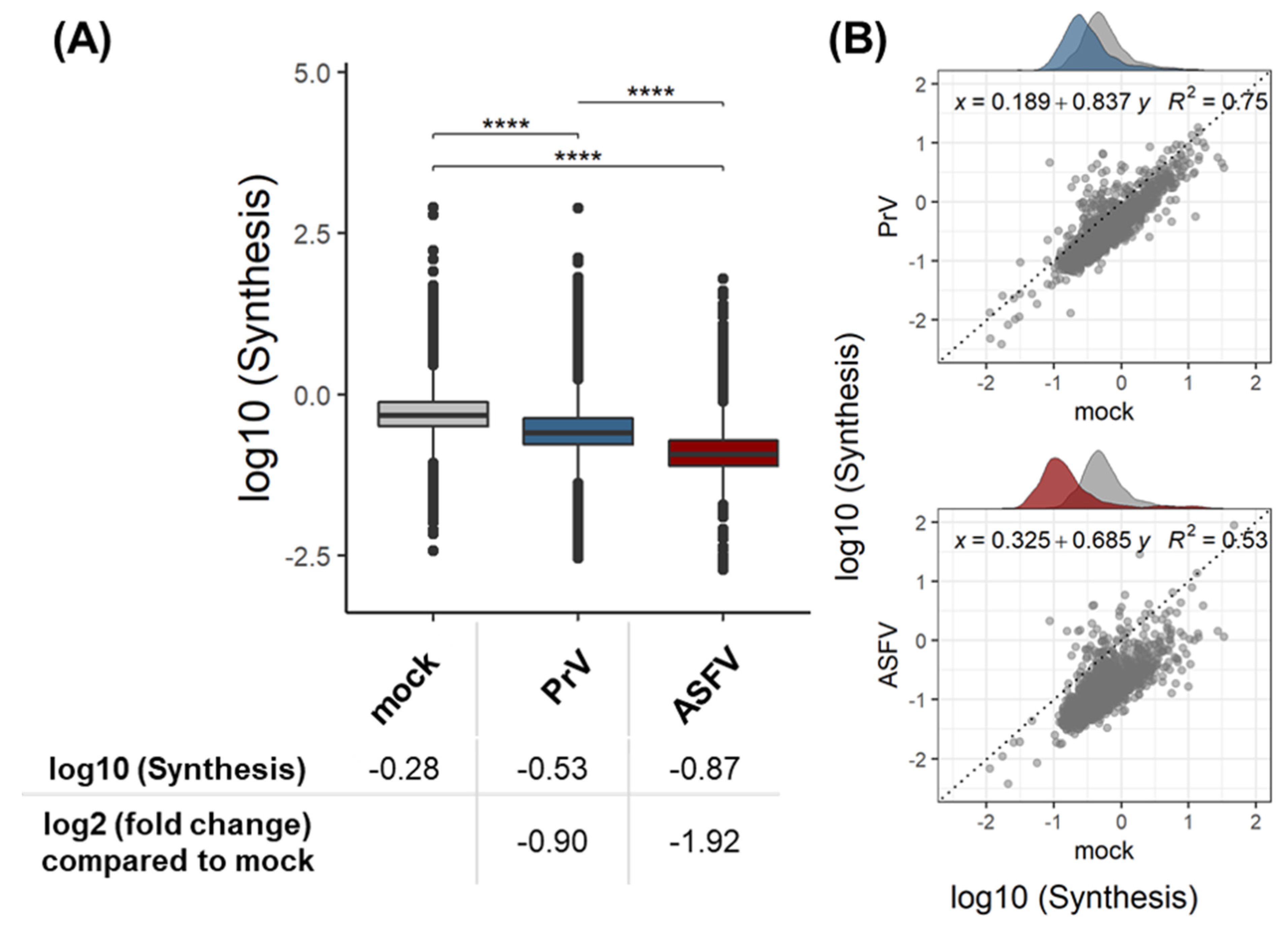
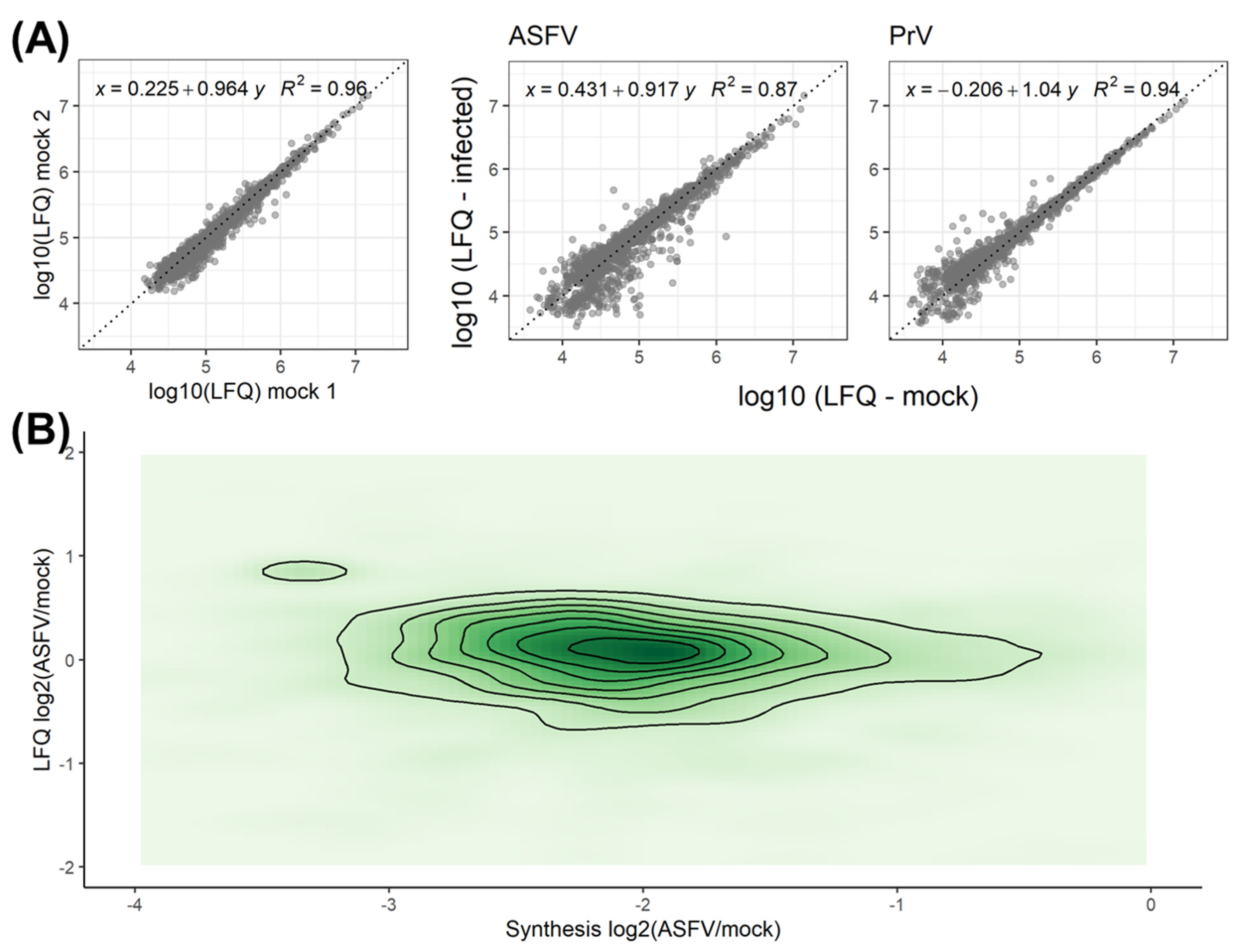
3.1. Validation of the Dynamic SILAC Approach for the Evaluation of Vhs Using PrV
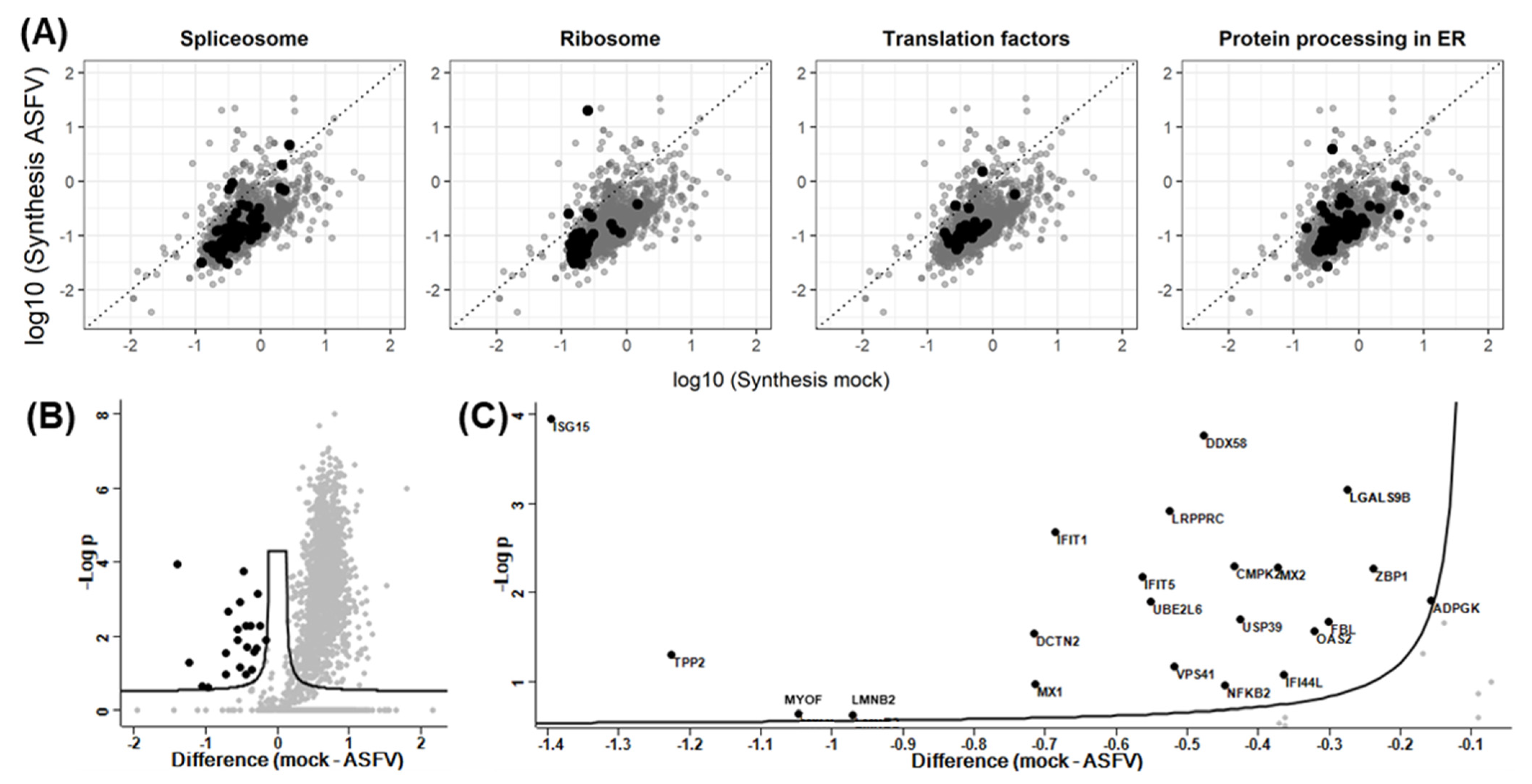
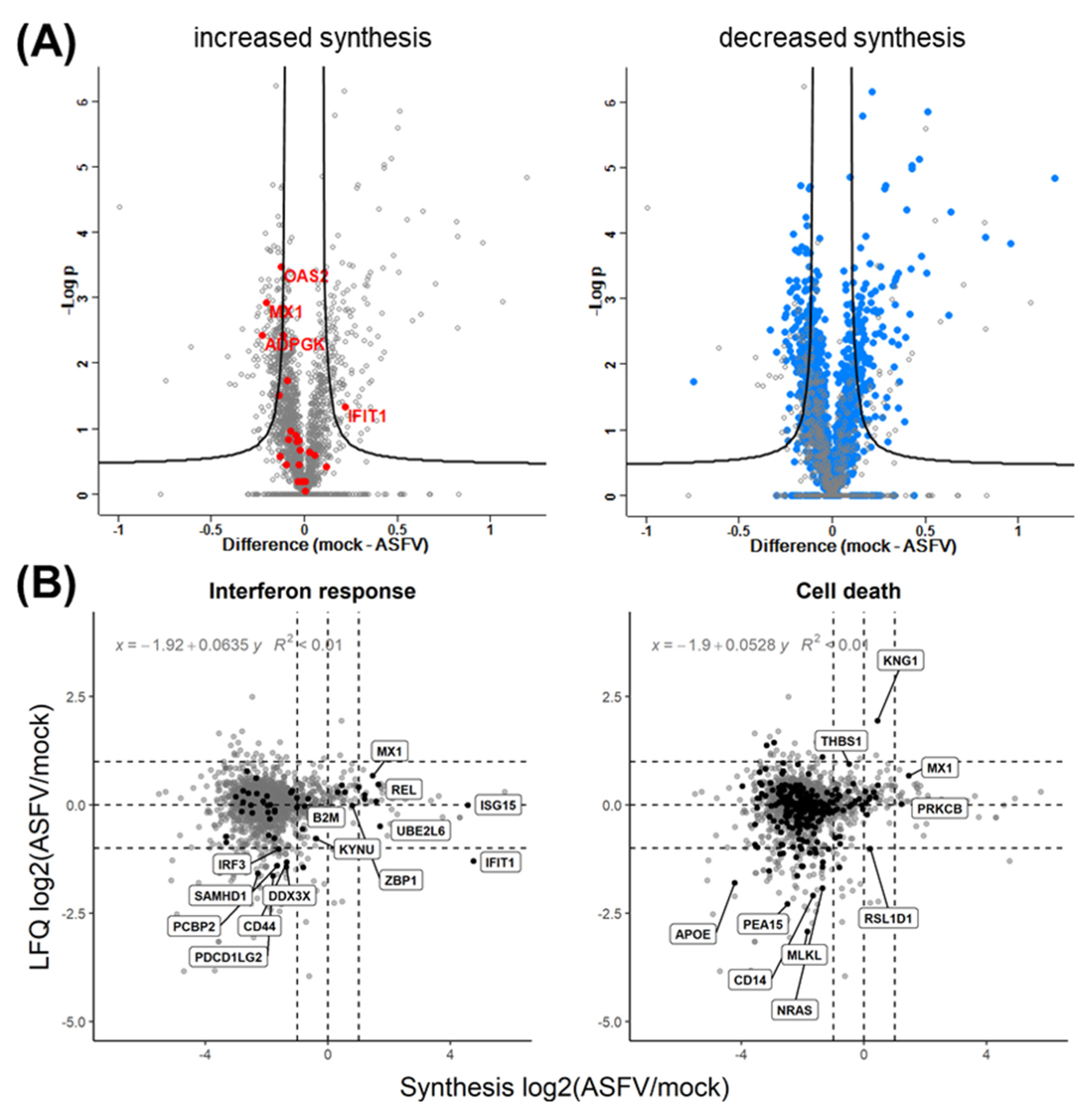
3.2. Quantitative Evaluation of the ASFV-Induced Vhs
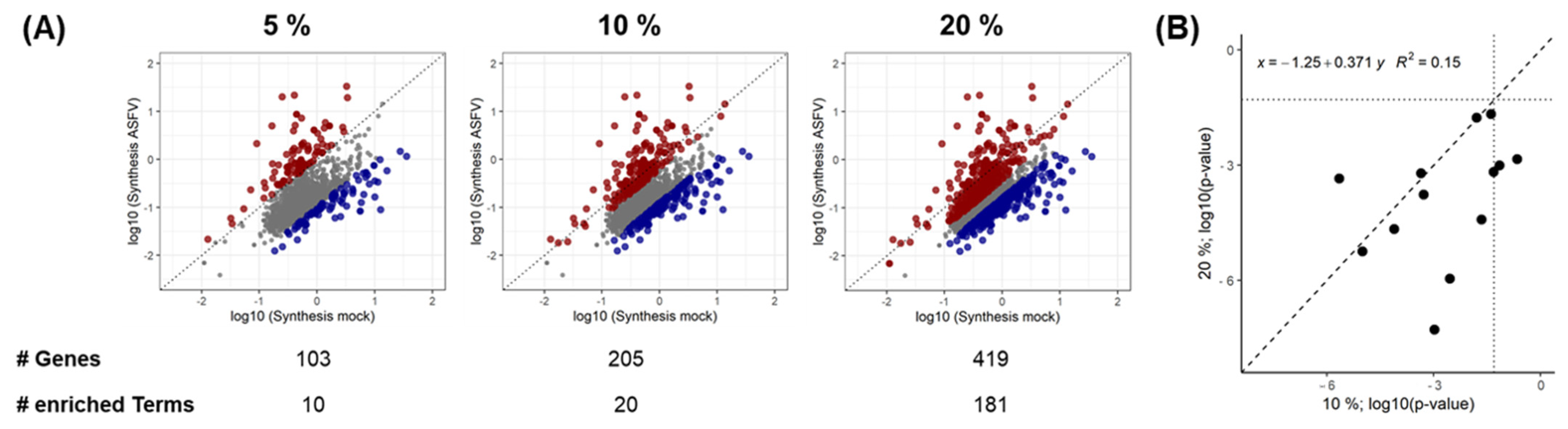
3.3. Effect of Synthesis Rates on Protein Abundance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stern-Ginossar, N.; Thompson, S.R.; Mathews, M.B.; Mohr, I. Translational Control in Virus-Infected Cells. Cold Spring Harb. Perspect. Biol. 2019, 11, a033001. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 2011, 9, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Dhungel, P.; Yang, Z. Going against the Tide: Selective Cellular Protein Synthesis during Virally Induced Host Shutoff. J. Virol. 2017, 91, e00071-17. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.C.; Link, A.J.; Graumann, J.; Tirrell, D.A.; Schuman, E.M. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl. Acad. Sci. USA 2006, 103, 9482–9487. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. MCP 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Doherty, M.K.; Hammond, D.E.; Clague, M.J.; Gaskell, S.J.; Beynon, R.J. Turnover of the human proteome: Determination of protein intracellular stability by dynamic SILAC. J. Proteome Res. 2009, 8, 104–112. [Google Scholar] [CrossRef]
- Schwanhausser, B.; Gossen, M.; Dittmar, G.; Selbach, M. Global analysis of cellular protein translation by pulsed SILAC. Proteomics 2009, 9, 205–209. [Google Scholar] [CrossRef]
- Ross, A.B.; Langer, J.D.; Jovanovic, M. Proteome Turnover in the Spotlight: Approaches, Applications, and Perspectives. Mol. Cell. Proteom. MCP 2021, 20, 100016. [Google Scholar] [CrossRef]
- Mathieson, T.; Franken, H.; Kosinski, J.; Kurzawa, N.; Zinn, N.; Sweetman, G.; Poeckel, D.; Ratnu, V.S.; Schramm, M.; Becher, I.; et al. Systematic analysis of protein turnover in primary cells. Nat. Commun. 2018, 9, 689. [Google Scholar] [CrossRef]
- Chen, X.; Wei, S.; Ji, Y.; Guo, X.; Yang, F. Quantitative proteomics using SILAC: Principles, applications, and developments. Proteomics 2015, 15, 3175–3192. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Q.-Q.; Zhou, M.-T.; Chen, X.; Guo, L. Protein turnover analysis in Salmonella Typhimurium during infection by dynamic SILAC, Topograph, and quantitative proteomics. J. Basic Microbiol. 2016, 56, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Kraft-Terry, S.D.; Engebretsen, I.L.; Bastola, D.K.; Fox, H.S.; Ciborowski, P.; Gendelman, H.E. Pulsed Stable Isotope Labeling of Amino Acids in Cell Culture Uncovers the Dynamic Interactions between HIV-1 and the Monocyte-Derived Macrophage. J. Proteome Res. 2011, 10, 2852–2862. [Google Scholar] [CrossRef]
- Bogdanow, B.; Wang, X.; Eichelbaum, K.; Sadewasser, A.; Husic, I.; Paki, K.; Budt, M.; Hergeselle, M.; Vetter, B.; Hou, J.; et al. The dynamic proteome of influenza A virus infection identifies M segment splicing as a host range determinant. Nat. Commun. 2019, 10, 5518. [Google Scholar] [CrossRef]
