Abstract
At present, there are few studies on the epidemiology of diseases in wild Chinese white shrimp Penaeus chinensis. In order to enrich the epidemiological information of the World Organisation for Animal Health (WOAH)-listed and emerging diseases in wild P. chinensis, we collected a total of 37 wild P. chinensis from the Yellow Sea in the past three years and carried out molecular detection tests for eleven shrimp pathogens. The results showed that infectious hypodermal and hematopoietic necrosis virus (IHHNV), Decapod iridescent virus 1 (DIV1), yellow head virus genotype 8 (YHV-8), and oriental wenrivirus 1 (OWV1) could be detected in collected wild P. chinensis. Among them, the coexistence of IHHNV and DIV1 was confirmed using qPCR, PCR, and sequence analysis with pooled samples. The infection with YHV-8 and OWV1 in shrimp was studied using molecular diagnosis, phylogenetic analysis, and transmission electron microscopy. It is worth highlighting that this study revealed the high prevalence of coinfection with YHV-8 and OWV1 in wild P. chinensis populations and the transmission risk of these viruses between the wild and farmed P. chinensis populations. This study enriches the epidemiological information of WOAH-listed and emerging diseases in wild P. chinensis in the Yellow Sea and raises concerns about biosecurity issues related to wild shrimp resources.
1. Introduction
The seed industry is a fundamental core supporting modern aquaculture. Viral diseases have been considered a vast hazard to the shrimp farming industry worldwide [1]. Wild shrimp stocks were often used for hatching and breeding to maintain the genetic diversity of farmed populations [2]. Meanwhile, the national stock enhancement program for natural fisheries resources requires artificial hatching of postlarvae from captured broodstock of local shrimp species [3], in which the interaction between the wild aquatic animal populations and aquaculture systems was highly involved. However, the epidemiological status of diseases in wild shrimp stocks is often overlooked. Pathogens, such as infectious hypodermal and hematopoietic necrosis virus (IHHNV), white spot syndrome virus (WSSV), and yellow head virus (YHV), which have caused substantial economic losses to the global shrimp farming industry, have been detected in wild black tiger shrimp Penaeus monodon and Indian white shrimp P. indicus [4,5,6,7]. Notably, coinfection with multiple pathogens is easily detected in wild shrimp populations. The individual-level coexistence of multiple pathogens in a susceptible species usually refers to coinfection with the pathogens, which may be a synergistic or antagonistic interaction between pathogens [8,9,10]. Various pathogens might have spread to oceans of the world through international trade, ocean transportation, and ocean current of water [5,11]. Shrimp can act as an asymptomatic carrier when the viral load is low and may remain infected for a long time [12,13]. However, to our knowledge, there has been little study on the coinfection or coexistence of multiple pathogens in wild Chinese white shrimp P. chinensis.
There are eight genotypes in yellow head complex viruses (YHVs) in the genus Okavirus of the family Roniviridae, of which three were accepted as virus species by the International Committee on Taxonomy of Viruses (ICTV). Yellow head virus (YHV; virus species Yellow head virus) is assigned to genotype 1 (YHV-1) [14]. Gill-associated virus (GAV; virus species Gill-associated virus) is assigned to genotype 2 [14]. Yellow head virus genotype 8 (YHV-8; virus species Okavirus 1) is assigned to genotype 8 [14]. Other genotypes (YHV-3 to YHV-7) have not been recognized as virus species by ICTV due to a lack of genome sequence [14]. Infection with YHV-1, listed as a notifiable disease by the World Organisation for Animal Health (WOAH) [15], has caused significant disease in P. monodon in Asia [16,17,18,19]. Infection with YHV-8 has been found to cause disease in farmed P. chinensis and P. vannamei in China and South Korea in recent years [20,21,22,23]. Oriental wenrivirus 1 (OWV1), identified from farmed P. chinensis, is a novel bunyavirus in the genus Wenrivirus of the family Phenuiviridae with a negative-stranded and 4-segmental RNA virus [24]. Although the virus was found in moribund P. chinensis, its pathogenicity is still unclear [24]. IHHNV, a member of the genus Penstyldensovirus in the family Parvoviridae, was first identified from P. stylirostris in Hawaii, where mortality has been notifiable to be greater than 90% [25]. Infection with IHHNV, listed as a notifiable crustacean disease by the WOAH [15], can lead to growth retardation and runt deformity syndrome [26,27,28]. Moreover, IHHNV can also be vertically transmitted [26,29]. Decapod iridescent virus 1 (DIV1) is the first virus in the new genus Decapodiridovirus of the family Iridoviridae [30]. DIV1 can infect a variety of crustaceans, resulting in significant mortality in cultured decapods and causing huge economic losses to the shrimp industry [31,32,33].
In facing the limited epidemiological information for WOAH-listed and emerging diseases in wild P. chinensis populations, this study collected wild P. chinensis individuals captured from the Yellow Sea for three consecutive years. Using molecular diagnostic methods, we confirmed the existence of IHHNV and DIV1 and coinfection with YHV-8 and OWV1 in wild P. chinensis populations. Consequently, the study provides epidemiological information for consideration of the biosecurity strategy related to wild P. chinensis resources as broodstock sources in hatching for the farmed shrimp larvae and the stock enhancement of natural resources.
2. Materials and Methods
2.1. Sample Information
Chinese white shrimp P. chinensis individuals of about 18–21 cm were sampled in the Yellow Sea, nine shrimp in November 2020, eight in November 2021 and twenty shrimp in March 2022. We pretreated the selected shrimp individuals on ice in advance, and all efforts were made to minimize the suffering of animals accordingly.
