Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
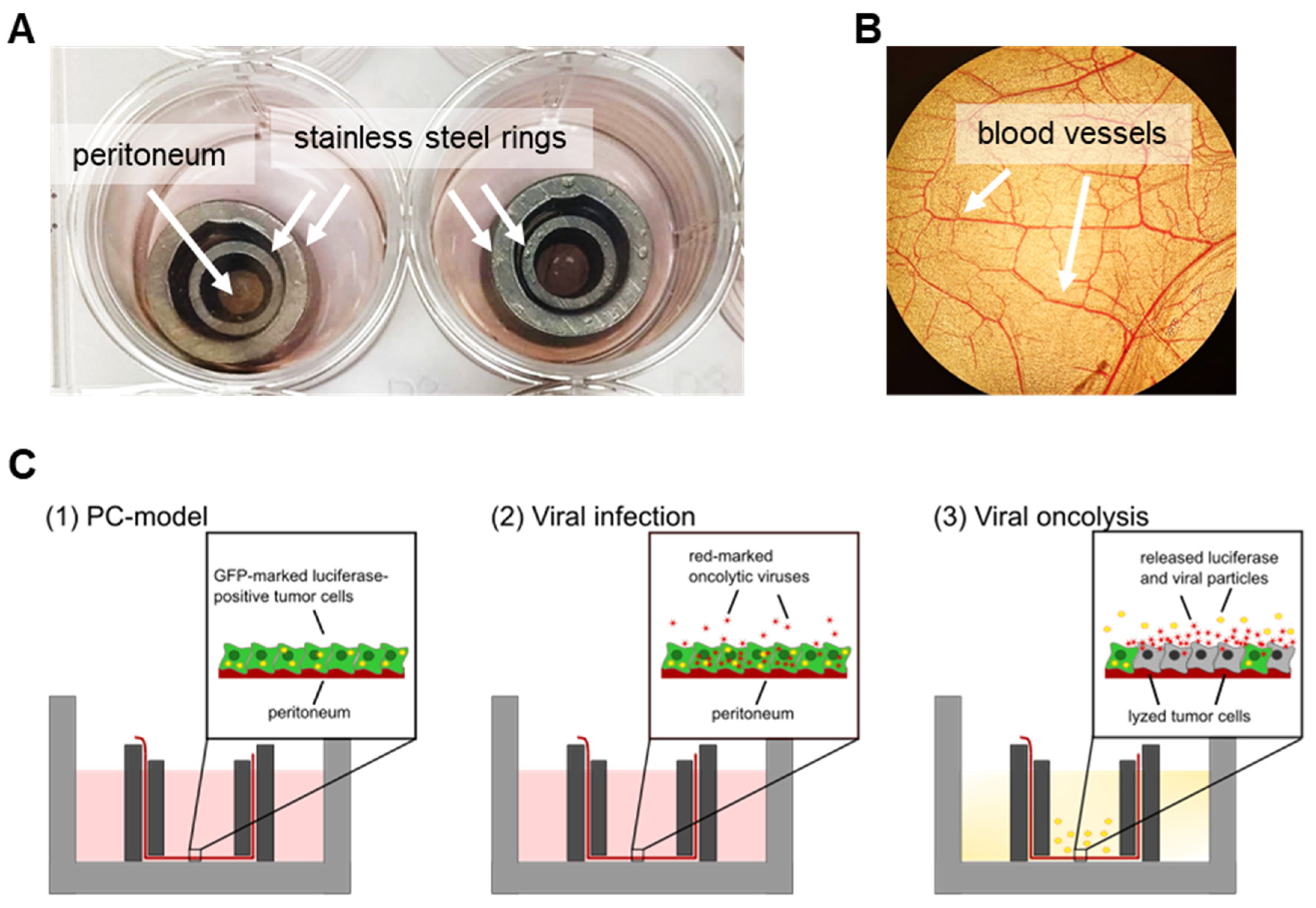
2.2. Preparation and Culture of Peritoneal Tissue
2.3. Preparation of Ex Vivo Peritoneum Co-Culture Model
2.4. Oncolytic Viral Constructs
2.5. Infection of GFP/luc–HT-29 Cells in Cell Culture or in Ex Vivo Peritoneum Co-Culture Model
2.6. Sulforhodamine B (SRB) Cell Viability Assay
2.7. Measurement of Luciferase Activity of GFP/luc–HT-29 Cells in Cell Culture or in Ex Vivo Peritoneum Co-Culture Models
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
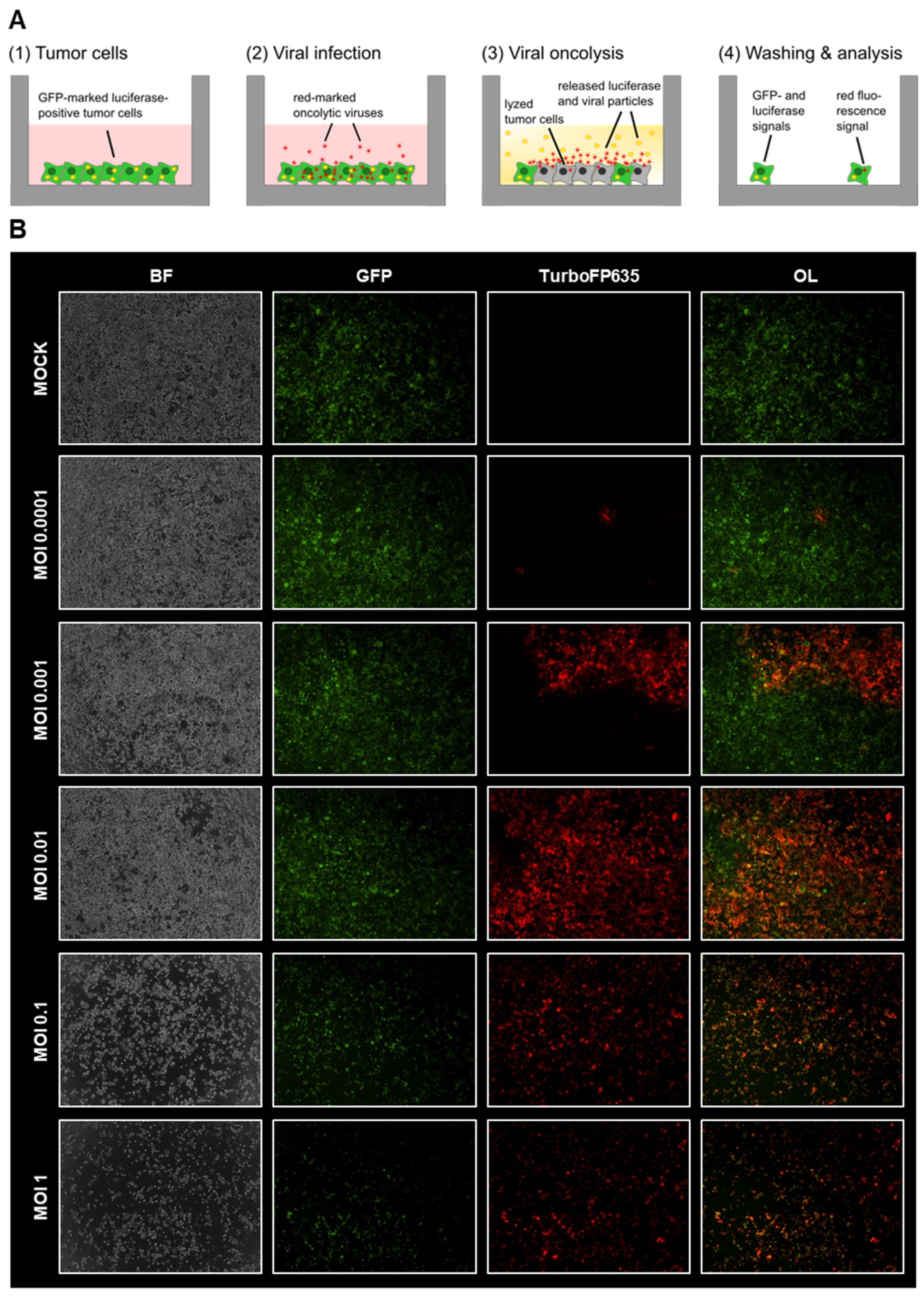
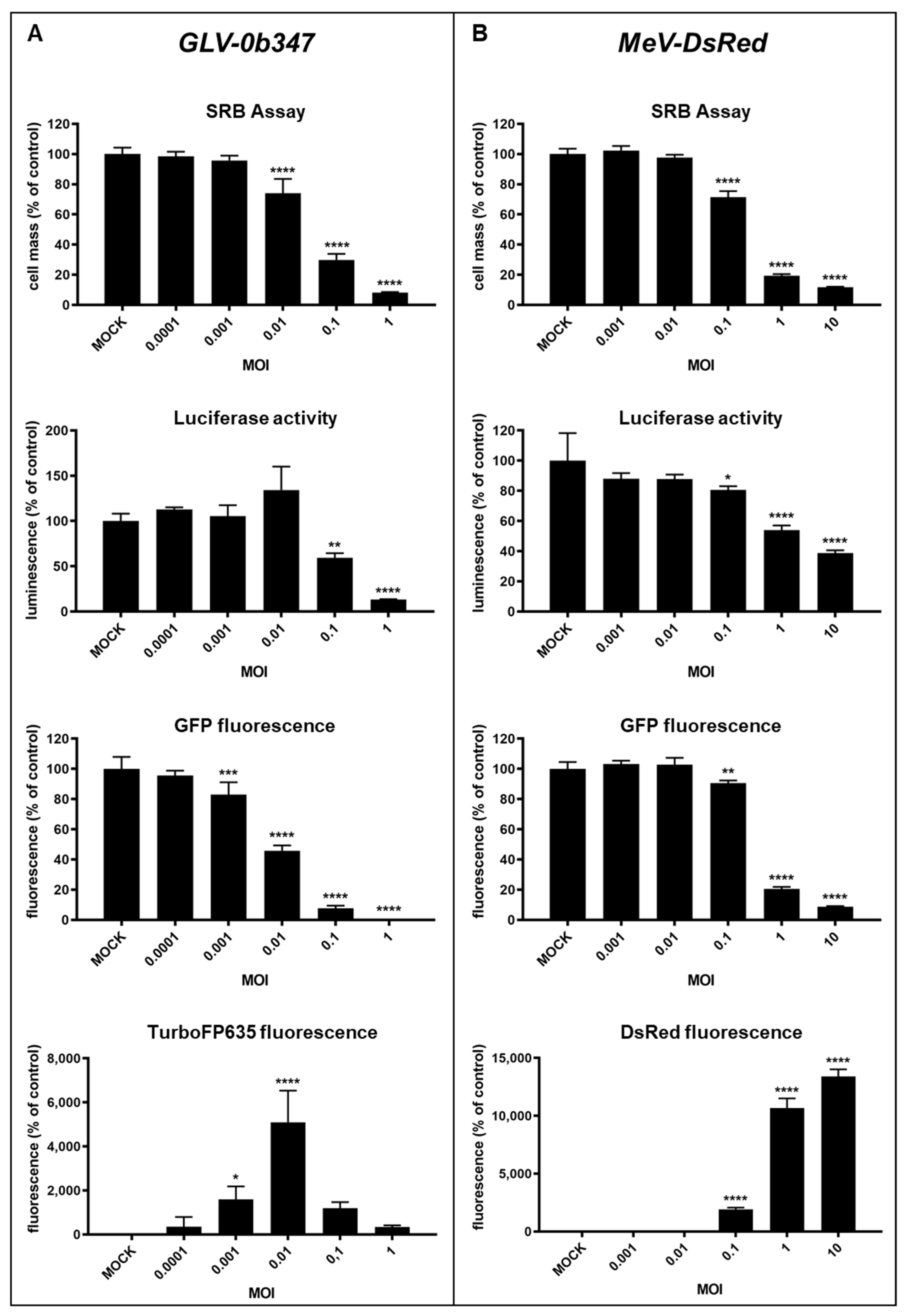
3.1. Virotherapeutic Treatment of GFP/luc-Labeled Human HT-29 Tumor Cells in Cell Culture
3.2. Comparison of Different Detection Options for the Oncolytic Activity of Virotherapeutic Compounds
3.3. Human Ex Vivo Peritoneum Model as a Platform for Virotherapeutic Applications
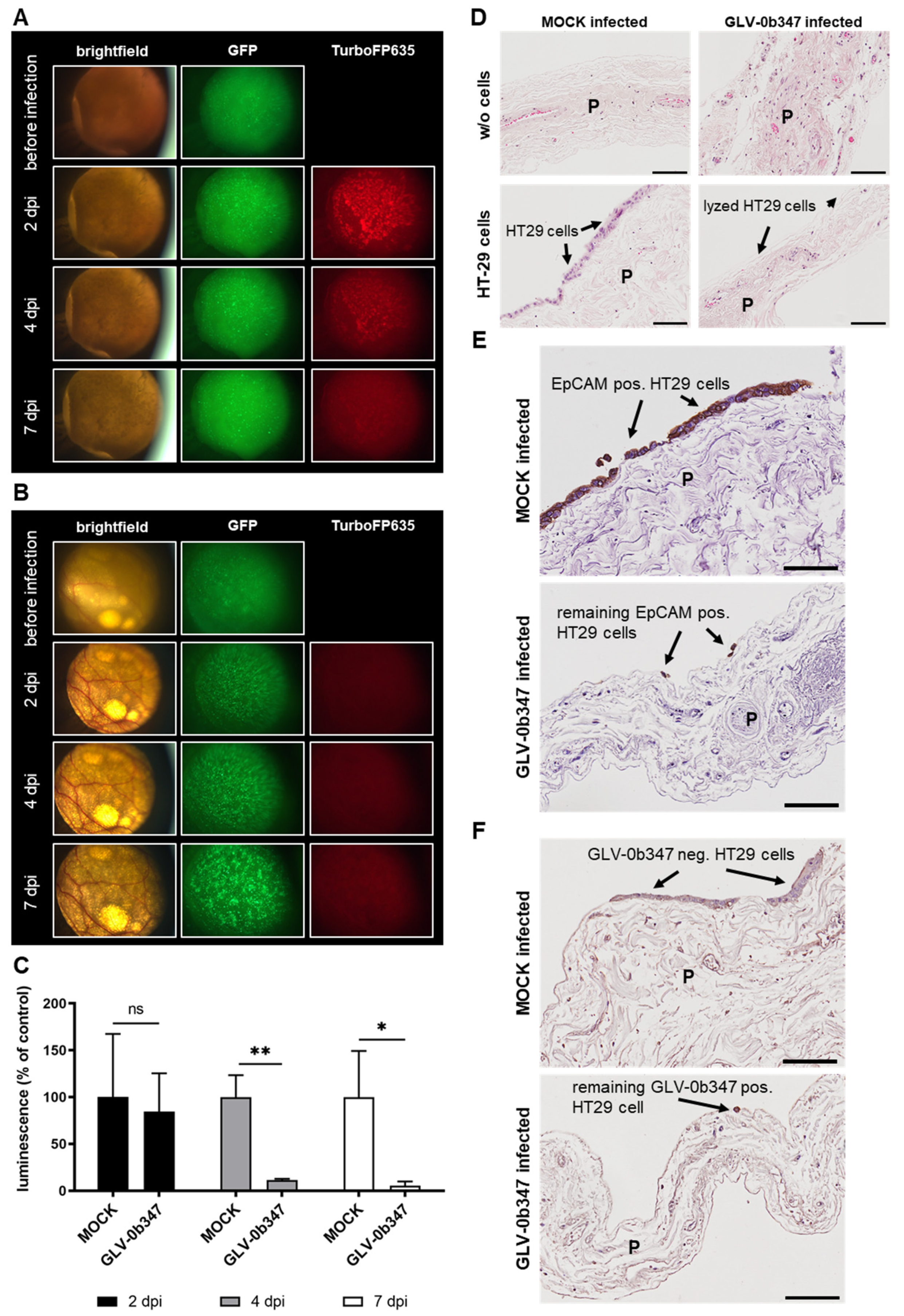
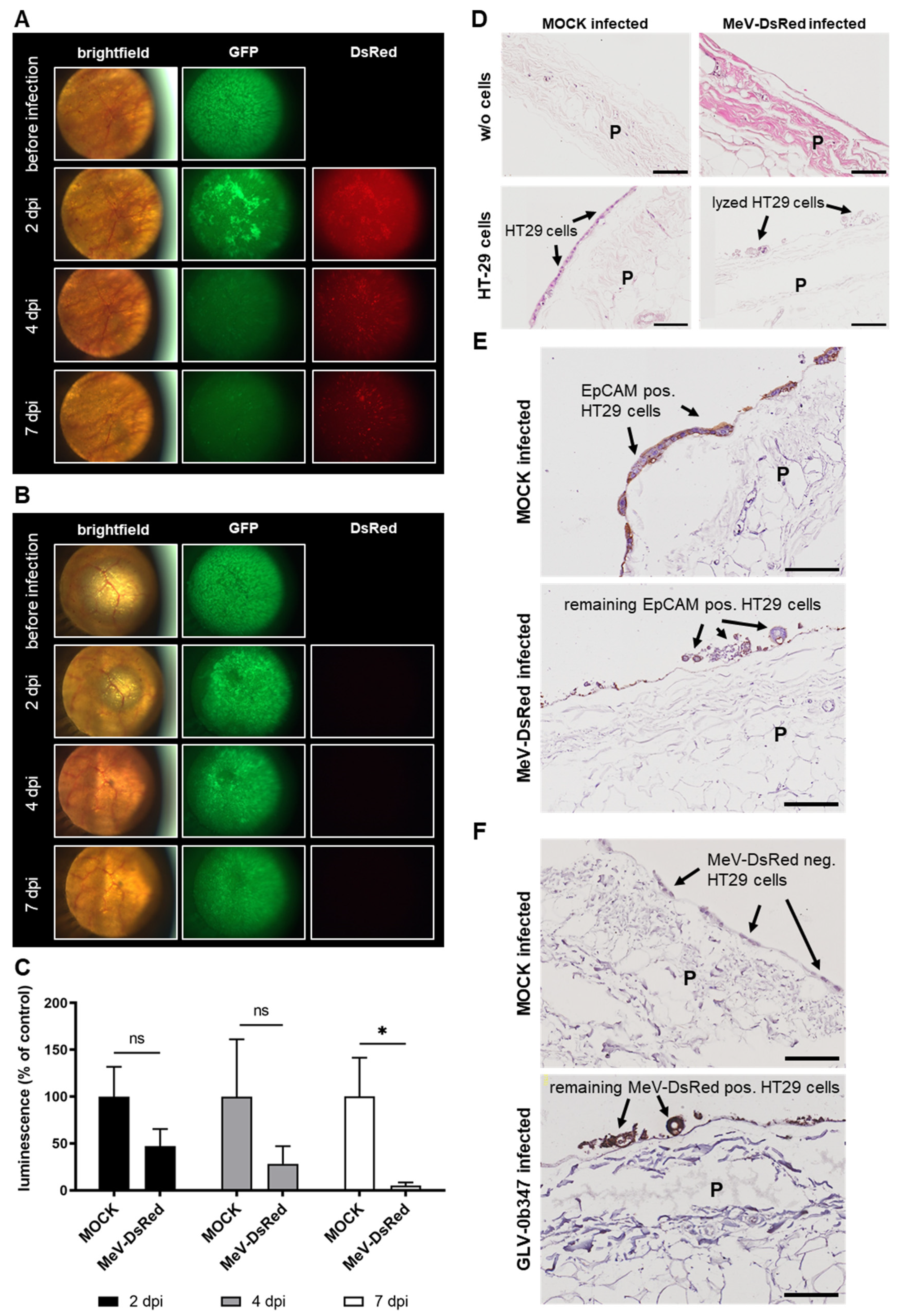
3.4. Virotherapeutic Treatment of PC Models with Recombinant Vaccinia Virus GLV-0b347
3.5. Virotherapeutic Treatment of PC Models with Recombinant Measles Vaccine Virus MeV-DsRed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Wal, J.B.C.; Jeekel, J. Biology of the peritoneum in normal homeostasis and after surgical trauma. Color. Dis. 2007, 9 (Suppl. 