- Nightingale, K.; Lin, K.-M.; Ravenhill, B.J.; Davies, C.; Nobre, L.; Fielding, C.A.; Ruckova, E.; Fletcher-Etherington, A.; Soday, L.; Nichols, H.; et al. High-Definition Analysis of Host Protein Stability during Human Cytomegalovirus Infection Reveals Antiviral Factors and Viral Evasion Mechanisms. Cell Host Microbe 2018, 24, 447–460.e11. [Google Scholar] [CrossRef]
- Rivas, H.G.; Schmaling, S.K.; Gaglia, M.M. Shutoff of Host Gene Expression in Influenza A Virus and Herpesviruses: Similar Mechanisms and Common Themes. Viruses 2016, 8, 102. [Google Scholar] [CrossRef]
- Dhungel, P.; Cantu, F.M.; Molina, J.A.; Yang, Z. Vaccinia Virus as a Master of Host Shutoff Induction: Targeting Processes of the Central Dogma and Beyond. Pathogens 2020, 9, 400. [Google Scholar] [CrossRef]
- Parrish, S.; Hurchalla, M.; Liu, S.-W.; Moss, B. The African swine fever virus g5R protein possesses mRNA decapping activity. Virology 2009, 393, 177–182. [Google Scholar] [CrossRef]
- Dixon, L.K.; Twigg, S.R.; Baylis, S.A.; Vydelingum, S.; Bristow, C.; Hammond, J.M.; Smith, G.L. Nucleotide sequence of a 55 kbp region from the right end of the genome of a pathogenic African swine fever virus isolate (Malawi LIL20/1). J. Gen. Virol. 1994, 75 Pt 7, 1655–1684. [Google Scholar] [CrossRef]
- Parrish, S.; Moss, B. Characterization of a second vaccinia virus mRNA-decapping enzyme conserved in poxviruses. J. Virol. 2007, 81, 12973–12978. [Google Scholar] [CrossRef]
- Parrish, S.; Resch, W.; Moss, B. Vaccinia virus D10 protein has mRNA decapping activity, providing a mechanism for control of host and viral gene expression. Proc. Natl. Acad. Sci. USA 2007, 104, 2139–2144. [Google Scholar] [CrossRef]
- Aujeszky, A. Über Eine Neue Infektionskrankheit bei Haustieren; Zbl f Bakt Abt 1; 1902; pp. 353–357. [Google Scholar]
- Mettenleiter, T.C. Aujeszky’s Disease and the Development of the Marker/DIVA Vaccination Concept. Pathogens 2020, 9, 563. [Google Scholar] [CrossRef]
- Nauwynck, H.J.; Pensaert, M.B. Virus production and viral antigen expression in porcine blood monocytes inoculated with pseudorabies virus. Arch. Virol. 1994, 137, 69–79. [Google Scholar] [CrossRef]
- Nauwynck, H.J.; Pensaert, M.B. Interactions of Aujeszky’s disease virus and porcine blood mononuclear cells in vivo and in vitro. Acta Vet. Hung. 1994, 42, 301–308. [Google Scholar]
- Berthomme, H.; Jacquemont, B.; Epstein, A. The pseudorabies virus host-shutoff homolog gene: Nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology 1993, 193, 1028–1032. [Google Scholar] [CrossRef]
- Lin, H.W.; Chang, Y.Y.; Wong, M.L.; Lin, J.W.; Chang, T.J. Functional analysis of virion host shutoff protein of pseudorabies virus. Virology 2004, 324, 412–418. [Google Scholar] [CrossRef]