2.2. DNA and RNA Extraction
The cephalothorax was snap-frozen in liquid nitrogen to extract DNA and RNA and then used for routine pathogen detection. Total DNA and RNA were extracted from 20–30 mg individual cephalothorax tissue of P. chinensis by TIANamp Marine Animal DNA Kit and RNAprep pure Tissue Kit (TIANGEN Biotech, Beijing, China), respectively, according to the manufacturer’s instructions. The concentration and quality of DNA and RNA were measured by NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA).
2.3. Pathogens Testing with Pooled Samples
We mixed DNA or RNA extracted from different batches of shrimp and detected seven pathogens, including WSSV, DIV1, IHHNV, Enterocytozoon hepatopenaei (EHP), covert mortality nodavirus (CMNV), infectious myonecrosis virus (IMNV), and Taura syndrome virus (TSV). Both PCR and qPCR methods targeting different sequence locations of each pathogen published in papers or recommended by the WOAH Aquatic Manual were used for molecular detection of the above seven pathogens [15,19,34,35,36,37,38,39,40,41,42,43,44,45,46,47].
2.4. YHVs and OWV1 Testing with Individual Samples
In order to explore whether there was coinfection with YHVs and OWV1 in wild P. chinensis, we detected GAV, YHV-1, YHV-8, and other YHVs by RT-PCR and OWV1 by RT-qPCR in every sampled P. chinensis [24,48]. When the YHVs test result is positive, the products need to be sent for Sanger sequencing, then Nucleotide-BLAST in the National Center for Biotechnology Information (NCBI) database or phylogenetic analysis to determine which genotype of YHVs. A positive control, a negative control, and a blank control were set for the PCR detection, and a positive control and a blank control were set for the qPCR detection. All primer information used in this study is listed in Table 1.

Table 1.
Primer sequences were used in this study.
2.5. Transmission Electron Microscopy
For transmission electron microscopy (TEM), samples were prepared in two ways: purifying viruses from gills for negative staining and embedding gills and lymphoid organs for ultrathin sections.
Small pieces (~1 mm3) of the lymphoid organ and gills were sampled and fixed in the TEM fixative (2% paraformaldehyde, 2.5% glutaraldehyde, 160 mM NaCl, and 4 mM CaCl2 in 200 mM PBS, pH 7.2). Ultrathin sections were cut, mounted on collodion-coated grids, and stained with aqueous uranyl acetate/lead citrate using standard procedures as we previously published [24].
2.6. Virus Purification
Fifteen grams of P. chinensis gill filaments from individuals collected in 2022 were chopped and added with 100 mL of SM buffer (50 mM Tris-HCl, 10 mM MgSO4, 100 mM NaCl, pH 7.5) with 0.5 mM 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) (Solarbio, Beijing, China). The tissue was homogenized by a homogenizer in an ice bath at a speed of 10,000 rpm for 5 s. These steps were repeated several times (> 3) until a uniform tissue homogenate was obtained. The homogenate was centrifuged at 1400 g for 15 min at 4 °C (CR21GIII, Hitachi, Tokyo, Japan). A 20 mL of SM buffer was added to the pellet and homogenized in an ice bath at 10,000 rpm for 10 s, followed by centrifugation at 6000 g for 15 min at 4 °C. These two supernatants obtained in the above steps were combined, filtered through a 38 μm mesh, and the filtrate was centrifuged at 10,000 g for 25 min at 4 °C. The final supernatant was again filtered through a 38 μm mesh, mixed with an equal volume of SM buffer, and centrifuged at 40,000 g for 2 h at 4 °C (CP100WX; Hitachi, Tokyo, Japan) to pellet viral particles [49]. Finally, 500 μL of SM buffer was added to the pellet to suspend it.
The supernatant and the pellet suspension were dropped on grids, negatively stained with 2% phosphotungstic acid (pH 6.5, Solarbio, Beijing, China), and observed under a TEM (HT7700, Hitachi, Tokyo, Japan) at 80 kV.
2.7. Phylogenetic Analyses
For the genus Okavirus, the approach proposed by Mohr was employed using primers YH30 m/31 m to get a partial sequence of the open reading frame 1b (ORF1b) region to clarify the genotype [19]. For the genus Wenrivirus, we constructed four pairs of primers (Table 2) with overlapping regions for the virus and obtained the full length of the RNA-directed RNA polymerase (RdRp) of the virus via Geneious version 2022.1.1 (Biomatters Ltd., Auckland, New Zealand) [50] splicing to obtain the sequence of the RdRp region of OWV1 in the sample. Then we used Geneious version 2022.1.1 to translate the obtained nucleotide sequences into amino acid sequences. For determining the evolutionary relationship of Wenrivirus in the samples collected in this paper, the entire RdRp region of Wenrivirus and some Phenuiviridae viruses were selected (Refer to another published article for virus names, etc. [24]). Multiple sequence alignments of Okavirus-related nucleic acid sequences were performed using the MUSCLE program in the MEGA version 11.0.10 software [51], and “Find Best DNA Models” were used to determine the most suitable models for Okavirus. Then based on the lowest Bayesian information criterion score, the Tamura-Nei model with discrete gamma distribution (TN93+G) was determined as the best model for Okavirus. Then based on the lowest Bayesian information criterion score, the LG with frequencies model gamma distributed with invariant sites (LG+G+I+F) was determined as the best model for Wenrivirus. The maximum likelihood phylogenetic tree was constructed using the best model for Okavirus and Wenrivirus. Phylogenetic testing was performed using the bootstrap method with 1000 replicates. All relevant sequences have been submitted to NCBI.

Table 2.
Testing results for seven pathogens with the pooled samples.
3. Results
3.1. Testing Results of Pooled Samples
To understand the pathogen information in wild P. chinensis populations, we detected the pooled samples for seven known pathogens, including DIV1, IHHNV, CMNV, EHP, WSSV, IMNV, and TSV. The PCR and qPCR results (Table 2) show that all shrimp samples collected in three years were negative for five pathogens, including CMNV, EHP, WSSV, IMNV, and TSV. The samples collected in 2020 showed DIV1 and IHHNV positive, and the samples collected in 2022 had IHHNV positive as well.