2), 9–13. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Uruski, P.; Tykarski, A.; Ksiazek, K. The peritoneal “soil” for a cancerous “seed”: A comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell Mol. Life Sci. 2018, 75, 509–525. [Google Scholar] [CrossRef]
- Liu, T.C.; Galanis, E.; Kirn, D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Kloker, L.D.; Yurttas, C.; Lauer, U.M. Three-dimensional tumor cell cultures employed in virotherapy research. Oncolytic Virother 2018, 7, 79–93. [Google Scholar] [CrossRef]
- Mönch, D.; Koch, J.; Maass, A.; Janssen, N.; Mürdter, T.; Renner, P.; Fallier-Becker, P.; Solass, W.; Schwab, M.; Dahlke, M.H.; et al. A human ex vivo coculture model to investigate peritoneal metastasis and innovative treatment options. Pleura Peritoneum 2021, 6, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Mönch, D.; Maass, A.; Mangold, A.; Guzvic, M.; Mürdter, T.; Leibold, T.; Dahlke, M.H.; Renner, P. Pharmacologic Targeting of MMP2/9 Decreases Peritoneal Metastasis Formation of Colorectal Cancer in a Human Ex Vivo Peritoneum Culture Model. Cancers 2022, 14, 3760. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, L.; Zhao, C.; Zheng, P.; Zhang, X.; Xu, J. Vaccinia virus-based vector against infectious diseases and tumors. Hum. Vaccines Immunother. 2021, 17, 1578–1585. [Google Scholar] [CrossRef]