- Romero, N.; Wuerzberger-Davis, S.M.; Van Waesberghe, C.; Jansens, R.J.; Tishchenko, A.; Verhamme, R.; Miyamoto, S.; Favoreel, H.W. Pseudorabies Virus Infection Results in a Broad Inhibition of Host Gene Transcription. J. Virol. 2022, 96, e0071422. [Google Scholar] [CrossRef]
- Finnen, R.L.; Banfield, B.W. Alphaherpesvirus Subversion of Stress-Induced Translational Arrest. Viruses 2016, 8, 81. [Google Scholar] [CrossRef]
- Penrith, M.L.; Kivaria, F.M. One hundred years of African swine fever in Africa: Where have we been, where are we now, where are we going? Transbound. Emerg. Dis. 2022, 69, e1179–e1200. [Google Scholar] [CrossRef]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef]
- Guo, Z.; Zhuo, Y.; Li, K.; Niu, S.; Dai, H. Recent advances in cell homeostasis by African swine fever virus-host interactions. Res. Vet. Sci. 2021, 141, 4–13. [Google Scholar] [CrossRef]
- Alfonso, P.; Rivera, J.; Hernaez, B.; Alonso, C.; Escribano, J.M. Identification of cellular proteins modified in response to African swine fever virus infection by proteomics. Proteomics 2004, 4, 2037–2046. [Google Scholar] [CrossRef]
- Castello, A.; Quintas, A.; Sanchez, E.G.; Sabina, P.; Nogal, M.; Carrasco, L.; Revilla, Y. Regulation of host translational machinery by African swine fever virus. PLoS Pathog. 2009, 5, e1000562. [Google Scholar] [CrossRef]
- Sanchez, E.G.; Quintas, A.; Nogal, M.; Castello, A.; Revilla, Y. African swine fever virus controls the host transcription and cellular machinery of protein synthesis. Virus Res. 2013, 173, 58–75. [Google Scholar] [CrossRef]
- Alonso, C.; Miskin, J.; Hernáez, B.; Fernandez-Zapatero, P.; Soto, L.; Cantó, C.; Rodríguez-Crespo, I.; Dixon, L.; Escribano, J.M. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 2001, 75, 9819–9827. [Google Scholar] [CrossRef]
- Esteves, A.; Marques, M.I.; Costa, J.V. Two-dimensional analysis of African swine fever virus proteins and proteins induced in infected cells. Virology 1986, 152, 192–206. [Google Scholar] [CrossRef]
- Galindo, I.; Hernáez, B.; Muñoz-Moreno, R.; Cuesta-Geijo, M.A.; Dalmau-Mena, I.; Alonso, C. The ATF6 branch of unfolded protein response and apoptosis are activated to promote African swine fever virus infection. Cell Death Dis. 2012, 3, e341. [Google Scholar] [CrossRef]
- Zhang, F.; Hopwood, P.; Abrams, C.C.; Downing, A.; Murray, F.; Talbot, R.; Archibald, A.; Lowden, S.; Dixon, L.K. Macrophage transcriptional responses following in vitro infection with a highly virulent African swine fever virus isolate. J. Virol. 2006, 80, 10514–10521. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, C.; Yang, Y.; Xie, Z.; Ao, Q.; Lv, L.; Zhang, S.; Chen, H.; Hu, R.; Chen, H.; et al. A novel function of African Swine Fever Virus pE66L in inhibition of host translation by the PKR/eIF2alpha pathway. J. Virol. 2020, 95, e01872-20. [Google Scholar]
- Rivera, J.; Abrams, C.; Hernaez, B.; Alcazar, A.; Escribano, J.M.; Dixon, L.; Alonso, C. The MyD116 African swine fever virus homologue interacts with the catalytic subunit of protein phosphatase 1 and activates its phosphatase activity. J. Virol. 2007, 81, 2923–2929. [Google Scholar] [CrossRef]