3.2. Testing Results of Individual Samples for YHVs and OWV1
All individual samples collected in three years were positive for two pathogens, including OWV1 and YHVs (Figure S1, Table 3). The Ct value of the individual OWV1 detection in three years changed from 9.2 ± 0.1 to 35.0 ± 1.7 cycles, in which the batch average Ct value of OWV1 was 19.2 ± 7.6, 16.3 ± 6.6, and 25.0 ± 6.7 in 2020, 2021, and 2022 (with significant differences to 2020 and 2021), respectively. After sequencing and BLAST in the NCBI database, we confirm that all YHVs positives are YHV-8.

Table 3.
Oriental wenrivirus 1 (OWV1) and yellow head complex viruses (YHVs) testing results of each sample.
3.3. TEM Examination
TEM performed with ultrathin sections of lymphoid organs and gills of P. chinensis showed spherical to oval virus particles (80 nm–115 nm) of OWV1 in the cytoplasm of the gill tissue (Figure 1A,B). However, no YHVs particle was observed in the gill and lymphoid organ tissues. Alternatively, the supernatant and pellet suspension from virus purification were observed with TEM. The results showed a few rod-like structures in the supernatant, with multiple linear structures but no envelope similar to our previous observation [49] (Figure 1C,D).
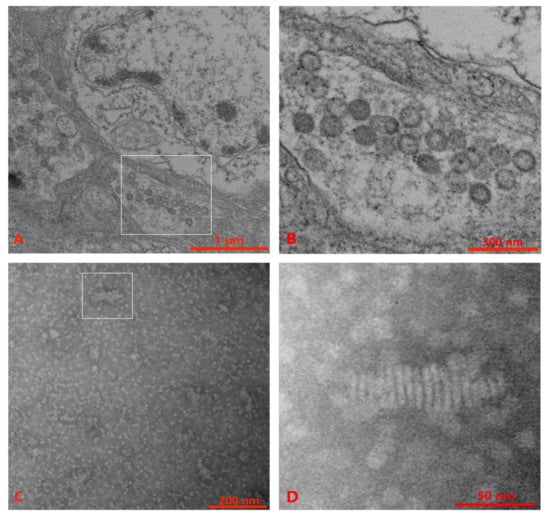
Figure 1.
Transmission electron microscopy (TEM) observations of wild P. chinensis. (A) Gill tissue with sample number 20211126007. (B) High magnification of the gill tissue section reveals abundant oriental wenrivirus 1 (OWV1) virions. (C) The supernatant was obtained after virus purification. (D) High magnification shows the obvious rod-like and multiple-linear structure with no envelope. Scale bars = (A) 1 μm, (B) 300 nm, (C) 200 nm, (D) 50 nm.
3.4. Homology and Phylogenetic Analyses
To unambiguously identify the genotype of YHVs, we performed a phylogenetic analysis of the samples collected in this study. The products of the YHV ORF1b region obtained were sent for sequencing. After NCBI Nucleotide-BLAST (Table 4), it shows that the ORF1b part of the YHV isolates collected in this study had a 98.32–99.24% similarity to the corresponding segment of YHV-8 20120706 (NC_048215.1). Furthermore, the results of the phylogenetic analysis show that YHVs sequenced in this study cluster with YHV-8 previously isolated from farmed P. chinensis populations [22] and are separated from the other seven genotypes (Figure 2). Therefore, YHV-8 was identified from the wild P. chinensis collected in this study.

Table 4.
Alignment of the open reading frame 1b (ORF1b) nucleotide sequence of yellow head virus genotype 8 (YHV-8) isolated from wild P. chinensis with the corresponding sequence of YHV-8 20120706 previously isolated from farmed P. chinensis.
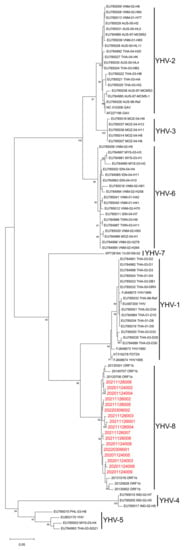
Figure 2.
Phylogenetic tree analysis of partial open reading frame 1b (ORF1b) region of yellow head complex viruses (YHVs). The maximum likelihood evolutionary trees of YHVs were constructed using MEGA version 11.0.10 software. The red font represents the samples collected in this experiment. All reference sequences are downloaded from GenBank. Bootstrap values were calculated with 1000 replicates of the alignment. Percentage bootstrap values (1000 replicates) >80% are shown. The partial nucleotide sequences of ORF1b from 20201124002, 20201124003, 20201124004, 20201124005, 20201124006, 20201124008, 20201124009, 20211126001, 20211126002, 20211126003, 20211126004, 20211126005, 20211126006, 20211126007, 20211126008, 20220309001 and 20220309002 have been submitted to the GenBank databases under accession number OP902176, OP902177, OP902178, OP902179, OP902180, OP902181, OP902182, OP902185, OP902186, OP902187, OP902200, OP902201, OP902202, OP902203, OP902204, OP902183 and OP902184, respectively.
The obtained product of OWV1 RdRp region was sent for sequencing and translated into the amino acid sequence using Geneious version 2022.1.1. After NCBI Protein-BLAST (Table 5), it can be seen that the RdRp of the OWV1 isolates collected in this study had a 99.14%–99.57% similarity to the corresponding segment of OWV1 (QHW05228.1). Moreover, the phylogenetic analysis results show that OWV1 isolates of wild P. chinensis cluster with the OWV1 reference sequence previously isolated from farmed P. chinensis [24] and then gather with Wenzhou shrimp virus 1 (WzSV-1) and Mourilyan virus (MoV) into the genus Wenrivirus (Figure 3).

