Virus–Host Protein Interaction Network of the Hepatitis E Virus ORF2-4 by Mammalian Two-Hybrid Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. High Throughput Screens
2.2. PPI Analysis
2.3. CRISPR-Cas9 Knockout of SHARPIN and RNF5
2.4. HEV ORF3 Transfection and IFN qRT-PCR Assays
2.5. HEV Infectious Virus Production
2.6. HEV Infection and HEV ORF2 Immunofluorescence Staining
2.7. SDS-PAGE and Western Blot
2.8. Reverse Transcription Quantitative PCR (RT-qPCR)
2.9. Statistical Analyses
3. Results
3.1. MAPPIT and KISS Are Functional Tools to Study HEV–Host Protein–protein Interactions
3.2. Functional Annotation Clustering for the HEV Proteins
3.3. SHARPIN Affects the Induction of Interferon upon ORF3 Transfection
3.4. RNF5 Interferes with IFN Induction upon ORF3 Transfection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Screen | Shared Protein Hits |
|---|---|
| Gt-1 ORF2 MAPPIT|Gt-3 ORF2 MAPPIT | CA12, ADCY3 |
| Gt-1 ORF3 MAPPIT|Gt-1 ORF3 KISS | SHARPIN |
| Gt-1 ORF3 KISS|Gt-3 ORF3 KISS | TMEM108, FAM171B, MCAM, TREML1, GPRC5D, SPN, MAG, HS1BP3, OPALIN, BSG, ERMAP, BTN3A2, CRB3, NUMB, PVRL4, TMEM184A, PTH1R, VSIG8, SLC7A8, PTGER4, SURF4 |
| Gt-1 ORF3 MAPPIT|Gt-3 ORF3 KISS | CX3CL1, CYBRD1, HAVCR1 |
| Gt-3 ORF3 MAPPIT|Gt-3 ORF3 KISS | NAT1, MAT1A, HSPA8, HPX |
| Gt-1 ORF3 KISS|Gt-3 ORF3 KISS|Gt-3 MAPPIT | TSG101 |
| ORF4 MAPPIT|ORF4 KISS | SHARPIN, C9orf169, MSI2, C3orf56, CELF5, TRAF1, LPXN, RBFOX1, THAP8, FAM168A, THAP11 |
| ORF2|ORF3 | TMEM154, FCGR2A, FTL |
| ORF3|ORF4 | FAM92A1, RBPMS, SHARPIN, SMN2, C9orf169, RCHY1, OLIG1, TSG101, CCDC170, METTL16 |
| ORF2|ORF4 | STUB1 |
| Annotation Category | GO Term | Gene Name |
|---|---|---|
| Biological Process | GO:0097531~mast cell migration | STAT5B, KITLG |
| GO:0044857~plasma membrane raft organization | COLEC12, FLOT1 | |
| GO:0060355~positive regulation of cell adhesion molecule production | COLEC12, FLOT1 | |
| GO:0061684~chaperone-mediated autophagy | HSP90AA1, STUB1 | |
| GO:0071360~cellular response to exogenous dsRNA | COLEC12, FLOT1 | |
| Cellular Compartment | GO:0016020~membrane | CA12, COLEC12, CYB5A, HSP90AA1, CMTM6, TOMM34, ADCY3, GBA2, EDA2R, TMEM154, KITLG, FUBP3, TMBIM4, CA4, FLOT1, C5ORF60, LGALS8, FTL |
| GO:0016021~integral component of Membrane | CA12, COLEC12, CYB5A, CMTM6, TOMM34, NDUFB1, ADCY3, TNFRSF10B, GBA2, EDA2R, TMEM154, KITLG, FCGR2A, TMBIM4, IGDCC3, CA4, C5ORF60, TIGIT | |
| GO:0005886~plasma membrane | CA12, COLEC12, HSP90AA1, ANXA4, CMTM6, ADCY3, TNFRSF10B, GBA2, EDA2R, KITLG, FCGR2A, RAB42, CAPNS2, IGDCC3, CA4, FLOT1, TIGIT | |
| GO:0009986~cell surface | TGIT, TNFRSF10B, ANXA4, CA4, HSP90AA1 | |
| Molecular Function | GO:0030911~TPR domain binding | HSP90AA1, STUB1 |
| GO:0004089~carbonate dehydratase activity | CA12, CA4 | |
| GO:0016836~hydro-lyase activity | CA12, CA4 |
| Annotation Category | GO Term | Gene Name |
|---|---|---|
| Biological Process | GO:0046718~viral entry into host cell | EFNB2, CD4, CXADR, BSG, AGTR1, NECTIN4, HAVCR1 |
| GO:1901379~regulation of potassium ion transmembrane transport | KCNE1, KCNIP1, KCNIP2, KCNAB3 | |
| GO:0007166~cell surface receptor signaling pathway | SPN, CD4, FCGR2A, ADGRF5, BSG, PTH2R, PTH1R, ADRB2, MS4A12, FCGR2B, IL27RA | |
| GO:0007155~cell adhesion | FGB, CD151, AMBP, SEMA4D, MCAM, CD99L2, CX3CL1, EFNB2, EFNB1, MAG, CD4, BSG, SSPN, NECTIN4 | |
| GO:0006955~immune response | PTGER4, SEMA4D, IL1R2, CXCR6, CX3CL1, IL27RA, SPN, CD4, CD7, FCGR2B, ICOS, CAMP, CMKLR1 | |
| GO:0007188~adenylate cyclase- modulating G-protein coupled receptor signaling pathway | PTGER4, FPR1, PTH2R, PTH1R, ADRB2 | |
| GO:0007204~positive regulation of cytosolic calcium ion concentration | PTGER4, FPR1, AGTR1, LPAR1, S1PR3, CXCR6, CMKLR1 | |
| GO:0030168~platelet activation | FGB, DGKG, TREML1, ENTPD2, PDPN | |
| GO:0050863~regulation of T cell activation | SPN, CD4, TREX1 | |
| GO:0043031~negative regulation of macrophage activation | ADGRF5, FCGR2B, CD200 | |
| GO:0006935~chemotaxis | SPN, FPR1, CXCR6, PLP2, CX3CL1, CMKLR1 | |
| GO:0050776~regulation of immune response | SPN, FCGR2A, FCGR2B, CD200 | |
| GO:0098915~membrane repolarization during ventricular cardiac muscle cell action potential | KCNE1, KCNE4, SCN2B | |
| GO:0007189~adenylate cyclase-activating G-protein coupled receptor signaling pathway | PTGER4, ADGRF5, LPAR1, S1PR3, PTH1R, ADRB2 | |
| GO:0097623~potassium ion export across plasma membrane | KCNE1, KCNIP2, KCNE4 | |
| GO:0019722~calcium-mediated signaling | TREML1, CD4, AGTR1, CXCR6, MCOLN1 | |
| GO:0007186~G-protein coupled receptor signaling pathway | ENTPD2, FZD7, LPAR1, FPR1, MRGPRF, GPBAR1, PTH2R, CXCR6, PTH1R, CX3CL1, GPR173, ADGRF5, AGTR1, APLNR, S1PR3, GPRC5D, CMKLR1 | |
| GO:1904659~glucose transmembrane transport | SLC2A12, SLC2A5, SLC2A6 | |
| GO:0007200~phospholipase C-activating G-protein coupled receptor signaling pathway | FPR1, AGTR1, PTH1R, CMKLR1 | |
| GO:0001817~regulation of cytokine Production | BTN3A3, BTN3A2, ERMAP, LITAF | |
| GO:0055085~transmembrane transport | SLC44A5, SLCO1B1, GJA3, FXYD2, CYBRD1, RNF5, SLC2A6 | |
| GO:0060326~cell chemotaxis | AGTR1, LPAR1, CXCR6, CX3CL1 | |
| GO:0006556~S-adenosylmethionine biosynthetic process | MAT1A, METTL16 | |
| GO:0071347~cellular response to interleukin-1 | FGB, MMP2, CAMP, CX3CL1 | |
| GO:0020027~hemoglobin metabolic process | HPX, AMBP | |
| GO:0032690~negative regulation of interleukin-1 alpha production | IL1R2, CX3CL1 | |
| GO:0071277~cellular response to calcium ion | SYT5, NEUROD2, BRAF, MCOLN1 | |
| GO:0031295~T cell costimulation | EFNB2, EFNB1, ICOS | |