- Zhang, F.; Moon, A.; Childs, K.; Goodbourn, S.; Dixon, L.K. The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2alpha and inhibits CHOP induction but is dispensable for these activities during virus infection. J. Virol. 2010, 84, 10681–10689. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.; Netherton, C.; Goatley, L.; Moon, A.; Goodbourn, S.; Dixon, L. Identification of residues within the African swine fever virus DP71L protein required for dephosphorylation of translation initiation factor eIF2alpha and inhibiting activation of pro-apoptotic CHOP. Virology 2017, 504, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.L.; Safrany, S.T.; Dixon, L.K.; Darzynkiewicz, E.; Stepinski, J.; Burke, R.; McLennan, A.G. The g5R (D250) gene of African swine fever virus encodes a Nudix hydrolase that preferentially degrades diphosphoinositol polyphosphates. J. Virol. 2002, 76, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Balaji, S.; Koonin, E.V.; Aravind, L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 2006, 117, 156–184. [Google Scholar] [CrossRef]
- Salas, M.L.; Kuznar, J.; Vinuela, E. Polyadenylation, methylation, and capping of the RNA synthesized in vitro by African swine fever virus. Virology 1981, 113, 484–491. [Google Scholar] [CrossRef]
- Simoes, M.; Rino, J.; Pinheiro, I.; Martins, C.; Ferreira, F. Alterations of Nuclear Architecture and Epigenetic Signatures during African Swine Fever Virus Infection. Viruses 2015, 7, 4978–4996. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Klupp, B.G.; Fuchs, W.; Kopp, M.; Teifke, J.P.; Mettenleiter, T.C. Influence of pseudorabies virus proteins on neuroinvasion and neurovirulence in mice. J. Virol. 2006, 80, 5571–5576. [Google Scholar] [CrossRef]
- Gabriel, C.; Blome, S.; Malogolovkin, A.; Parilov, S.; Kolbasov, D.; Teifke, J.P.; Beer, M. Characterization of African swine fever virus Caucasus isolate in European wild boars. Emerg. Infect. Dis. 2011, 17, 2342–2345. [Google Scholar] [CrossRef]
- Wöhnke, E.; Fuchs, W.; Hartmann, L.; Blohm, U.; Blome, S.; Mettenleiter, T.C.; Karger, A. Comparison of the Proteomes of Porcine Macrophages and a Stable Porcine Cell Line after Infection with African Swine Fever Virus. Viruses 2021, 13, 2198. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Haynes, S.E.; Teo, G.C.; Avtonomov, D.M.; Polasky, D.A.; Nesvizhskii, A.I. Fast Quantitative Analysis of timsTOF PASEF Data with MSFragger and IonQuant. Mol. Cell. Proteom. MCP 2020, 19, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Aken, B.L.; Achuthan, P.; Akanni, W.; Amode, M.R.; Bernsdorff, F.; Bhai, J.; Billis, K.; Carvalho-Silva, D.; Cummins, C.; Clapham, P.; et al. Ensembl 2017. Nucleic Acids Res. 2017, 45, D635–D642. [Google Scholar] [CrossRef]