Table 5.
Alignment of the RNA-directed RNA polymerase (RdRp) amino acid sequence of oriental wenrivirus 1 (OWV1) isolated from wild P. chinensis with the corresponding sequence of OWV1 previously isolated from farmed P. chinensis.
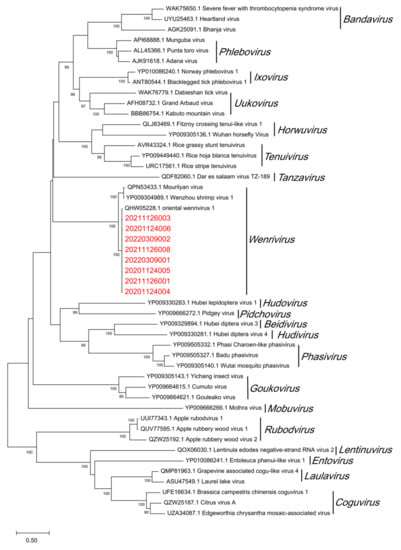
Figure 3.
Phylogenetic tree of the RNA-directed RNA polymerase (RdRp) protein sequences of oriental wenrivirus 1 (OWV1) and some viruses in the family Phenuiviridae. The maximum likelihood evolutionary trees of Phenuiviridae were constructed using MEGA version 11.0.10 software. The red font represents the samples collected in this experiment. All reference sequences are downloaded from GenBank. Bootstrap values were calculated with 1000 replicates of the alignment. Percentage bootstrap values (1000 replicates) >80% are shown. The partial nucleotide sequences of RdRp from 20201124004, 20201124005, 20201124006, 20211126001, 20211126003, 20211126008, 20220309001 and 20220309002 have been submitted to the GenBank databases under accession number OP902199, OP902198, OP902197, OP902196, OP902194, OP902204, OP902189 and OP902188, respectively.
4. Discussion
Shrimp diseases have become an important factor hindering the shrimp industry’s green development of the shrimp farming industry [52]. This study revealed that multiple pathogens, including YHV-8, OWV1, DIV1, and IHHNV, can be detected in wild P. chinensis populations in the Yellow Sea. Furthermore, we observed coinfection with YHV-8 and OWV1 in all P. chinensis individuals by molecular diagnosis, phylogenetic analysis, and TEM. As the P. chinensis farming industry still largely relays on the wild P. chinensis broodstock [3], the consistently high prevalence of YHV-8 and the existence of DIV1 in wild P. chinensis reveal significant risks in the shrimp farming industry and the stock enhancement program for fishery resources of P. chinensis in East Asia.
Unlike human health, surveillance for aquatic animal health usually emphasizes the population-based approach. Detection of a lower prevalence in a larger population requires a larger sample size [53], which requires more tests. Therefore, pooling individual samples before a diagnostic test is a commonly used strategy. WOAH standard recommends a pooling rate of less than 5:1 [15], which may still result in a large number of tests for a large population. Our previous study using the TaqMan-qPCR method to evaluate the pooling rate revealed that a 50:1 pooling rate could have a similar diagnostic sensitivity to the pooling rate of 5:1 [54]. All the pooling modes from 5:1 to 150:1 have good diagnostic specificity. The tests of our present study using a pooling rate of no more than 20:1 should not significantly impact the diagnostic specificity and sensitivity.
Compared with the farmed samples, the accessibility of captured wild P. chinensis is much more difficult due to the declined fishery resources and uncertainty of seasonal migration caused by climate change [55,56]. Meanwhile, weak and diseased shrimp individuals may much easier be predated by carnivorous fishes or sink to the deep bottom. In facing these challenges, we used two different PCR methods targeting two sequence locations for each of the seven pathogens except YHVs and OWV1. This strategy can verify false results due to low specificity, low sensitivity, and contamination in sample preparation or PCR methods. Nevertheless, the PCR and qPCR results for each pathogen were highly coincident, which indicated that the molecular results were reliable.
Unlike the research on the viral diseases of farmed crustaceans, the reports on the spread of wild crustacean viruses and coinfection with multi-pathogen are still very limited [7]. Spann et al. [57] reported that coinfection with MoV and GAV is very common in diseased P. monodon. Notably, YHV-8 and GAV belong to Okavirus, and MoV and OWV1 belong to Wenrivirus. The similarity of the virus taxonomy and the coinfection in Spann et al.’s and our results raise the interesting issue of whether the Okavirus and Wenrivirus have a synergistic mechanism in the concomitance of coinfection. Teixeira-Lopes et al. [58] revealed that viral coinfection could regulate the viral load in the host, and there may be a negative correlation between the two viruses when they coexist. On the other hand, a number of studies reported that shrimp previously infected with IHHNV gained resistance to infection with WSSV and significantly reduced the mortality of shrimp. However, the survival rate of shrimp infected with TSV and YHV was significantly higher than that of shrimp infected only with YHV [10,59]. Further study on interactions between concomitant YHV-8 and OWV1 in P. chinensis populations may help to discover the deep infection mechanism of shrimp viruses.
The confirmatory diagnosis of a suspected case of infection with a specific pathogen is generally based on meeting the criterium of two or more independent tests in the WOAH Manual [15]. The diagnosis of infection with DIV1 also follows the same principle [60]. With qPCR and nested PCR, this study confirmed that wild P. chinensis in the Yellow Sea has been infected with IHHNV and DIV1. IHHNV natively originated from the Western Hemisphere. It was first detected from farmed P. vannamei in 2001 in China [61]. P. chinensis has not been listed as a susceptible species of IHHNV [15]. However, IHHNV positives have been detected from farmed P. chinensis in the National Target Surveillance Program of China in 2019 [62]. IHHNV detected in wild populations of P. chinensis implies the transmission risk of viruses between farmed and wild shrimp populations. DIV1 was detected in the wild P. monodon captured from the northeastern Indian Ocean [6]. DIV1 detected from wild P. chinensis indicated a broader virus spread in the wild. We did not find viral particles like DIV1 or IHHNV with TEM, which may be related to the selected tissues, centrifugal force, and low viral load. After subsequent virus purification, a large number of suspected nucleocapsid complex structures, likely YHV-8 virions, were observed in the supernatant. However, no complete complex structure was found, which might be caused by a large centrifugal force [49].