| Cellular Compartment | GO:0005886~plasma membrane | DGKG, MRGPRF, NCMAP, IL27RA, GPR173, GJA3, BSG, MS4A12, CMKLR1, ENTPD2, CXADR, IL1R2, SYTL1, SLC9B2, MAG, AGTR1, APLNR, KCNE1, TSG101, TTYH3, CD151, GYPB, SLC22A1, FPR1, LPAR1, LYPD3, CD99L2, ADRB2, CBARP, EFNB2, SLCO1B1, EFNB1, EPB41L2, EPB41L3, ICOS, TMEM184A, SYT5, HSPA8, MPIG6B, AMBP, CAV2, MCAM, FZD7, SURF4, CYBRD1, TNFRSF10A, BRAF, TREML1, LRFN4, FXYD2, SSPN, CD200, SCN2B, SLC44A5, PTH1R, SLC2A5, SLC6A1, LITAF, SLC2A6, CX3CL1, SPN, HAVCR1, ERMAP, C11ORF24, FGB, OPALIN, MMP2, SLC2A12, GPBAR1, PTH2R, BTN3A3, BTN3A2, KCNAB3, SLC7A8, ADGRF5, NUMB, GPRC5D, PTGER4, PLPPR1, STAU2, PACC1, CXCR6, ACVR1B, RNF5, SLITRK3, PDPN, S1PR3, CACNG1, CACNG2, RELT, LAIR1, JAM2, SEMA4D, KCNIP1, KCNIP2, KIR3DL2, MCOLN1, CD4, FCGR2A, PIK3IP1, CD6, CD7, RAB9A, PLP2, FCGR2B, NECTIN4, CRB3 |
| GO:0016021~integral component of membrane | ZNF451, DPY19L2P2, MRGPRF, IL27RA, GPR173, SYNPR, GJA3, BSG, MS4A12, VSIG8, CMKLR1, STARD3, ENTPD2, IL1R2, SLC9B2, MAG, AGTR1, APLNR, KCNE1, RTN1, CD151, KCNE4, GYPB, SLC22A1, FPR1, LPAR1, LYPD3, SLC38A11, CD99L2, ADRB2, CBARP, SLCO1B1, EPB41L3, ICOS, C16ORF54, TMEM184A, SYT5, MPIG6B, CAV2, RHBDD1, TREX1, MCAM, FZD7, SURF4, CYBRD1, TNFRSF10A, DNAJC14, TREML1, LRFN4, FAM163A, CD200, SLC44A5, PTH1R, SLC2A5, SLC6A1, LITAF, SLC2A6, CX3CL1, SPN, TMEM108, HAVCR1, ERMAP, C11ORF24, OPALIN, SLC2A12, GPBAR1, PTH2R, BTN3A3, C1ORF162, BTN3A2, SYP, SLC7A8, ADGRF5, RFT1, GPRC5D, PTGER4, PLPPR1, SLC43A3, PACC1, TMEM72, CXCR6, RNF5, PDPN, RELT, LAIR1, JAM2, RPRM, CMTM4, TMEM176B, KIR3DL2, MCOLN1, TMEM154, CD4, FCGR2A, PIK3IP1, CD6, LPCAT4, CD7, FAM171B, PLP2, FCGR2B, NECTIN4, CRB3 | |
| GO:0005887~integral component of plasma membrane | CD151, PLPPR1, PACC1, GYPB, SLC22A1, LPAR1, ADRB2, CXCR6, PTH1R, SLC6A1, ACVR1B, SLC2A5, NCMAP, IL27RA, EFNB2, SPN, EFNB1, SLCO1B1, GJA3, PDPN, BSG, S1PR3, CACNG1, JAM2, CMKLR1, CXADR, SEMA4D, CAV2, PTH2R, KIR3DL2, MCOLN1, MAG, CD4, FCGR2A, SLC7A8, CD6, SSPN, AGTR1, APLNR, FCGR2B, CD200 | |
| GO:0009986~cell surface | FGB, KCNE1, CD151, AMBP, PACC1, LPAR1, CD99L2, TNFRSF10A, SLC6A1, ACVR1B, CX3CL1, SPN, TREML1, EFNB1, LRFN4, CD6, APOH, ADGRF5, SLITRK3, CACNG2, HAVCR1, CD200, JAM2 | |
| GO:0016020~membrane | LC44A5, DGKG, PTH1R, SLC6A1, SLC2A5, SLC2A6, CX3CL1, SPN, SYNPR, BSG, H4C1, VSIG8, CMKLR1, H3C8, ENTPD2, IL1R2, SLC2A12, BTN3A3, SYP, C1ORF162, BTN3A2, DNAJC7, ADGRF5, AGTR1, VDAC3, FTL, PTGER4, CD151, STAU2, SLC22A1, TMEM72, FPR1, SLC38A11, ADRB2, ACVR1B, EFNB2, SLCO1B1, PDPN, RPRM, C16ORF54, HSPA8, CMTM4, CAV2, FZD7, CYBRD1, DNAJC14, MCOLN1, TMEM154, TREML1, CD4, CD6, LPCAT4, FAM163A, AGO2, CD7, FAM171B, PLP2, CD200 | |
| GO:0016323~basolateral plasma membrane | SLCO1B1, CXADR, SLC7A8, SLC43A3, SLC22A1, PDPN, BSG, NUMB, PTH1R, SLC9B2 | |
| GO:0043005~neuron projection | SYNPR, CXADR, PLPPR1, RTN1, STAU2, SMN2, BRAF, SYP, SLC6A1, MARK4, CX3CL1, CD200 | |
| GO:0045121~membrane raft | EFNB1, MAG, CD4, KCNE1, CXADR, CAV2, PDPN, BSG, TNFRSF10A | |
| GO:0042383~sarcolemma | STAC, CAV2, BSG, SSPN, CACNG1, SLC2A5 | |
| GO:0016324~apical plasma membrane | KCNE1, SLC7A8, KCNE4, SLC22A1, PDPN, CYBRD1, PTH1R, ADRB2, SLC2A5, CRB3, SLC9B2 | |
| GO:0033270~paranode region of axon | MAG, EPB41L3, NCMAP | |
| GO:0036477~somatodendritic compartment | TMEM108, CACNG2, JAM2 | |
| GO:0009897~external side of plasma membrane | FGB, SPN, CD4, CD6, MCAM, BTN3A3, CXCR6, BTN3A2, FCGR2B, ERMAP, IL27RA | |
| GO:0070062~extracellular exosome | TSG101, TTYH3, SLC2A5, CST4, SPN, RAB43, EFNB1, HPX, CYSRT1, APOH, EPB41L2, BSG, H4C1, CAMP, FGB, H3C8, HSPA8, ENTPD2, AMBP, SYTL1, CYBRD1, DNAJC7, FXYD2, AGO2, VDAC3, RAB9A, ALDOB, NECTIN4, GPRC5D, CRB3, FTL | |
| GO:0043235~receptor complex | GPBAR1, PTH1R, ADRB2, GPRC5D, MCOLN1, ACVR1B, IL27RA | |
| GO:0005925~focal adhesion | EFNB2, HSPA8, TLE2, CD151, CAV2, MCAM, EPB41L2, BSG, NUMB, CD99L2 | |
| GO:0030672~synaptic vesicle membrane | SYT5, SYNPR, SYP, CBARP, SLC9B2 | |
| GO:0044297~cell body | CXADR, BRAF, FCGR2B, CD200 | |
| GO:0031528~microvillus membrane | SLC7A8, PDPN, SYTL1 | |
| GO:0008076~voltage-gated potassium channel complex | KCNE1, KCNIP1, KCNIP2, KCNAB3 | |
| GO:0098982~GABA-ergic synapse | LRFN4, SLITRK3, LPAR1, SLC6A1 | |
| GO:0072562~blood microparticle | FGB, HSPA8, HPX, PRSS1, AMBP | |
| GO:0030425~dendrite | EFNB2, HSPA8, SHARPIN, RTN1, KCNIP1, KCNIP2, AGO2, TMEM108, MARK4 | |
| Molecular Function | GO:0005515~protein binding | ZNF451, IL27RA, RAB43, SYNPR, CYSRT1, RUSC1, BSG, BANF1, MS4A12, H4C1, PDK1, CMKLR1, STARD3, TLE2, ENTPD2, CXADR, IL1R2, SYTL1, MAT1A, KCTD4, SLC9B2, NPAS4, AGTR1, APLNR, VDAC3, FTL, NAA60, ZNF792, KCNE1, TSG101, RTN1, CD151, KCNE4, GYPB, CUL2, SLC22A1, FPR1, LPAR1, SLC38A11, CD99L2, ADRB2, EFNB2, EFNB1, HPX, SHARPIN, APOH, EPB41L2, EPB41L3, SMN2, DMAP1, ICOS, MARK4, C16ORF54, HSPA8, NIBAN3, MPIG6B, AMBP, RBPMS, CAV2, STAC, RHBDD1, FZD7, SURF4, CYBRD1, TNFRSF10A, BRAF, TREML1, KIF18A, LRFN4, FAM163A, AGO2, CIBAR1, CIBAR2, CD200, SLC44A5, PTH1R, SLC2A5, SLC6A1, LITAF, SLC2A6, CX3CL1, SPN, TMEM108, HAVCR1, ERMAP, C11ORF24, TGIF1, FGB, H3C8, STRBP, MMP2, PTH2R, OLIG1, BTN3A2, SYP, KCNAB3, DNAJC7, SLC7A8, GFOD1, ADGRF5, RFT1, NUMB, ALDOB, GPRC5D, PTGER4, PLPPR1, SLC43A3, STAU2, PACC1, TMEM72, RCHY1, ACVR1B, RNF5, CST4, SLITRK3, PDPN, HS1BP3, S1PR3, CACNG1, CACNG2, RELT, LAIR1, JAM2, RPRM, KRTAP10-5, SEMA4D, KCNIP1, CMTM4, KCNIP2, TMEM176B, CIMAP1A, KIR3DL2, MCOLN1, DHRS2, TMEM154, CD4, FCGR2A, PIK3IP1, CD6, CD7, CCDC170, RAB9A, PLP2, FCGR2B, NECTIN4, CRB3 |
| GO:0015459~potassium channel regulator activity | KCNE1, KCNIP1, KCNIP2, KCNE4, ADRB2, KCNAB3 | |
| GO:0001618~virus receptor activity | EFNB2, CD4, CXADR, BSG, NECTIN4, HAVCR1 | |
| GO:0044325~ion channel binding | KCNE1, KCNIP1, STAC, KCNIP2, KCNE4, KCNAB3, CBARP | |
| GO:0022857~transmembrane transporter activity | SLC44A5, SLCO1B1, SLC7A8, SLC43A3, SLC22A1, SLC2A5, SLC2A6 | |
| GO:1902282~voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization | KCNE1, KCNE4, SCN2B | |
| GO:0005355~glucose transmembrane transporter activity | SLC2A12, SLC2A5, SLC2A6 | |
| GO:0004888~transmembrane signaling receptor activity | SPN, TREML1, CD4, FCGR2A, SEMA4D, FCGR2B, IL27RA | |