- Klupp, B.G.; Hengartner, C.J.; Mettenleiter, T.C.; Enquist, L.W. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 2004, 78, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dowle, M.; Srinivasan, A. Data.Table: Extension of ‘Data.Frame’. R package Version 1.14.8. 2023. Available online: https://CRAN.R-project.org/package=data.table (accessed on 30 March 2023).
- Tweedie, S.; Braschi, B.; Gray, K.; Jones, T.E.M.; Seal, R.L.; Yates, B.; Bruford, E.A. Genenames.org: The HGNC and VGNC resources in 2021. Nucleic Acids Res. 2021, 49, D939–D946. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Vilo, J.; Peterson, H. gprofiler2-an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Research 2020, 9, ELIXIR-709. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; p. 260. [Google Scholar]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, W.; Huang, C.; Narayanan, K.; Lokugamage, K.G.; Makino, S. A two-pronged strategy to suppress host protein synthesis by SARS coronavirus Nsp1 protein. Nat. Struct. Mol. Biol. 2009, 16, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Y.; Gluck, A.; Nachshon, A.; Winkler, R.; Fisher, T.; Rozman, B.; Mizrahi, O.; Lubelsky, Y.; Zuckerman, B.; Slobodin, B.; et al. SARS-CoV-2 uses a multipronged strategy to impede host protein synthesis. Nature 2021, 594, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Karger, A.; Perez-Nunez, D.; Urquiza, J.; Hinojar, P.; Alonso, C.; Freitas, F.B.; Revilla, Y.; Le Potier, M.F.; Montoya, M. An Update on African Swine Fever Virology. Viruses 2019, 11, 864. [Google Scholar] [CrossRef]
- Keßler, C. Proteomanalyse der Partikel des Virus der Afrikanischen Schweinepest und ASFV Infizierter Säugetierzellen; University of Greifswald: Greifswald, Germany, 2019. [Google Scholar]
- Wohnke, E.; Cackett, G.; Werner, F.; Blome, S.; Mettenleiter, T.C.; Karger, A. Proteome Analysis of Swine Macrophages after Infection with Two Genotype II African Swine Fever Isolates of Different Pathogenicity. Viruses 2022, 14, 2140. [Google Scholar] [CrossRef]
- Urzainqui, A.; Tabares, E.; Carrasco, L. Proteins synthesized in African swine fever virus-infected cells analyzed by two-dimensional gel electrophoresis. Virology 1987, 160, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Goatley, L.C.; Nash, R.; Netherton, C.L. Primary Macrophage Culture from Porcine Blood and Lungs. Methods Mol. Biol. 2022, 2503, 63–72. [Google Scholar] [PubMed]
- Zhu, J.J.; Ramanathan, P.; Bishop, E.A.; O’Donnell, V.; Gladue, D.P.; Borca, M.V. Mechanisms of African swine fever virus pathogenesis and immune evasion inferred from gene expression changes in infected swine macrophages. PLoS ONE 2019, 14, e0223955. [Google Scholar] [CrossRef]