Okavirus isolates from captured wild P. chinensis cluster with YHV-8 previously identified from farmed P. chinensis [22] and separated from the other seven genotypes in the phylogenetic analysis. Similarly, the phylogenetic analysis of OWV1 showed that OWV1 isolates from wild and farmed shrimp [24] cluster together. These phylogenetic analyses provide information for tracing the source of YHV-8 and OWV1 infection in wild and farmed P. chinensis. Conclusively, the sequence similarity of the isolates from farmed and wild P. chinensis for both viruses implies the transmission risk of viruses between farmed and wild shrimp populations.
This study has detected multiple pathogens in wild P. chinensis, which reminds pathogenic risks in wild shrimp populations are nonnegligible. Wild shrimp used for broodstock must be strictly inspected and quarantined to select specific pathogen-free stocks [4,29,63]. Diseased and virus-carrying wild shrimp should be strictly prevented from entering the aquaculture systems. The national surveillance plan targeting specific pathogens may need to be extended to wild shrimp [64]. On the other hand, hatcheries for stock enhancement should implement strictly high-quality biosecurity measures to ensure pathogens from aquaculture systems will not be released into wild populations [3]. Implementation of biosecurity measures is critical not only for preventing disease risks in shrimp farms but also for securing the ecosystem of wild shrimp populations.
5. Conclusions
This study is the first to report the coexistence of multiple viruses, including DIV1, IHHNV, YHV-8, and OWV1. Notably, the results reveal a consistently high prevalence of coinfection with YHV-8 and OWV1 in wild P. chinensis populations and the transmission risk of viruses between wild and farmed populations. The findings imply that we should pay attention to the epidemiology of diseases in wild P. chinensis, implement disease surveillance on wild shrimp, and introduce biosecurity policies and measures to prevent disease risks both in shrimp farms and hatcheries for stock enhancement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15020361/s1, Figure S1: Agarose gel electrophoresis of positive pathogens by PCR detection. Figure S2: Agarose gel electrophoresis of negative pathogens by PCR detection.
Author Contributions
Conceptualization, J.Q., X.D. and J.H.; formal analysis, J.Q. and F.Z.; funding acquisition, X.D. and J.H.; sampling, J.Q., F.M. and G.W.; methodology, J.Q., X.D., G.W. and J.H.; sample testing, J.Q., Y.C. and F.M.; project administration, X.D., J.H. and J.Q.; resources, J.Q., F.M. and C.L.; supervision, X.D. and J.H.; writing—original draft, J.Q. and X.D.; revision, X.D., G.W., Y.C. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shinan District Science and Technology Foundation (Qingdao) (2022-2-027-ZH), Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS (20603022022005), Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO.2020TD39), and the earmarked fund for China Agriculture Research System (CARS-48).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Qiu, L.; Chen, X.; Zhao, R.H.; Li, C.; Gao, W.; Zhang, Q.L.; Huang, J. Description of a natural infection with decapod iridescent virus 1 in farmed giant freshwater prawn, Macrobrachium rosenbergii. Viruses 2019, 11, 354. [Google Scholar] [CrossRef]
- Benzie, J.A.H. Use and exchange of genetic resources of penaeid shrimps for food and aquaculture. Rev. Aquac. 2009, 1, 232–250. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, Z.; Deng, J.; Ye, Y. Stock enhancement and translocation of the shrimp Penaeus chinensis in China. Fish. Res. 2006, 80, 67–79. [Google Scholar] [CrossRef]
- Arbon, P.M.; Condon, K.; Andrade Martinez, M.; Jerry, D.R. Molecular detection of six viral pathogens from Australian wild sourced giant black tiger shrimp (Penaeus monodon) broodstock. Aquaculture 2022, 548, 737651. [Google Scholar] [CrossRef]
- Gholamhosseini, A.; Mohammadi, A.; Akbari, S.; Banaee, M. Molecular, histopathologic and electron microscopic analysis of white spot syndrome virus in wild shrimp (Fenneropenaeus indicus) in the coastal waters of Iran. Arch. Virol. 2020, 165, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Srisala, J.; Sanguanrut, P.; Thaiue, D.; Laiphrom, S.; Siriwattano, J.; Khudet, J.; Thaiue, D.; Powtongsook, S.; Flegel, T.W.; Sritunyalucksana, K. Infectious myonecrosis virus (IMNV) and decapod iridescent virus 1 (DIV1) detected in captured, wild Penaeus monodon from the Indian Ocean. Aquaculture 2021, 545, 737262. [Google Scholar] [CrossRef]
- Hamano, K.; Maeno, Y.; Klomkling, S.; Aue-Umneoy, D.; Tsutsui, I. Presence of viral pathogens amongst wild Penaeus monodon in Thailand. Jpn. Agric. Res. Q. 2017, 51, 191–197. [Google Scholar] [CrossRef]
- Kotob, M.H.; Gorgoglione, B.; Kumar, G.; Abdelzaher, M.; Saleh, M.; El-Matbouli, M. The impact of Tetracapsuloides bryosalmonae and Myxobolus cerebralis co-infections on pathology in rainbow trout. Parasites Vectors 2017, 10, 442. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Durand, S.V.; White, B.L.; Redman, R.M.; Mohney, L.L.; Lightner, D.V. Induced resistance to white spot syndrome virus infection in Penaeus stylirostris through pre-infection with infectious hypodermal and hematopoietic necrosis virus—A preliminary study. Aquaculture 2003, 216, 19–29. [Google Scholar] [CrossRef]