- Yang, Z.; Gray, M.; Winter, L. Why do poxviruses still matter? Cell Biosci. 2021, 11, 96. [Google Scholar] [CrossRef]
- Carter, M.E.; Hartkopf, A.D.; Wagner, A.; Volmer, L.L.; Brucker, S.Y.; Berchtold, S.; Lauer, U.M.; Koch, A. A Three-Dimensional Organoid Model of Primary Breast Cancer to Investigate the Effects of Oncolytic Virotherapy. Front. Mol. Biosci. 2022, 9, 826302. [Google Scholar] [CrossRef]
- Lange, S.; Lampe, J.; Bossow, S.; Zimmermann, M.; Neubert, W.; Bitzer, M.; Lauer, U.M. A novel armed oncolytic measles vaccine virus for the treatment of cholangiocarcinoma. Hum. Gene 2013, 24, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Falk, P.; Ruiz-Jasbon, F.; Strigard, K.; Gunnarsson, U.; Ivarsson, M.L. An ex vivo model using human peritoneum to explore mesh-tissue integration. Biol. Open 2017, 6, 1391–1395. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Castro, C.; Roberts, L.D.; Griffin, J.L.; Smith, G.L. A role for vaccinia virus protein C16 in reprogramming cellular energy metabolism. J. Gen. Virol. 