- Taddeo, B.; Esclatine, A.; Roizman, B. Post-transcriptional processing of cellular RNAs in herpes simplex virus-infected cells. Biochem. Soc. Trans. 2004, 32 Pt 5, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Tidu, A.; Janvier, A.; Schaeffer, L.; Sosnowski, P.; Kuhn, L.; Hammann, P.; Westhof, E.; Eriani, G.; Martin, F. The viral protein NSP1 acts as a ribosome gatekeeper for shutting down host translation and fostering SARS-CoV-2 translation. RNA 2020, 27, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Makino, S. Mechanisms of Coronavirus Nsp1-Mediated Control of Host and Viral Gene Expression. Cells 2021, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Quintas, A.; Perez-Nunez, D.; Sanchez, E.G.; Nogal, M.L.; Hentze, M.W.; Castello, A.; Revilla, Y. Characterization of the African swine fever virus decapping enzyme during infection. J. Virol. 2017, 91, e00990-17. [Google Scholar] [CrossRef] [PubMed]
- Cackett, G.; Portugal, R.; Matelska, D.; Dixon, L.; Werner, F. African Swine Fever Virus and host response—Transcriptome profiling of the Georgia 2007/1 strain and porcine macrophages. J. Virol. 2022, 96, e01939-21. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Salas, M.L.; Santaren, J.F. African swine fever virus-induced polypeptides in porcine alveolar macrophages and in Vero cells: Two-dimensional gel analysis. Proteomics 2001, 1, 1447–1456. [Google Scholar] [CrossRef]
- Fornasiero, E.F.; Savas, J.N. Determining and interpreting protein lifetimes in mammalian tissues. Trends Biochem. Sci. 2022, 48, 106–118. [Google Scholar] [CrossRef]
- McShane, E.; Sin, C.; Zauber, H.; Wells, J.N.; Donnelly, N.; Wang, X.; Hou, J.; Chen, W.; Storchova, Z.; Marsh, J.A.; et al. Kinetic Analysis of Protein Stability Reveals Age-Dependent Degradation. Cell 2016, 167, 803–815.e21. [Google Scholar] [CrossRef]
- Perng, Y.C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Afonso, C.L.; Piccone, M.E.; Zaffuto, K.M.; Neilan, J.; Kutish, G.F.; Lu, Z.; Balinsky, C.A.; Gibb, T.R.; Bean, T.J.; Zsak, L.; et al. African swine fever virus multigene family 360 and 530 genes affect host interferon response. J. Virol. 2004, 78, 1858–1864. [Google Scholar] [CrossRef]
- Jaing, C.; Rowland, R.R.R.; Allen, J.E.; Certoma, A.; Thissen, J.B.; Bingham, J.; Rowe, B.; White, J.R.; Wynne, J.W.; Johnson, D.; et al. Gene expression analysis of whole blood RNA from pigs infected with low and high pathogenic African swine fever viruses. Sci. Rep. 2017, 7, 10115. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.L.; Simpson, J.; Haller, O.; Wileman, T.E.; Takamatsu, H.H.; Monaghan, P.; Taylor, G. Inhibition of a large double-stranded DNA virus by MxA protein. J. Virol. 2009, 83, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Islam, M.; Nash, R.; Reis, A.L. African swine fever virus evasion of host defences. Virus Res. 2019, 266, 25–33. [Google Scholar] [CrossRef]
- Riera, E.; Pérez-Núñez, D.; García-Belmonte, R.; Miorin, L.; García-Sastre, A.; Revilla, Y. African Swine Fever Virus Induces STAT1 and STAT2 Degradation to Counteract IFN-I Signaling. Front. Microbiol. 2021, 12, 2467. [Google Scholar] [CrossRef]