- Kotob, M.H.; Kumar, G.; Saleh, M.; Gorgoglione, B.; Abdelzaher, M.; El-Matbouli, M. Differential modulation of host immune genes in the kidney and cranium of the rainbow trout (Oncorhynchus mykiss) in response to Tetracapsuloides bryosalmonae and Myxobolus cerebralis co-infections. Parasites Vectors 2018, 11, 326. [Google Scholar] [CrossRef]
- Saravanan, K.; Praveenraj, J.; Kiruba-Sankar, R.; Devi, V.; Biswas, U.; Kumar, T.S.; Sudhagar, A.; El-Matbouli, M.; Kumar, G. Co-Infection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) and white spot syndrome virus (WSSV) in the wild crustaceans of Andaman and Nicobar Archipelago, India. Viruses 2021, 13, 1378. [Google Scholar] [CrossRef]
- Lightner, D.V. The penaeid shrimp viral pandemics due to IHHNV, WSSV, TSV and YHV: History in the Americas and current status. In Proceedings of the 32nd Joint Meeting of the United States-Japan Cooperative Program in Natural Resource, Davis and Santa Barbara, CA, USA, 17, 18 and 20 November 2003. [Google Scholar]
- Morales-Covarrubias, M.S.; Nunan, L.M.; Lightner, D.V.; Mota-Urbina, J.C.; Garza-Aguirre, M.C.; Chávez-Sánchez, M.C. Prevalence of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in wild adult blue shrimp Penaeus stylirostris from the Northern Gulf of California, Mexico. J. Aquat. Anim. Health 1999, 11, 296–301. [Google Scholar] [CrossRef]
- Walker, P.J.; Cowley, J.A.; Dong, X.; Huang, J.; Moody, N.; Ziebuhr, J.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Roniviridae. J. Gen. Virol. 2020, 102, jgv001514. [Google Scholar] [CrossRef]
- WOAH. Manual of Diagnostic Tests for Aquatic Animals. World Organisation for Aquatic Animal Health. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/ (accessed on 9 January 2023).
- Walker, P.J.; Cowley, J.A.; Spann, K.; Hodgson, R.; Hall, M.; Withyachumnarnkul, B. Yellow Head Complex Viruses: Transmission Cycles and Topographical Distribution in the Asia-Pacific Region; World Aquaculture Society: Baton Rouge, LA, USA, 2001; pp. 292–302. Available online: http://hdl.handle.net/102.100.100/201029?index=1 (accessed on 18 January 2023).
- Wijegoonawardane, P.K.M.; Cowley, J.A.; Phan, T.; Hodgson, R.A.J.; Nielsen, L.; Kiatpathomchai, W.; Walker, P.J. Genetic diversity in the yellow head nidovirus complex. Virology 2008, 380, 213–225. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, W.C.; Wang, X.H.; Zhang, Q.L.; Ren, Y.B.; Song, J.P.; Wang, X.P.; Dong, X.; Huang, J. First detection of yellow head virus genotype 3 (YHV-3) in cultured Penaeus monodon, mainland China. J. Fish Dis. 2018, 41, 1449–1451. [Google Scholar] [CrossRef]
- Mohr, P.G.; Moody, N.J.; Hoad, J.G.; Williams, L.M.; Bowater, R.O.; Cummins, D.M.; Cowley, J.A.; Crane, M.S. New yellow head virus genotype (YHV7) in giant tiger shrimp Penaeus monodon indigenous to northern Australia. Dis. Aquat. Organ. 2015, 115, 263–268. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Yang, H.L.; Yang, B.; Liu, S.; Wang, H.L.; Wang, Q.T.; Liu, F.; Zhang, Q.L. Detection of a new genotype of yellow head virus in farmed shrimp suspicious of EMS/AHPNS infection. Oceanol. Limnol. Sin. 2014, 45, 703–709. [Google Scholar] [CrossRef]
- Zhu, L.L.; Zhang, Q.L.; Wan, X.Y.; Qiu, L.; Huang, J. Molecular epidemiology of a new yellow head virus strain in China. Prog. Fish. Sci. 2016, 37, 68–77. [Google Scholar] [CrossRef]
- Dong, X.; Liu, S.; Zhu, L.L.; Wan, X.Y.; Liu, Q.; Qiu, L.; Zhou, P.Z.; Zhang, Q.L.; Huang, J. Complete genome sequence of an isolate of a novel genotype of yellow head virus from Fenneropenaeus chinensis indigenous in China. Arch. Virol. 2017, 162, 1149–1152. [Google Scholar] [CrossRef]
- Kim, S.R.; Cwr, G.; Shmp, W.; Shin, G.W. Detection and genetic characteristic of Yellow-head virus genotype 8 (YHV-8) cultured Litopanaeus vanamei, in Korea. J. Fish Pathol. 2020, 33, 77–81. [Google Scholar] [CrossRef]
- Dong, X.; Hu, T.; Ren, Y.b.; Meng, F.Z.; Li, C.; Zhang, Q.L.; Chen, J.Y.; Song, J.P.; Wang, R.Y.; Shi, M.; et al. A novel bunyavirus discovered in oriental shrimp (Penaeus chinensis). Front. Microbiol. 2021, 12, 751112. [Google Scholar] [CrossRef]
- Lightner, D.V.; Redman, R.M.; Bell, T.A. Infectious hypodermal and hematopoietic necrosis, a newly recognized virus disease of penaeid shrimp. J. Invertebr. Pathol. 1983, 42, 62–70. [Google Scholar] [CrossRef]
- Bell, T.A.; Lightner, D.V. IHHN virus: Infectivity and pathogenicity studies in Penaeus styliostris and Penaeus vannamei. Aquaculture 1984, 38, 185–194. [Google Scholar] [CrossRef]
- Kalagayan, H.; Godin, D.; Kanna, R.; Hagino, G.; Sweeney, J.; Wyban, J.; James, B. IHHN virus as an etiological factor in runt-deformity syndrome of juvenile Penaeus vannamei culturted in Hawaii. World Aquac. Soc. 1991, 22, 235–243. [Google Scholar] [CrossRef]
- Primavera, J.H.; Quinitio, E.T. Runt-deformity syndrome in cultured fiant tiger prawn Penaeus monodon. J. Crustac. Biol. 2000, 20, 796–802. [Google Scholar] [CrossRef]
- Lotz, J.M. Viruses, biosecurity and specific pathogen-free stocks in shrimp aquaculture. World J. Microbiol. Biotechnol. 1997, 13, 405–413. [Google Scholar] [CrossRef]
- ICTV. Iridoviridae, Subfamily: Betairidovirinae, Genus: Decapodiridovirus. The ICTV Report on Virus Classification and Taxon Nomenclature. Available online: https://ictv.global/report/chapter/iridoviridae/iridoviridae/decapodiridovirus (accessed on 9 January 2023).