2015, 96 Pt 2, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Puzanov, I.; Collichio, F.; Milhem, M.M.; Hauschild, A.; Chen, L.; Sharma, A.; Garbe, C.; Singh, P.; Mehnert, J.M. Patterns of response with talimogene laherparepvec in combination with ipilimumab or ipilimumab alone in metastatic unresectable melanoma. Br. J. Cancer 2019, 121, 417–420. [Google Scholar] [CrossRef]
- Noll, M.; Berchtold, S.; Lampe, J.; Malek, N.P.; Bitzer, M.; Lauer, U.M. Primary resistance phenomena to oncolytic measles vaccine viruses. Int. J. Oncol. 2013, 43, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, S.; Beil, J.; Raff, C.; Smirnow, I.; Schell, M.; D’Alvise, J.; Gross, S.; Lauer, U.M. Assessing and Overcoming Resistance Phenomena against a Genetically Modified Vaccinia Virus in Selected Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 7618. [Google Scholar] [CrossRef]
- Lauer, U.M.; Beil, J. Oncolytic viruses: Challenges and considerations in an evolving clinical landscape. Future Oncol. 2022, 18, 2713–2732. [Google Scholar] [CrossRef]
- Ji, Q.; Wu, Y.; Albers, A.; Fang, M.; Qian, X. Strategies for Advanced Oncolytic Virotherapy: Current Technology Innovations and Clinical Approaches. Pharmaceutics 2022, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Nettelbeck, D.M.; Leber, M.F.; Altomonte, J.; Angelova, A.; Beil, J.; Berchtold, S.; Delic, M.; Eberle, J.; Ehrhardt, A.; Engeland, C.E.; et