| GO:0042802~identical protein binding | ZNF792, SLC22A1, ADRB2, SLC6A1, RNF5, LITAF, SHARPIN, CYSRT1, APOH, SMN2, BANF1, KRTAP10-5, CXADR, SEMA4D, KCNIP2, CYBRD1, TNFRSF10A, BRAF, SYP, MAT1A, MCOLN1, SLC9B2, CD4, CD6, ALDOB, NECTIN4, FTL | |
| GO:0005102~receptor binding | FGB, MAG, CXADR, SEMA4D, PIK3IP1, PDPN, BTN3A3, BTN3A2, ERMAP, CX3CL1 | |
| GO:0004991~parathyroid hormone receptor activity | PTH2R, PTH1R | |
| GO:0038023~signaling receptor activity | CD4, SEMA4D, BSG, CD7, APLNR, TNFRSF10A, CMKLR1 | |
| GO:0044877~macromolecular complex binding | NPAS4, KCNE1, TSG101, SHARPIN, KCNIP2, CUL2, ADRB2, FCGR2B, RNF5 | |
| GO:0004930~G-protein coupled receptor activity | FZD7, LPAR1, FPR1, MRGPRF, PTH2R, CXCR6, ADRB2, GPR173, ADGRF5, AGTR1, APLNR, S1PR3, GPRC5D, CMKLR1 | |
| GO:0008289~lipid binding | DGKG, STARD3, CD4, APOH, S1PR3, MCOLN1 | |
| GO:0042803~protein homodimerization activity | STARD3, NAA60, TSG101, AMBP, RBPMS, CAV2, TREX1, PTH1R, ADRB2, RCHY1, MAG, CD4, BANF1 |
| Annotation Category | GO Term | Gene Name |
|---|---|---|
| Biological Process | GO:0000381~regulation of alternative mRNA splicing, via spliceosome | RBFOX1, RBFOX2, CELF4, CELF5 |
| GO:0045862~positive regulation of Proteolysis | FBXW11, STUB1, BTRC | |
| GO:0045892~negative regulation of transcription, DNA-templeted | LBH, RBFOX2, HEY1, FBXW11, EPC1, BTRC, WTIP, GATAD2A | |
| GO:0009615~response to virus | RPS15A, PRKRA, ADAR, CCT5 | |
| GO:0043161~proteasome-mediated ubiquitin-dependent protein catabolic process | SHARPIN, FBXW11, STUB1, BTRC, KLHL20 | |
| GO:0006397~mRNA processing | RBFOX1, RBFOX2, CELF4, SMN2, ADAR | |
| GO:0016567~protein ubiquitination | FBXW11, MKRN3, STUB1, BTRC, LZTR1, RCHY1, KLHL20 | |
| GO:0007010~cytoskeleten organization | DES, ZMYM6, WTIP, KLHL20 | |
| GO:0016236~macroautophagy | TSG101, CHMP4C, ZFYVE1 | |
| GO:0000209~protein polyubiquitination | FBXW11, MKRN3, STUB1, BTRC | |
| GO:0042753~positive regulation of circadian rhythm | FBXW11, BTRC | |
| GO:0035455~response to interferon-alpha | ADAR, KLHL20 | |
| GO:0000122~negative regulation of transcription from RNA polymerase II promotor | TSG101, HEY1, DDX20, ZBTB42, EPC1, ZNF3, MEIS2, GATAD2A, THAP11 | |
| Cellular Compartment | GO:0005737~cytoplasm | ITSN2, HSPB6, CELF4, CELF5, MSI1, ADAR, MSI2, PPP1CC, LBH, HEY1, EPC1, METTL16, LZTS2, BTRC, CEP55, SH3GLB2, RBFOX1, RBFOX2, DYNLT3, TRAF1, PRKAR1B, PSME1, BLZF1, TSG101, FBLIM1, CAMK2A, DDX20, C10ORF88, DAZ3, ZBTB42, RCHY1, KLC1, SAV1, RPS15A, GNA11, SMN2, LPXN, CTAG1B, PDLIM5, ZC3H14, PIBF1, CCT5, CARD9, KLHL20, DES, IMPDH1, PRKRA, STUB1, CIBAR1 |
| GO:0005829~cytosol | ITSN2, TSG101, HSPB6, FBLIM1, CAMK2A, DDX20, MSI2, RCHY1, KLC1, SAV1, KRT83, PPP1CC, RPS15A, SHARPIN, SMN2, CEP72, PLXNA1, LPXN, LZTS2, BTRC, PDLIM5, CCT5, SH3GLB2, RBFOX2, RBPMS, FBXW11, KRTAP10-9, CARD9, TRAF1, KLHL20, THAP11, DES, PRKAR1B, DDAH2, IMPDH1, PRKRA, CHMP4C, PSME1, STUB1 | |
| GO:0005634~nucleus | HSPB6, CAMK2A, NAB2, DDX20, CELF4, CELF5, DAZ3, ZBTB42, MSI1, ADAR, ZNF3, RCHY1, SAV1, PPP1CC, LBH, HEY1, ZMYM6, SMN2, EPC1, LPXN, METTL16, BTRC, ZC3H14, PIBF1, RBFOX1, RBFOX2, FBXW11, DYNLT3, OLIG1, WTIP, MEIS2, GATAD2A, DES, IMPDH1, PRKRA, STUB1, CIBAR1, BLZF1 | |
| GO:0090543~Flemming body | TSG101, CHMP4C, CEP55 | |
| GO:0000151~ubiquitin ligase complex | SHARPIN, FBXW11, STUB1, RCHY1 | |
| GO:0005771~multivesicular body | TSG101, PRKAR1B, CHMP4C | |
| GO:0030018~Z-disc | DES, SMN2, STUB1, PDLIM5 | |
| GO:0005813~centrosome | ITSN2, FBXW11, CEP72, LZTS2, PIBF1, CCT5, CEP55 | |
| GO:0000776~kinetochore | PPP1CC, FBXW11, DYNLT3, CHMP4C | |
| GO:0005654~nucleoplasm | SH3GLB2, RBFOX2, RBPMS, CAMK2A, NAB2, DDX20, CELF4, ZBTB42, ADAR, RCHY1, GATAD2A, THAP11, PPP1CC, RPS15A, HEY1, PRKRA, SMN2, PSME1, CEP72, PLXNA1, EPC1, STUB1, BTRC, BLZF1 | |
| GO:1990904~ribonucleoprotein complex | CELF4, MKRN3, CELF5, ZC3H14 | |
| GO:0030496~midbody | PPP1CC, CHMP4C, LZTS2, CEP55 | |
| GO:0032797~SMN complex | DDX20, SMN2 | |
| GO:0097504~Gemini of coiled bodies | DDX20, SMN2 | |
| Molecular Function | GO:0005515~protein binding | ITSN2, NAB2, IHH, CELF5, MSI1, ADAR, MSI2, ZFYVE1, PPP1CC, LBH, CYSRT1, HEY1, EPC1, LZTS2, BTRC, SMCO1, CEP55, LINC02875, SH3GLB2, RBFOX1, RBFOX2, FBXW11, DYNLT3, OLIG1, TRAF1, THAP8, PRKAR1B, DDAH2, PPP1R3B, CHMP4C, PSME1, PRR23E, NUP54, METTL27, BLZF1, TSG101, FBLIM1, CAMK2A, MOSPD2, DDX20, C10ORF88, DAZ3, ZBTB42, ZNF3, RCHY1, KLC1, KRT83, SAV1, FAM168A, RPS15A, SHARPIN, GNA11, MGAT5B, SMN2, CEP72, LPXN, CTAG1B, PDLIM5, ZC3H14, PIBF1, CCT5, RBPMS, KRTAP10-9, MIIP, CARD9, WTIP, MEIS2, GATAD2A, KLHL20, THAP11, DES, PRKRA, CCDC170, CORO6, MKRN3, NMUR2, STUB1, LZTR1, CIBAR1 |
| GO:0042802~identical protein binding | SH3GLB2, DYNLT3, CARD9, CAMK2A, NAB2, C10ORF88, MSI1, TRAF1, MSI2, ZNF3, SAV1, SHARPIN, DES, CYSRT1, IMPDH1, PRKRA, SMN2, MKRN3, NUP54, CTAG1B, PIBF1, CEP55 | |
| GO:0003723~RNA binding | RBFOX1, RBFOX2, RBPMS, DDX20, CELF4, CELF5, ALG13, ADAR, MSI1, MSI2, PPP1CC, RPS15A, IMPDH1, PRKRA, SMN2, MKRN3, METTL16, ZC3H14 | |
| GO:0003729~mRNA binding | RBFOX1, RBFOX2, RBPMS, CELF4, CELF5, MSI1, MSI2 | |
| GO:0004842~ubiquitin-protein transferase activity | SHARPIN, FBXW11, STUB1, TRAF1, BTRC, RCHY1, KLHL20 | |
| GO:0042803~protein homodimerization activity | TSG101, RBPMS, HSPB6, PRKRA, CARD9, CAMK2A, CHMP4C, STUB1, RCHY1 | |
| GO:0046983~protein dimerization activity | HEY1, FBXW11, OLIG1, BTRC |
| Annotation cluster 1 | Enrichment score: 10.14 | Amount of hits |
| GOTERM_CC_DIRECT | plasma membrane | 138 |
| UP_KW_CELLULAR_COMPONENT | cell membrane | 103 |
| UP_KW_CELLULAR_COMPONENT | Membrane | 170 |
| Annotation cluster 2 | Enrichment score: 2.38 | Amount of hits |
| GOTERM_BP_DIRECT | viral entry into host cell | 7 |
| UP_KW_MOLECULAR_FUNCTION | host cell receptor for virus entry | 6 |
| GOTERM_MF_DIRECT | virus receptor activity | 6 |
| Annotation cluster 3 | Enrichment score: 1.9 | Amount of hits |