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Wang, R.Y.; Cheng, D.Y.; Dong, X.; Yang, B.; Wang, X.H.; et al. Characterization of a new member of Iridoviridae, Shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei). Sci. Rep. 2017, 7, 11834. [Google Scholar] [CrossRef]
- Sun, W.F.; Huang, X.S.; Hu, X.J.; Wen, G.L.; Cao, Y.C.; Zhang, J.S. Detection and analysis of Enterocytozoon hepatopenaei (EHP), Vibrio parahaemolyticus acutehepatopancreatic necrosis disease (VPAHPND) and shrimp hemocyte iridescent virus (SHIV) from Litopenaeus vannamei in coastal areas of Guangdong Province. J. South. Agric. 2019, 50, 2343–2349. [Google Scholar]
- Qiu, L.; Chen, X.; Gao, W.; Li, C.; Guo, X.M.; Zhang, Q.L.; Yang, B.; Huang, J. Molecular epidemiology and histopathological study of a natural infection with decapod iridescent virus 1 in farmed white leg shrimp, Penaeus vannamei. Aquaculture 2021, 533, 736105. [Google Scholar] [CrossRef]
- Lo, C.F.; Leu, J.H.; Ho, C.H.; Chen, C.H.; Peng, S.E.; Chen, Y.T.; Chou, C.M.; Yeh, P.Y.; Huang, C.J.; Chou, H.Y. Detection of baculovirus associated with white spot syndrome (WSBV) in penaeid shrimps using polymerase chain reaction. Dis. Aquat. Org. 1996, 24, 133–141. [Google Scholar] [CrossRef]
- Durand, S.V.; Lightner, D.V. Quantitative real time PCR for the measurement of white spot syndrome virus in shrimp. J. Fish Dis. 2002, 25, 381–389. [Google Scholar] [CrossRef]
- Dhar, A.K.; Roux, M.M.; Klimpel, K.R. Detection and quantification of infectious hypodermal and hematopoietic necrosis virus and white spot virus in shrimp using real-time quantitative PCR and SYBR green chemistry. J. Clin. Microbiol. 2001, 39, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Qiu, L.; Sheng, A.Z.; Wan, X.Y.; Cheng, D.Y.; Huang, J. Quantitative detection method of Enterocytozoon hepatopenaei using TaqMan probe real-time PCR. J. Invertebr. Pathol. 2018, 151, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.F.J.; Durand, S.V.; White, B.L.; Redman, R.M.; Pantoja, C.R.; Lightner, D.V. Postlarvae and juveniles of a selected line of Penaeus stylirostris are resistant to infectious hypodermal and hematopoietic necrosis virus infection. Aquaculture 2000, 190, 203–210. [Google Scholar] [CrossRef]
- Jaroenlak, P.; Sanguanrut, P.; Williams, B.A.P.; Stentiford, G.D.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. A Nested PCR assay to avoid false positive detection of the microsporidian Enterocytozoon hepatopenaei (EHP) in environmental samples in shrimp farms. Public Libr. Sci. 2016, 11, e0166320. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.P.D.; Srisuvan, T.; Tang, K.F.J.; Lightner, D.V. Real-time reverse transcription polymerase chain reaction assay using TaqMan probe for detection and quantification of infectious myonecrosis virus (IMNV). Aquaculture 2007, 264, 9–15. [Google Scholar] [CrossRef]
- Senapin, S.; Phewsaiya, K.; Briggs, M.; Flegel, T.W. Outbreaks of infectious myonecrosis virus (IMNV) in Indonesia confirmed by genome sequencing and use of an alternative RT-PCR detection method. Aquaculture 2007, 266, 32–38. [Google Scholar] [CrossRef]
- Li, X.P.; Wan, X.Y.; Xu, T.T.; Huang, J.; Zhang, Q.L. Development and validation of a TaqMan RT-qPCR for the detection of convert mortality nodavirus (CMNV). J. Virol. Methods 2018, 262, 65–71. [Google Scholar] [CrossRef]
- Poulos, B.T.; Lightner, D.V. Detection of infectious myonecrosis virus (IMNV) of penaeid shrimp by reverse-transcriptase polymerase chain reaction (RT-PCR). Dis. Aquat. Org. 2006, 73, 69–72. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liu, Q.; Liu, S.; Yang, H.L.; Liu, S.; Zhu, L.L.; Yang, B.; Jin, J.T.; Ding, L.X.; Wang, X.H.; et al. A new nodavirus is associated with covert mortality disease of shrimp. J. Gen. Virol. 2014, 95, 2700–2709. [Google Scholar] [CrossRef]
- Navarro, S.A.; Tang, K.F.J.; Lightner, D.V. An improved Taura syndrome virus (TSV) RT-PCR using newly designed primers. Aquaculture 2009, 293, 290–292. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Wang, J.; Lightner, D.V. Quantitation of Taura syndrome virus by real-time RT-PCR with a TaqMan assay. J. Virol. Methods 2004, 115, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, X.; Guo, X.M.; Gao, W.; Huang, J. A TaqMan probe based real-time PCR for the detection of Decapod iridescent virus 1. J. Invertebr. Pathol. 2020, 173, 107367. [Google Scholar] [CrossRef]