al. Virotherapy in Germany-Recent Activities in Virus Engineering, Preclinical Development, and Clinical Studies. Viruses 2021, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Lauer, U.M.; Schell, M.; Beil, J.; Berchtold, S.; Koppenhöfer, U.; Glatzle, J.; Königsrainer, A.; Möhle, R.; Nann, D.; Fend, F.; et al. Phase I Study of Oncolytic Vaccinia Virus GL-ONC1 in Patients with Peritoneal Carcinomatosis. Clin. Cancer Res. 2018, 24, 4388–4398. [Google Scholar] [CrossRef]
- Tate, S.J.; Van de Sande, L.; Ceelen, W.P.; Torkington, J.; Parker, A.L. The Feasibility of Pressurised Intraperitoneal Aerosolised Virotherapy (PIPAV) to Administer Oncolytic Adenoviruses. Pharmaceutics 2021, 13, 2043. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Chen, K.; Gu, S.; Wang, J.; Meng, Z.; Li, Y.; Wang, P. Intraperitoneal oncolytic virotherapy for patients with malignant ascites: Characterization of clinical efficacy and antitumor immune response. Mol. Ther. Oncolytics 2022, 25, 31–422022. [Google Scholar] [CrossRef]
- Manyam, M.; Stephens, A.J.; Kennard, J.A.; LeBlanc, J.; Ahmad, S.; Kendrick, J.E.; Holloway, R.W. A phase 1b study of intraperitoneal oncolytic viral immunotherapy in platinum-resistant or refractory ovarian cancer. Gynecol. Oncol. 2021, 163, 481–489. [Google Scholar] [CrossRef]