| GOTERM_BP_DIRECT | miRNA loading onto RISC involved in gene silencing by miRNA | 3 |
| UP_KW_BIOLOGICAL_PROCESS | RNA-mediated gene silencing | 5 |
| GOTERM_BP_DIRECT | pre-miRNA processing | 3 |
| GOTERM_MF_DIRECT | double-stranded RNA binding | 5 |
| Annotation cluster 4 | Enrichment score: 1.72 | Amount of hits |
| GOTERM_MF_DIRECT | mRNA binding | 11 |
| UP_KW_MOLECULAR_FUNCTION | RNA-binding | 21 |
| GOTERM_MF_DIRECT | RNA binding | 27 |
| Annotation cluster 5 | Enrichment score: 1.7 | Amount of hits |
| UP_KW_MOLECULAR_FUNCTION | voltage-gated channel | 8 |
| GOTERM_BP_DIRECT | membrane repolarization during ventricular cardiac muscle cell action potential | 3 |
| GOTERM_MF_DIRECT | voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization | 3 |
| GOTERM_BP_DIRECT | regulation of heart rate by cardiac conduction | 3 |
| Annotation cluster 6 | Enrichment score: 1.62 | Amount of hits |
| GOTERM_MF_DIRECT | potassium channel regulator activity | 6 |
| GOTERM_BP_DIRECT | regulation of potassium ion transmembrane transport | 4 |
| GOTERM_MF_DIRECT | ion channel binding | 8 |
| UP_KW_MOLECULAR_FUNCTION | voltage-gated channel | 8 |
| GOTERM_BP_DIRECT | potassium ion export across plasma membrane | 3 |
| UP_KW_BIOLOGICAL_PROCESS | Ion transport | 17 |
| UP_KW_BIOLOGICAL_PROCESS | Potassium transport | 6 |
| UP_KW_MOLECULAR_FUNCTION | Ion channel | 11 |
| UP_KW_MOLECULAR_FUNCTION | Potassium channel | 4 |
| GOTERM_CC_DIRECT | voltage-gated potassium channel complex | 4 |
| GOTERM_MF_DIRECT | voltage-gated potassium channel activity | 3 |
| GOTERM_BP_DIRECT | potassium ion transmembrane transport | 3 |
| Annotation cluster 7 | Enrichment score: 1.53 | Amount of hits |
| UP_KW_MOLECULAR_FUNCTION | receptor | 43 |
| GOTERM_BP_DIRECT | positive regulation of cytosolic calcium ion concentration | 8 |
| UP_KW_MOLECULAR_FUNCTION | G-protein coupled receptor | 19 |
| UP_KW_MOLECULAR_FUNCTION | transducer | 20 |
| GOTERM_BP_DIRECT | G-protein coupled receptor signaling pathway | 20 |
| GOTERM_MF_DIRECT | G-protein coupled receptor activity | 16 |
| Annotation cluster 8 | Enrichment score: 1.5 | Amount of hits |
| GOTERM_MF_DIRECT | glucose transmembrane transporter activity | 3 |
| UP_KW_BIOLOGICAL_PROCESS | sugar transport | 4 |
| GOTERM_BP_DIRECT | glucose transmembrane transport | 3 |
| Annotation cluster 9 | Enrichment score: 1.04 | Amount of hits |
| GOTERM_BP_DIRECT | chemotaxis | 7 |
| GOTERM_BP_DIRECT | chemokine-mediated signaling pathway | 3 |
| GOTERM_BP_DIRECT | inflammatory response | 8 |
| Annotation cluster 10 | Enrichment score: 0.96 | Amount of hits |
| UP_KW_MOLECULAR_FUNCTION | developmental protein | 21 |
| UP_KW_MOLECULAR_FUNCTION | developmental protein | 21 |
| UP_KW_BIOLOGICAL_PROCESS | differentiation | 16 |
| Annotation cluster 11 | Enrichment score: 0.94 | Amount of hits |
| GOTERM_BP_DIRECT | positive regulation of proteolysis | 3 |
| GOTERM_BP_DIRECT | post-translational protein modification | 4 |
| GOTERM_BP_DIRECT | SCF-dep. proteasomal ubiquitin-dep. protein catabolic process | 4 |
| GOTERM_MF_DIRECT | ubiquitin-protein transferase activity | 8 |
| GOTERM_BP_DIRECT | protein polyubiquitination | 6 |
| GOTERM_BP_DIRECT | proteasome-mediated ubiquitin-dep. protein catabolic process | 6 |
| GOTERM_MF_DIRECT | ubiquitin protein ligase activity | 7 |
| Annotation cluster 12 | Enrichment score: 0.79 | Amount of hits |
| GOTERM_MF_DIRECT | protein binding involved in protein folding | 4 |
| GOTERM_BP_DIRECT | protein refolding | 3 |
| UP_KW_MOLECULAR_FUNCTION | chaperone | 7 |
| GOTERM_MF_DIRECT | unfolded protein binding | 5 |
| GOTERM_BP_DIRECT | protein folding | 5 |
| UP_KW_BIOLOGICAL_PROCESS | stress response | 3 |
| GOTERM_MF_DIRECT | ATPase activity | 6 |
| Annotation cluster 13 | Enrichment score: 0.68 | Amount of hits |
| GOTERM_CC_DIRECT | ubiquitin ligase complex | 5 |
| GOTERM_MF_DIRECT | ubiquitin-protein transferase activity | 8 |
| GOTERM_BP_DIRECT | protein destabilization | 3 |
| GOTERM_BP_DIRECT | proteasome-mediated ubiquitin-dep. protein catabolic process | 6 |
| GOTERM_BP_DIRECT | protein ubiquitination | 10 |
| UP_KW_BIOLOGICAL_PROCESS | Ubl conjugation pathway | 13 |
| GOTERM_MF_DIRECT | ubiquitin protein ligase activity | 7 |
| GOTERM_BP_DIRECT | ubiquitin-dependent protein catabolic process | 5 |
| Annotation cluster 14 | Enrichment score: 0.62 | Amount of hits |
| GOTERM_BP_DIRECT | cell projection organization | 4 |
| GOTERM_CC_DIRECT | ciliary basal body | 5 |
| UP_KW_BIOLOGICAL_PROCESS | cilium biogenesis/degradation | 5 |
| GOTERM_CC_DIRECT | centriole | 4 |
| GOTERM_BP_DIRECT | cilium assembly | 4 |
| Annotation cluster 15 | Enrichment score: 0.59 | Amount of hits |
| GOTERM_BP_DIRECT | regulation of alternative mRNA splicing, via spliceosome | 4 |
| GOTERM_BP_DIRECT | mRNA processing | 6 |
| UP_KW_BIOLOGICAL_PROCESS | mRNA processing | 8 |
| UP_KW_BIOLOGICAL_PROCESS | mRNA splicing | 6 |
| GOTERM_BP_DIRECT | RNA splicing | 4 |
| Annotation cluster 16 | Enrichment score: 0.59 | Amount of hits |
| UP_KW_BIOLOGICAL_PROCESS | differentiation | 16 |
| UP_KW_BIOLOGICAL_PROCESS | spermatogenesis | 6 |
| GOTERM_BP_DIRECT | cell differentiation | 9 |
| GOTERM_BP_DIRECT | spermatogenesis | 6 |
| Annotation cluster 17 | Enrichment score: 0.34 | Amount of hits |
| GOTERM_BP_DIRECT | microtubule-based movement | 3 |
| UP_KW_CELLULAR_COMPONENT | microtubule | 6 |
| UP_KW_MOLECULAR_FUNCTION | motor protein | 3 |
| Annotation cluster 18 | Enrichment score: 0.26 | Amount of hits |
| GOTERM_CC_DIRECT | midbody | 5 |
| UP_KW_BIOLOGICAL_PROCESS | cell division | 7 |
| GOTERM_BP_DIRECT | cell cycle | 6 |
| UP_KW_BIOLOGICAL_PROCESS | mitosis | 5 |
| GOTERM_BP_DIRECT | cell division | 6 |
| UP_KW_BIOLOGICAL_PROCESS | cell cycle | 8 |
| Annotation cluster 19 | Enrichment score: 0.21 | Amount of hits |
| UP_KW_BIOLOGICAL_PROCESS | adaptive immunity | 9 |