- Cowley, J.A.; Cadogan, L.C.; Wongteerasupaya, C.; Hodgson, R.; Boonsaeng, V.; Walker, P.J. Multiplex RT-nested PCR differentiation of gill-associated virus (Australia) from yellow head virus (Thailand) of Penaeus monodon. J. Virol. Methods 2004, 117, 49–59. [Google Scholar] [CrossRef]
- Li, C.; Ren, Y.B.; Dong, X.; Wang, C.M.; Huang, J. Extraction of assembling complexes of viral capsomers from shrimp tissue infected with yellow head virus genotype 8 (YHV-8). J. Fish Dis. 2019, 42, 613–616. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Koichiro, T.; Glen, S.; Sudhir, K. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Dhar, A.K.; Cowley, J.A.; Hasson, K.W.; Walker, P.J. Genomic organization, biology, and diagnosis of Taura syndrome virus and yellow head virus of penaeid shrimp. Adv. Virus Res. 2004, 63, 353–421. [Google Scholar] [CrossRef]
- Ossiander, F.J.; Wedemeyer, G. Computer program for sample sizes required to determine disease incidence in fish populations. J. Fish. Res. Board Can. 1973, 30, 1383–1384. [Google Scholar] [CrossRef]
- Song, Z.L.; Dong, X.; Zhao, R.H.; Wang, X.H.; Wu, H.Y.; Yu, D.H.; Xie, G.S.; Huang, J. Evaluation on the detection of Enterocytozoon hepatopenaei in pooled DNA samples of Litopenaeus vannamei based on TaqMan qPCR. Prog. Fish. Sci. 2019, 40, 122–132. [Google Scholar] [CrossRef]
- Wang, W.J.; Lyu, D.; Wang, M.S.; Liu, K.F.; Kong, J.; Shan, X.J.; Jin, X.S. Research in migration route of hatchery released Chinese shrimp (Fenneropenaeus chinensis) in the Bohai Bay using method of SSR marker. Acta Oceanol. Sin. 2020, 39, 76–81. [Google Scholar] [CrossRef]
- Li, P.F.; Zhang, H.; Zhang, X.M.; Gao, T.X.; Han, Z.Q. Study on the genetic variability of the hatchery-released and wild populations of Chinese white shrimp Fenneropenaeus chinensis in the Yellow Sea and Bohai Sea. Aquac. Int. 2017, 25, 2117–2126. [Google Scholar] [CrossRef]
- Spann, K.M.; Cowley, J.A.; Walker, P.J.; Lester, R.J.G. A yellow-head-like virus from Penaeus monodon cultured in Australia. Dis. Aquat. Org. 1997, 31, 169–179. [Google Scholar] [CrossRef]
- Teixeira-Lopes, M.A.; Vieira-Girão, P.R.N.; Freire, J.E.D.C.; Rocha, Í.R.C.B.; Costa, F.H.F.; Rádis-Baptista, G. Natural co-infection with infectious hypodermal and hematopoietic necrosis virus (IHHNV) and infectious myonecrosis virus (IMNV) in Litopenaeus vannamei in Brazil. Aquaculture 2011, 312, 212–216. [Google Scholar] [CrossRef]
- Aranguren, L.F.; Tang, K.F.J.; Lightner, D.V. Protection from yellow head virus (YHV) infection in Penaeus vannamei pre-infected with Taura syndrome virus (TSV). Dis. Aquat. Org. 2012, 98, 185–192. [Google Scholar] [CrossRef] [PubMed]
- WOAH. Disease Card: Infection with Decapod Iridescent Virus 1 (A DIV1) Disease Card. World Organisation for Aquatic Animal Health. Available online: https://www.woah.org/app/uploads/2021/03/a-div1-disease-card.pdf (accessed on 8 January 2023).
- Yang, B.; Song, X.L.; Huang, J.; Shi, C.Y.; Liu, L. Evidence of existence of infectious hypodermal and hematopoietic necrosis virus in penaeid shrimp cultured in China. Vet. Microbiol. 2007, 120, 63–70. [Google Scholar] [CrossRef]
- Dong, X.; Li, F.J.; Xie, G.S.; Yang, B.; Zhang, Q.L.; Huang, J. The analysis of infectious hypodermal and hematopoietic necrosis in 2019. In 2020 Analysis of Major Aquatic Animal Diseases in China; BOF and NFTEC; China Agriculture Press: Beijing, China, 2020; pp. 150–165. ISBN 978-7-109-27064-0. [Google Scholar]
- Browdy, C.L. Recent developments in penaeid broodstock and seed production technologies: Improving the outlook for superior captive stocks. Aquaculture 1998, 164, 3–21. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Fejzic, N.; Mackinnon, B.; Huchzermeyer, D.; Seric-Haracic, S.; Mardones, F.O.; Mohan, C.V.; Taylor, N.; Jansen, M.D.; Tavornpanich, S.; et al. A 12-point checklist for surveillance of diseases of aquatic organisms: A novel approach to assist multidisciplinary teams in developing countries. Rev. Aquac. 2021, 13, 1469–1487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).