- Holloway, R.; Mendivil, A.; Kendrick, J.; Abain, L.; Brown, J.; Fitzsimmons, C.; Kennard, J.; King, M.; LeBlanc, J.; Lopez, K.; et al. Oncolytic vaccinia (OLVI-VEC) primed immunochemotherapy in platinum-resistant/refractory ovarian cancer. Int. J. Gynecol. Cancer 2020, 30, A9–A10. [Google Scholar]
- Galanis, E.; Atherton, P.J.; Maurer, M.J.; Knutson, K.L.; Dowdy, S.C.; Cliby, W.A.; Haluska, P., Jr.; Long, H.J.; Oberg, A.; Aderca, I.; et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015, 75, 22–30. [Google Scholar] [CrossRef]
- Cohn, D.E.; Sill, M.W.; Walker, J.L.; O’Malley, D.; Nagel, C.I.; Rutledge, T.L.; Bradley, W.; Richardson, D.L.; Moxley, K.M.; Aghajanian, C. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin(R)) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 146, 477–483. [Google Scholar] [CrossRef]
- Bennett, J.J.; Delman, K.A.; Burt, B.M.; Mariotti, A.; Malhotra, S.; Zager, J.; Petrowsky, H.; Mastorides, S.; Federoff, H.; Fong, Y. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene 2002, 9, 935–945. [Google Scholar] [CrossRef]