| UP_KW_BIOLOGICAL_PROCESS | immunity | 13 |
| GOTERM_BP_DIRECT | adaptive immune response | 6 |
| Annotation cluster 20 | Enrichment score: 0.08 | Amount of hits |
| GOTERM_MF_DIRECT | kinase activity | 4 |
| GOTERM_BP_DIRECT | protein phosphorylation | 7 |
| UP_KW_MOLECULAR_FUNCTION | kinase | 9 |
| UP_KW_MOLECULAR_FUNCTION | serine/threonine-protein kinase | 4 |
| GOTERM_MF_DIRECT | protein serine/threonine kinase activity | 4 |
| Annotation cluster 21 | Enrichment score: 0.08 | Amount of hits |
| GOTERM_CC_DIRECT | extracellular region | 28 |
| GOTERM_CC_DIRECT | extracellular space | 25 |
| UP_KW_CELLULAR_COMPONENT | secreted | 23 |
| Annotation cluster 22 | Enrichment score: 0.05 | Amount of hits |
| UP_KW_MOLECULAR_FUNCTION | repressor | 11 |
| GOTERM_BP_DIRECT | positive regulation of transcription from RNA polymerase II promoter | 15 |
| GOTERM_BP_DIRECT | regulation of transcription, DNA-templated | 12 |
| GOTERM_MF_DIRECT | transcriptional activator activity, RNA polymerase II transcription regulatory region sequence-specific binding | 6 |
| GOTERM_CC_DIRECT | chromatin | 10 |
| UP_KW_BIOLOGICAL_PROCESS | transcription regulation | 26 |
| UP_KW_MOLECULAR_FUNCTION | DNA-binding | 22 |
| UP_KW_BIOLOGICAL_PROCESS | transcription | 26 |
| GOTERM_MF_DIRECT | RNA polymerase II transcription factor activity, sequence-specific DNA binding | 12 |
| GOTERM_MF_DIRECT | RNA polymerase II core promoter proximal region sequence-specific DNA binding | 11 |
| GOTERM_MF_DIRECT | sequence-specific double-stranded DNA binding | 4 |
| GOTERM_MF_DIRECT | transcription factor activity, sequence-specific DNA binding | 4 |
| GOTERM_BP_DIRECT | regulation of transcription from RNA polymerase II promoter | 13 |
| Gene Symbol | HEV Interacting Protein | Function in Viral Infection | Reference |
|---|---|---|---|
| UBR2 | ORF2 |
| [68] |
| FTL | ORF2, ORF3 |
| [26,28,69,70] |
| TNFRSF10B | ORF2 |
| [71,72,73] |
| TOMM34 | ORF2 |
| [74,75,76] |
| TIGIT | ORF2 |
| [77,78,79] |
| FCGR2A | ORF2, ORF3 |
| [80,81] |
| ANXA4 | ORF2 |
| [82,83,84] |
| CMTM6 | ORF2 |
| [85] |
| TTC1 | ORF2 |
| [86] |
| CAPNS2 | ORF2 |
| [87,88] |
| PSMA5 | ORF2 |
| [89,90] |
| FUBP3 | ORF2 |
| [91,92,93] |
| LGALS8 | ORF2 |
| [94] |
| STUB1 | ORF2, ORF4 |
| [95,96,97] |
| STAT5B | ORF2 |
| [98,99,100,101] |
| ADRB2 | ORF3 |
| [102,103] |
| PDPN | ORF3 |
| [104] |
| IL27RA | ORF3 |
| [105,106] |
| ICOS | ORF3 |
| [107] |
| SLITRK3 | ORF3 |
| [108,109] |
| FCGR2B | ORF3 |
| [110,111,112] |
| CD7 | ORF3 |
| [113,114,115,116] |
| SLC9B2 | ORF3 |
| [117] |
| LPAR1 | ORF3 |
| [118] |
| RBPMS | ORF3, ORF4 |
| [119,120] |
| CMKLR1 | ORF3 |
| [121,122] |
| APLNR | ORF3 |
| [123] |
| SLCO1B1 | ORF3 |
| [124] |
| PRRS1 | ORF3 |
| [125,126] |
| EPB41L3 | ORF3 |
| [127,128] |
| CACNG1 | ORF3 |
| [129] |
| DNAJC14 | ORF3 |
| [130,131] |
| SLC2A5 | ORF3 |
| [132] |
| RTN1 | ORF3 |
| [133] |
| STARD3 | ORF3 |
| [134] |
| CXCR6 | ORF3 |
| [135,136,137,138] |
| CST4 | ORF3 |
| [139] |
| FPR1 | ORF3 |
| [140,141,142] |
| TMEM176B | ORF3 |
| [143] |
| RNF5 | ORF3 |
| [66,67,144,145] |
| CXADR | ORF3 |
| [44] |
| ZAR1L | ORF3 |
| [146] |
| PDK1 | ORF3 |
| [147,148] |
| TGIF1 | ORF3 |
| [149,150,151] |
| FZD7 | ORF3 |
| [152] |
| SHARPIN | ORF3, ORF4 |
| [58,59,153,154] |
| TNFRSF10A | ORF3 |
| [155,156,157] |
| SCN2B | ORF3 |
| [158,159] |
| OCT1 | ORF3 |
| [160] |
| PLPPR1 | ORF3 |
| [161] |
| ADGRF5 | ORF3 |
| [162] |
| ENTPD2 | ORF3 |
| [163] |
| SLC38A11 | ORF3 |
| [164] |
| SLC44A5 | ORF3 |
| [5] |
| EPB41L2 | ORF3 |
| [165,166] |
| GPBAR1 | ORF3 |
| [167,168,169] |
| LITAF | ORF3 |
| [170,171,172] |
| APOM | ORF3 |
| [173,174] |
| SYP | ORF3 |
| [175,176,177] |
| NEUROD2 | ORF3 |
| [178] |
| RCHY1 | ORF3, ORF4 |
| [179,180,181,182] |
| S1PR3 | ORF3 |
| [183] |
| ACVR1B | ORF3 |
| [184] |
| EFNB1 | ORF3 |
| [185] |
| DNAJC7 | ORF3 |
| [186,187] |
| CAV2 | ORF3 |
| [188,189,190] |
| VDAC3 | ORF3 |
| [191,192] |
| SPN | ORF3 |
| [193,194] |
| MAG | ORF3 |
| [195,196] |
| BSG | ORF3 |
| [45,46] |
| BTN3A2 | ORF3 |
| [197] |
| CRB3 | ORF3 |
| [198] |
| NUMB | ORF3 |
| [199,200] |
| NECTIN4 | ORF3 |
| [48] |
| TSG101 | ORF3, ORF4 |
| [15] |
| PTH1R | ORF3 |
| [201] |
| PTGER4 | ORF3 |
| [202] |
| SURF4 | ORF3 |
| [203] |
| CX3CL1 | ORF3 |
| [204,205,206] |
| CYBRD1 | ORF3 |
| [207] |
| HAVCR1 | ORF3 |
| [49,50] |
| AGTR1 | ORF3 |
| [47] |
| BANF1 | ORF3 |
| [208,209,210] |
| BRAF | ORF3 |
| [211,212] |
| BTN3A3 | ORF3 |
| [197,213] |
| CAMP | ORF3 |
| [214,215,216] |
| CD6 | ORF3 |
| [217] |
| CUL2 | ORF3 |
| [218,219,220] |
| AGO2 | ORF3 |
| [221,222,223,224] |
| FXYD2 | ORF3 |
| [225] |
| H3C8 | ORF3 |
| [226,227] |
| H4C1 | ORF3 |
| [228,229] |
| IL1R2 | ORF3 |
| [230,231] |
| KIF18A | ORF3 |
| [232] |
| KIR3DL2 | ORF3 |
| [233,234] |
| LAIR1 | ORF3 |
| [235,236] |
| MMP2 | ORF3 |
| [237] |
| NAA60 | ORF3 |
| [238] |
| NPAS4 | ORF3 |
| [239] |
| PIWIL4 | ORF3 |
| [240] |
| RAB43 | ORF3 |
| [241] |
| RAB9A | ORF3 |
| [242,243,244] |
| SEMA4D | ORF3 |
| [245,246,247] |
| STAU2 | ORF3 |
| [248] |
| TREX1 | ORF3 |
| [249] |
| MSI2 | ORF4 |
| [250] |
| CAMK2A | ORF4 |
| [251,252,253] |
| DDAH2 | ORF4 |
| [254,255] |
| LBH | ORF4 |
| [256] |
| TRAF1 | ORF4 |
| [32,257,258] |
| DYNLT3 | ORF4 |
| [259] |
| GATAD2A | ORF4 |
| [260] |
| PPP1CC | ORF4 |
| [261] |
| CCT5 | ORF4 |
| [262,263] |
| ZFYVE1 | ORF4 |
| [264,265] |
| DDX20 | ORF4 |
| [266] |
| HEY1 | ORF4 |
| [267] |
| CEP72 | ORF4 |
| [268] |
| CEP55 | ORF4 |
| [269] |
| KLHL20 | ORF4 |
| [270] |
| NAB2 | ORF4 |
| [271] |
| RBFOX2 | ORF4 |
| [272] |
| SH3GLB2 | ORF4 |
| [273] |
| PSME1 | ORF4 |
| [274] |
| MSI1 | ORF4 |
| [275] |
| FBXW11 | ORF4 |
| [276,277] |
| BTRC | ORF4 |
| [278,279] |
| RPS15A | ORF4 |
| [280,281] |
| PRKAR1B | ORF4 |
| [282] |
| ADAR | ORF4 |
| [55,283] |
| CARD9 | ORF4 |
| [284,285,286] |
| PRKRA | ORF4 |
| [287,288,289] |
| DES | ORF4 |
| [290,291,292] |
| IMPDH1 | ORF4 |
| [293] |
| ZMYM6 | ORF4 |