- Wang, Z.; Ai, Q.; Huang, S.; Ou, Y.; Gao, Y.; Tong, T.; Fan, H. Immune Escape Mechanism and Vaccine Research Progress of African Swine Fever Virus. Vaccines 2022, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.L.; Salas, J.; Vinuela, E. Phosphorylation of African swine fever virus proteins in vitro and in vivo. Biochimie 1988, 70, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Baylis, S.A.; Banham, A.H.; Vydelingum, S.; Dixon, L.K.; Smith, G.L. African swine fever virus encodes a serine protein kinase which is packaged into virions. J. Virol. 1993, 67, 4549–4556. [Google Scholar] [CrossRef]
- Iyer, L.M.; Aravind, L.; Koonin, E.V. Common origin of four diverse families of large eukaryotic DNA viruses. J. Virol. 2001, 75, 11720–11734. [Google Scholar] [CrossRef]
- Freitas, F.B.; Frouco, G.; Martins, C.; Ferreira, F. African swine fever virus encodes for an E2-ubiquitin conjugating enzyme that is mono- and di-ubiquitinated and required for viral replication cycle. Sci. Rep. 2018, 8, 3471. [Google Scholar] [CrossRef]
- Barrado-Gil, L.; Del Puerto, A.; Munoz-Moreno, R.; Galindo, I.; Cuesta-Geijo, M.A.; Urquiza, J.; Nistal-Villan, E.; Maluquer de Motes, C.; Alonso, C. African Swine Fever Virus Ubiquitin-Conjugating Enzyme Interacts With Host Translation Machinery to Regulate the Host Protein Synthesis. Front. Microbiol. 2020, 11, 622907. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef] [PubMed]
| Term ID | Term Name | Increased Synthesis | Decreased Synthesis | ||||
|---|---|---|---|---|---|---|---|
| 5% | 10% | 20% | 5% | 10% | 20% | ||
| KEGG:00010 | Glycolysis/Gluconeogenesis | - | 3.20 × 10−2 | - | - | 1 | - |
| KEGG:00511 | Other glycan degradation | - | 1 | 1 | - | 2.88 × 10−3 | 1.12 × 10−6 |
| KEGG:00970 | Aminoacyl-tRNA biosynthesis | - | 2.95 × 10−2 | - | - | 1 | - |
| KEGG:01200 | Carbon metabolism | - | 2.25 × 10−2 | - | - | 1 | - |
| KEGG:04142 | Lysosome | - | 1 | 1 | - | 6.92 × 10−6 | 3.34 × 10−17 |
| KEGG:04612 | Antigen processing and presentation | - | 4.11 × 10−2 | 2.11 × 10−2 | - | 1 | 1 |
| KEGG:05162 | Measles | 2.99 × 10−2 | 1.55 × 10−2 | - | 1 | 1 | - |
| KEGG:05164 | Influenza A | 1.16 × 10−2 | 1.34 × 10−2 | - | 1 | 1 | - |
| KEGG:05169 | Epstein-Barr virus infection | 3.08 × 10−2 | 4.82 × 10−2 | 6.68 × 10−4 | 1 | 1 | 1 |
| KEGG:05171 | Coronavirus disease—COVID-19 | 7.85 × 10−3 | 2.22 × 10−2 | 3.84 × 10−5 | 1 | 1 | 1 |
| REAC:R-HSA-1169408 | ISG15 antiviral mechanism | 2.44 × 10−4 | 4.44 × 10−4 | 6.19 × 10−4 | 1 | 1 | 1 |
| REAC:R-HSA-1169410 | Antiviral mechanism by IFN-stimulated genes | 2.98 × 10−5 | 1.01 × 10−5 | 5.69 × 10−6 | 1 | 1 | 1 |
| REAC:R-HSA-1280215 | Cytokine Signaling in Immune system | 2.96 × 10−3 | 1.05 × 10−3 | 5.27 × 10−8 | 1 | 1 | 1 |
| REAC:R-HSA-168249 | Innate Immune System | - | 4.30 × 10−1 | 2.85 × 10−3 | - | 1.30 × 10−2 | 1.57 × 10−8 |
| REAC:R-HSA-168256 | Immune System | 3.69 × 10−2 | 7.77 × 10−5 | 3.27 × 10−7 | 1 | 1 | 4.30 × 10−5 |
| REAC:R-HSA-379716 | Cytosolic tRNA aminoacylation | - | 5.21 × 10−4 | 1.72 × 10−4 | - | 1 | 1 |
| REAC:R-HSA-379724 | tRNA Aminoacylation | - | 1.62 × 10−2 | 1.70 × 10−2 | - | 1 | 1 |
| REAC:R-HSA-6798695 | Neutrophil degranulation | - | 1.42 × 10−1 | 1.96 × 10−3 | - | 1.63 × 10−4 | 2.40 × 10−13 |