- Ishikawa, W.; Kikuchi, S.; Ogawa, T.; Tabuchi, M.; Tazawa, H.; Kuroda, S.; Noma, K.; Nishizaki, M.; Kagawa, S.; Urata, Y.; et al. Boosting Replication and Penetration of Oncolytic Adenovirus by Paclitaxel Eradicate Peritoneal Metastasis of Gastric Cancer. Mol. Ther. Oncolytics 2020, 18, 262–271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, J.; Beil, J.; Berchtold, S.; Mönch, D.; Maaß, A.; Smirnow, I.; Schenk, A.; Carter, M.E.; Kloker, L.D.; Leibold, T.; et al. Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model. Viruses 2023, 15, 363. https://doi.org/10.3390/v15020363
Koch J, Beil J, Berchtold S, Mönch D, Maaß A, Smirnow I, Schenk A, Carter ME, Kloker LD, Leibold T, et al. Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model. Viruses. 2023; 15(2):363. https://doi.org/10.3390/v15020363
Chicago/Turabian StyleKoch, Jana, Julia Beil, Susanne Berchtold, Dina Mönch, Annika Maaß, Irina Smirnow, Andrea Schenk, Mary E. Carter, Linus D. Kloker, Tobias Leibold, and et al. 2023. "Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model" Viruses 15, no. 2: 363. https://doi.org/10.3390/v15020363
APA StyleKoch, J., Beil, J., Berchtold, S., Mönch, D., Maaß, A., Smirnow, I., Schenk, A., Carter, M. E., Kloker, L. D., Leibold, T., Renner, P., Dahlke, M.-H., & Lauer, U. M. (2023). Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model. Viruses, 15(2), 363. https://doi.org/10.3390/v15020363