| [294] |
| CHMP4C | ORF4 |
| [295,296,297] |
References
- Titeca, K.; Lemmens, I.; Tavernier, J.; Eyckerman, S. Discovering cellular protein-protein interactions: Technological strategies and opportunities. Mass Spectrom. Rev. 2019, 38, 79–111. [Google Scholar] [CrossRef] [PubMed]
- Eyckerman, S.; Verhee, A.; der Heyden, J.V.; Lemmens, I.; Ostade, X.V.; Vandekerckhove, J.; Tavernier, J. Design and application of a cytokine-receptor-based interaction trap. Nat. Cell Biol. 2001, 3, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Lievens, S.; Gerlo, S.; Lemmens, I.; De Clercq, D.J.; Risseeuw, M.D.; Vanderroost, N.; De Smet, A.S.; Ruyssinck, E.; Chevet, E.; Van Calenbergh, S.; et al. Kinase Substrate Sensor (KISS), a mammalian in situ protein interaction sensor. Mol. Cell. Proteom. MCP 2014, 13, 3332–3342. [Google Scholar] [CrossRef] [PubMed]
- Rolland, T.; Tasan, M.; Charloteaux, B.; Pevzner, S.J.; Zhong, Q.; Sahni, N.; Yi, S.; Lemmens, I.; Fontanillo, C.; Mosca, R.; et al. A proteome-scale map of the human interactome network. Cell 2014, 159, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Van Royen, T.; Sedeyn, K.; Moschonas, G.D.; Toussaint, W.; Vuylsteke, M.; Van Haver, D.; Impens, F.; Eyckerman, S.; Lemmens, I.; Tavernier, J.; et al. An Unexpected Encounter: Respiratory Syncytial Virus Nonstructural Protein 1 Interacts with Mediator Subunit MED25. J. Virol. 2022, 96, e0129722. [Google Scholar] [CrossRef] [PubMed]
- Van Schoubroeck, B.; Van Acker, K.; Dams, G.; Jochmans, D.; Clayton, R.; Berke, J.M.; Lievens, S.; Van der Heyden, J.; Tavernier, J. MAPPIT as a high-throughput screening assay for modulators of protein-protein interactions in HIV and HCV. Methods Mol. Biol. 2012, 812, 295–307. [Google Scholar] [CrossRef]
- Webb, G.W.; Dalton, H.R. Hepatitis E: An expanding epidemic with a range of complications. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 828–832. [Google Scholar] [CrossRef]
- Zhu, F.C.; Zhang, J.; Zhang, X.F.; Zhou, C.; Wang, Z.Z.; Huang, S.J.; Wang, H.; Yang, C.L.; Jiang, H.M.; Cai, J.P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef]
- Sayed, I.M.; Vercouter, A.S.; Abdelwahab, S.F.; Vercauteren, K.; Meuleman, P. Is hepatitis E virus an emerging problem in industrialized countries? Hepatology 2015, 62, 1883–1892. [Google Scholar] [CrossRef]
- Kenney, S.P.; Meng, X.J. Hepatitis E Virus Genome Structure and Replication Strategy. Cold Spring Harb. Perspect. Med. 2019, 9. [Google Scholar] [CrossRef]
- Montpellier, C.; Wychowski, C.; Sayed, I.M.; Meunier, J.C.; Saliou, J.M.; Ankavay, M.; Bull, A.; Pillez, A.; Abravanel, F.; Helle, F.; et al. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology 2018, 154, 211–223.e8. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Heller, B.; Capuccino, J.M.; Song, B.; Nimgaonkar, I.; Hrebikova, G.; Contreras, J.E.; Ploss, A. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc. Natl. Acad. Sci. USA 2017, 114, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Ma, Z.; Wang, R.; Yu, Y.; Kannan, H.; Fredericksen, B.; Zhang, Y.J. Enhancement of interferon induction by ORF3 product of hepatitis E virus. J. Virol. 2014, 88, 8696–8705. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Takahashi, M.; Jirintai, S.; Tanaka, T.; Nishizawa, T.; Yasuda, J.; Okamoto, H. Tumour susceptibility gene 101 and the vacuolar protein sorting pathway are required for the release of hepatitis E virions. J. Gen. Virol. 2011, 92, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.P.; Anang, S.; Subramani, C.; Madhvi, A.; Bakshi, K.; Srivastava, A.; Shalimar; Nayak, B.; Ranjith Kumar, C.T.; Surjit, M. Endoplasmic Reticulum Stress Induced Synthesis of a Novel Viral Factor Mediates Efficient Replication of Genotype-1 Hepatitis E Virus. PLoS Pathog. 2016, 12, e1005521. [Google Scholar] [CrossRef]
- Lievens, S.; Van der Heyden, J.; Masschaele, D.; De Ceuninck, L.; Petta, I.; Gupta, S.; De Puysseleyr, V.; Vauthier, V.; Lemmens, I.; De Clercq, D.J.; et al. Proteome-scale Binary Interactomics in Human Cells. Mol. Cell. Proteom. MCP 2016, 15, 3624–3639. [Google Scholar] [CrossRef]
- Masschaele, D.; Gerlo, S.; Lemmens, I.; Lievens, S.; Tavernier, J. KISS: A Mammalian Two-Hybrid Method for In Situ Analysis of Protein-Protein Interactions. Methods Mol. Biol. 2018, 1794, 269–278. [Google Scholar] [CrossRef]
- Lievens, S.; Vanderroost, N.; Van der Heyden, J.; Gesellchen, V.; Vidal, M.; Tavernier, J. Array MAPPIT: High-throughput interactome analysis in mammalian cells. J. Proteome Res. 2009, 8, 877–886. [Google Scholar] [CrossRef]
- Masschaele, D.; De Ceuninck, L.; Wauman, J.; Defever, D.; Stenner, F.; Lievens, S.; Peelman, F.; Tavernier, J. RNF41 interacts with the VPS52 subunit of the GARP and EARP complexes. PLoS ONE 2017, 12, e0178132. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudie, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; Verhoye, L.; Cocquerel, L.; Abravanel, F.; Foquet, L.; Montpellier, C.; Debing, Y.; Farhoudi, A.; Wychowski, C.; Dubuisson, J.; et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut 2017, 66, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Miao, J.; Zhao, M.; Tang, M.; Yeo, A.E.; Yu, H.; Zhang, J.; Xia, N.S. Role of heat-shock protein 90 in hepatitis E virus capsid trafficking. J. Gen. Virol. 2010, 91, 1728–1736. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, W.; Kang, Y.; Chen, Y.; Cui, L.; Yang, Z.; Hua, X. HEV-Capsid Protein Interacts With Cytochrome P4502C8 and Retinol-Binding Protein 4. Hepat. Mon. 2011, 11, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Huang, W.; Yang, J.; Wen, Z.; Geng, Y.; Zhao, C.; Zhang, H.; Wang, Y. Systematic identification of hepatitis E virus ORF2 interactome reveals that TMEM134 engages in ORF2-mediated NF-kappaB pathway. Virus Res. 2017, 228, 102–108. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Wen, Z.; Zhang, F.; Qi, Y.; Huang, W.; Zhang, H.; Wang, Y. Asialoglycoprotein receptor facilitates infection of PLC/PRF/5 cells by HEV through interaction with ORF2. J. Med. Virol. 2016, 88, 2186–2195. [Google Scholar] [CrossRef]