| REAC:R-HSA-909733 | Interferon alpha/beta signaling | 1.61 × 10−8 | 2.26 × 10−6 | 4.48 × 10−4 | 1 | 1 | 1 |
| REAC:R-HSA-913531 | Interferon Signaling | 2.77 × 10−8 | 3.12 × 10−7 | 3.17 × 10−9 | 1 | 1 | 1 |
| Term ID | Term Name | Decreased | Increased |
|---|---|---|---|
| KEGG:01100 | Metabolic pathways | ||
| KEGG:04142 | Lysosome | ||
| KEGG:05022 | Pathways of neurodegeneration—multiple diseases | ||
| REAC:R-HSA-168249 | Innate immune system | ||
| REAC:R-HSA-168256 | Immune system | ||
| REAC:R-HSA-1169410 | Antiviral mechanism by IFN-stimulated genes | ||
| REAC:R-HSA-1169408 | ISG15 antiviral mechanism | ||
| REAC:R-HSA-1280215 | Cytokine signaling in immune system | ||
| REAC:R-HSA-913531 | Interferon signaling | ||
| REAC:R-HSA-909733 | Interferon alpha/beta signaling | ||
| REAC:R-HSA-199991 REAC:R-HSA-5653656 | Membrane trafficking | ||
| REAC:R-HSA-199977 REAC:R-HSA-6807878 | ER to Golgi anterograde transport | ||
| REAC:R-HSA-8953854 REAC:R-HSA-194441 | Metabolism of RNA | ||
| REAC:R-HSA-72203 | Processing of capped intron-containing pre-mRNA | ||
| REAC:R-HSA-72163 REAC:R-HSA-72172 | mRNA splicing | ||
| REAC:R-HSA-379724 REAC:R-HSA-379716 | tRNA aminoacylation | ||
| REAC:R-HSA-6784531 | tRNA processing in the nucleus | ||
| REAC:R-HSA-72202 | Transport of mature transcript to cytoplasm | ||
| KEGG:03013 | Nucleocytoplasmic transport | ||
| REAC:R-HSA-9615933 REAC:R-HSA-3301854 | Nuclear Pore Complex (NPC) disassembly and reformation | ||
| REAC:R-HSA-4085377 REAC:R-HSA-4615885 REAC:R-HSA-3232142 | SUMOylation of proteins | ||
| REAC:R-HSA-5213460 REAC:R-HSA-9686347 | RIPK1-mediated regulated necrosis | ||
| REAC:R-HSA-5675482 REAC:R-HSA-5218859 | Regulation of necroptotic cell death | ||
| KEGG:04210 | Apoptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wöhnke, E.; Klupp, B.G.; Blome, S.; Mettenleiter, T.C.; Karger, A. Mass-Spectrometric Evaluation of the African Swine Fever Virus-Induced Host Shutoff Using Dynamic Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC). Viruses 2023, 15, 1283. https://doi.org/10.3390/v15061283
Wöhnke E, Klupp BG, Blome S, Mettenleiter TC, Karger A. Mass-Spectrometric Evaluation of the African Swine Fever Virus-Induced Host Shutoff Using Dynamic Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC). Viruses. 2023; 15(6):1283. https://doi.org/10.3390/v15061283
Chicago/Turabian StyleWöhnke, Elisabeth, Barbara G. Klupp, Sandra Blome, Thomas C. Mettenleiter, and Axel Karger. 2023. "Mass-Spectrometric Evaluation of the African Swine Fever Virus-Induced Host Shutoff Using Dynamic Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC)" Viruses 15, no. 6: 1283. https://doi.org/10.3390/v15061283
APA StyleWöhnke, E., Klupp, B. G., Blome, S., Mettenleiter, T. C., & Karger, A. (2023). Mass-Spectrometric Evaluation of the African Swine Fever Virus-Induced Host Shutoff Using Dynamic Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC). Viruses, 15(6), 1283. https://doi.org/10.3390/v15061283