- Geng, Y.; Yang, J.; Huang, W.; Harrison, T.J.; Zhou, Y.; Wen, Z.; Wang, Y. Virus host protein interaction network analysis reveals that the HEV ORF3 protein may interrupt the blood coagulation process. PLoS ONE 2013, 8, e56320. [Google Scholar] [CrossRef]
- Tyagi, S.; Surjit, M.; Roy, A.K.; Jameel, S.; Lal, S.K. The ORF3 protein of hepatitis E virus interacts with liver-specific alpha1-microglobulin and its precursor alpha1-microglobulin/bikunin precursor (AMBP) and expedites their export from the hepatocyte. J. Biol. Chem. 2004, 279, 29308–29319. [Google Scholar] [CrossRef]
- Venkatesan, K.; Rual, J.F.; Vazquez, A.; Stelzl, U.; Lemmens, I.; Hirozane-Kishikawa, T.; Hao, T.; Zenkner, M.; Xin, X.; Goh, K.I.; et al. An empirical framework for binary interactome mapping. Nat. Methods 2009, 6, 83–90. [Google Scholar] [CrossRef]
- LeDesma, R.; Nimgaonkar, I.; Ploss, A. Hepatitis E Virus Replication. Viruses 2019, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.K.; Lole, K.S. Hepatitis E virus ORF1 encoded non structural protein-host protein interaction network. Virus Res. 2016, 213, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, K.; Ferrie, M.; Ankavay, M.; Montpellier, C.; Camuzet, C.; Alexandre, V.; Dembele, A.; Lecoeur, C.; Foe, A.T.; Bouquet, P.; et al. An Arginine-Rich Motif in the ORF2 capsid protein regulates the hepatitis E virus lifecycle and interactions with the host cell. PLoS Pathog. 2022, 18, e1010798. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.; Moffat, K.; Brooks, E.; Wileman, T. A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication. Adv. Virus Res. 2007, 70, 101–182. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Suzuki, R.; Kataoka, C.; Watashi, K.; Aizaki, H.; Kato, N.; Matsuura, Y.; Suzuki, T.; Wakita, T. Alternative endocytosis pathway for productive entry of hepatitis C virus. J. Gen. Virol. 2014, 95, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Serrano, E.E.; Gonzalez-Lopez, O.; Das, A.; Lemon, S.M. Cellular entry and uncoating of naked and quasi-enveloped human hepatoviruses. eLife 2019, 8, e43983. [Google Scholar] [CrossRef]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef]
- Raab-Traub, N.; Dittmer, D.P. Viral effects on the content and function of extracellular vesicles. Nat. Reviews. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef]
- Stravalaci, M.; Pagani, I.; Paraboschi, E.M.; Pedotti, M.; Doni, A.; Scavello, F.; Mapelli, S.N.; Sironi, M.; Perucchini, C.; Varani, L.; et al. Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules. Nat. Immunol. 2022, 23, 275–286. [Google Scholar] [CrossRef]
- Dansako, H.; Yamane, D.; Welsch, C.; McGivern, D.R.; Hu, F.; Kato, N.; Lemon, S.M. Class A scavenger receptor 1 (MSR1) restricts hepatitis C virus replication by mediating toll-like receptor 3 recognition of viral RNAs produced in neighboring cells. PLoS Pathog. 2013, 9, e1003345. [Google Scholar] [CrossRef] [PubMed]
- Bonaparte, M.I.; Dimitrov, A.S.; Bossart, K.N.; Crameri, G.; Mungall, B.A.; Bishop, K.A.; Choudhry, V.; Dimitrov, D.S.; Wang, L.F.; Eaton, B.T.; et al. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl. Acad. Sci. USA 2005, 102, 10652–10657. [Google Scholar] [CrossRef] [PubMed]
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell binding and entry. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, K. The coxsackievirus and adenovirus receptor: Virological and biological beauty. FEBS Lett. 2020, 594, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Vanarsdall, A.L.; Pritchard, S.R.; Wisner, T.W.; Liu, J.; Jardetzky, T.S.; Johnson, D.C. CD147 Promotes Entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and Endothelial Cells. mBio 2018, 9, e00781-18. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Yoneda, M.; Ikeda, F.; Terao-Muto, Y.; Sato, H.; Kai, C. CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells. J. Virol. 2010, 84, 4183–4193. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.P.; Zhou, R.; Chan, K.H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2021, 184, 2212–2228.e12. [Google Scholar] [CrossRef]
- Muhlebach, M.D.; Mateo, M.; Sinn, P.L.; Prufer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Corneillie, L.; Lemmens, I.; Montpellier, C.; Ferrie, M.; Weening, K.; Van Houtte, F.; Hanoulle, X.; Cocquerel, L.; Amara, A.; Tavernier, J.; et al. The phosphatidylserine receptor TIM1 promotes infection of enveloped hepatitis E virus. Cell. Mol. Life Sci. CMLS 2023, 80, 326. [Google Scholar] [CrossRef]
- Dong, C.; Zafrullah, M.; Mixson-Hayden, T.; Dai, X.; Liang, J.; Meng, J.; Kamili, S. Suppression of interferon-alpha signaling by hepatitis E virus. Hepatology 2012, 55, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, M.; Huang, Y.; Peng, W.; Zheng, Z.; Xia, N.; Xu, J.; Tian, D. The ORF3 Protein of Genotype 1 Hepatitis E Virus Suppresses TLR3-induced NF-kappaB Signaling via TRADD and RIP1. Sci. Rep. 2016, 6, 27597. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Ding, Q. Hepatitis E Virus Assembly and Release. Viruses 2019, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Subramani, C.; Nair, V.P.; Anang, S.; Mandal, S.D.; Pareek, M.; Kaushik, N.; Srivastava, A.; Saha, S.; Shalimar; Nayak, B.; et al. Host-Virus Protein Interaction Network Reveals the Involvement of Multiple Host Processes in the Life Cycle of Hepatitis E Virus. mSystems 2018, 3. [Google Scholar] [CrossRef]
- Samuel, C.E. Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral. Virology 2011, 411, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of protein kinase PKR in cell biology: From antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. MMBR 2006, 70, 1032–1060. [Google Scholar] [CrossRef] [PubMed]
- Edilova, M.I.; Abdul-Sater, A.A.; Watts, T.H. TRAF1 Signaling in Human Health and Disease. Front. Immunol. 2018, 9, 2969. [Google Scholar] [CrossRef]
- MacDuff, D.A.; Baldridge, M.T.; Qaqish, A.M.; Nice, T.J.; Darbandi, A.D.; Hartley, V.L.; Peterson, S.T.; Miner, J.J.; Iwai, K.; Virgin, H.W. HOIL1 Is Essential for the Induction of Type I and III Interferons by MDA5 and Regulates Persistent Murine Norovirus Infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Belgnaoui, S.M.; Paz, S.; Samuel, S.; Goulet, M.L.; Sun, Q.; Kikkert, M.; Iwai, K.; Dikic, I.; Hiscott, J.; Lin, R. Linear ubiquitination of NEMO negatively regulates the interferon antiviral response through disruption of the MAVS-TRAF3 complex. Cell Host Microbe 2012, 12, 211–222. [Google Scholar] [CrossRef]
- Inn, K.S.; Gack, M.U.; Tokunaga, F.; Shi, M.; Wong, L.Y.; Iwai, K.; Jung, J.U. Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction. Mol. Cell 2011, 41, 354–365. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Ambardekar, C.; Hu, Z.; Lhomme, S.; Feng, Z. Hepatitis E virus persists in the presence of a type III interferon response. PLoS Pathog. 2017, 13, e1006417. [Google Scholar] [CrossRef] [PubMed]
- Sooryanarain, H.; Heffron, C.L.; Meng, X.J. The U-Rich Untranslated Region of the Hepatitis E Virus Induces Differential Type I and Type III Interferon Responses in a Host Cell-Dependent Manner. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dao Thi, V.L.; Liu, P.; Takacs, C.N.; Xiang, K.; Andrus, L.; Gouttenoire, J.; Moradpour, D.; Rice, C.M. Pan-Genotype Hepatitis E Virus Replication in Stem Cell-Derived Hepatocellular Systems. Gastroenterology 2018, 154, 663–674.e667. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Syed, G.H.; Kim, S.J.; Siddiqui, A. Hepatitis B Virus-Induced Parkin-Dependent Recruitment of Linear Ubiquitin Assembly Complex (LUBAC) to Mitochondria and Attenuation of Innate Immunity. PLoS Pathog. 2016, 12, e1005693. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xu, S.; Wei, Y.L.; Zhang, X.G.; Wang, Q.; Jia, Y.N.; Wang, W.B.; Han, L.; Chen, Z.S.; Wang, Z.X.; et al. The PB1 protein of influenza A virus inhibits the innate immune response by targeting MAVS for NBR1-mediated selective autophagic degradation. PLoS Pathog. 2021, 17, e1009300. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, H.; Yu, S.; Ding, Y.; Wu, W.; Mao, X.; Liao, Y.; Meng, C.; Ur Rehman, Z.; Tan, L.; et al. Newcastle Disease Virus V Protein Degrades Mitochondrial Antiviral Signaling Protein To Inhibit Host Type I Interferon Production via E3 Ubiquitin Ligase RNF5. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Zhang, L.; Lei, C.Q.; Li, Y.; Mao, A.P.; Yang, Y.; Wang, Y.Y.; Zhang, X.L.; Shu, H.B. The Ubiquitin Ligase RNF5 Regulates Antiviral Responses by Mediating Degradation of the Adaptor Protein MITA. Immunity 2009, 30, 397–407. [Google Scholar] [CrossRef]
- Boso, G.; Tasaki, T.; Kwon, Y.T.; Somia, N.V. The N-end rule and retroviral infection: No effect on integrase. Virol. J. 2013, 10, 233. [Google Scholar] [CrossRef]
- Ojha, N.K.; Lole, K.S. Hepatitis E virus ORF1 encoded macro domain protein interacts with light chain subunit of human ferritin and inhibits its secretion. Mol. Cell. Biochem. 2016, 417, 75–85. [Google Scholar] [CrossRef]
- Hao, Z.; Zheng, L.; Kluwe, L.; Huang, W. Ferritin light chain and squamous cell carcinoma antigen 1 are coreceptors for cellular attachment and entry of hepatitis B virus. Int. J. Nanomed. 2012, 7, 827–834. [Google Scholar] [CrossRef]
- Du, J.; Liang, X.; Liu, Y.; Qu, Z.; Gao, L.; Han, L.; Liu, S.; Cui, M.; Shi, Y.; Zhang, Z.; et al. Hepatitis B virus core protein inhibits TRAIL-induced apoptosis of hepatocytes by blocking DR5 expression. Cell Death Differ. 2009, 16, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sola-Riera, C.; Gupta, S.; Maleki, K.T.; Gonzalez-Rodriguez, P.; Saidi, D.; Zimmer, C.L.; Vangeti, S.; Rivino, L.; Leo, Y.S.; Lye, D.C.; et al. Hantavirus Inhibits TRAIL-Mediated Killing of Infected Cells by Downregulating Death Receptor 5. Cell Rep. 2019, 28, 2124–2139.e6. [Google Scholar] [CrossRef]
- Shin, G.C.; Kang, H.S.; Lee, A.R.; Kim, K.H. Hepatitis B virus-triggered autophagy targets TNFRSF10B/death receptor 5 for degradation to limit TNFSF10/TRAIL response. Autophagy 2016, 12, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Geiss, G.K.; Bumgarner, R.E.; An, M.C.; Agy, M.B.; van’t Wout, A.B.; Hammersmark, E.; Carter, V.S.; Upchurch, D.; Mullins, J.I.; Katze, M.G. Large-scale monitoring of host cell gene expression during HIV-1 infection using cDNA microarrays. Virology 2000, 266, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Alrefai, H.G.; Hussein, M.H.; Helal, G.M.; Khashana, M.S.; Fawzy, M.S. Overexpression of heat shock protein HSP90AA1 and translocase of the outer mitochondrial membrane TOM34 in HCV-induced hepatocellular carcinoma: A pilot study. Clin. Biochem. 2019, 63, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakul, J.; Thongtan, T.; Pannengpetch, S.; Wikan, N.; Kantamala, D.; Kumrapich, B.; Suwan, W.; Smith, D.R. Phosphoproteomic analysis of dengue virus infected U937 cells and identification of pyruvate kinase M2 as a differentially phosphorylated phosphoprotein. Sci. Rep. 2020, 10, 14493. [Google Scholar] [CrossRef] [PubMed]
- Holder, K.A.; Burt, K.; Grant, M.D. TIGIT blockade enhances NK cell activity against autologous HIV-1-infected CD4(+) T cells. Clin. Transl. Immunol. 2021, 10, e1348. [Google Scholar] [CrossRef] [PubMed]
- Schorer, M.; Rakebrandt, N.; Lambert, K.; Hunziker, A.; Pallmer, K.; Oxenius, A.; Kipar, A.; Stertz, S.; Joller, N. TIGIT limits immune pathology during viral infections. Nat. Commun. 2020, 11, 1288. [Google Scholar] [CrossRef]
- Wang, J.; Hou, H.; Mao, L.; Wang, F.; Yu, J.; Luo, Y.; Lin, Q.; Sun, Z. TIGIT Signaling Pathway Regulates Natural Killer Cell Function in Chronic Hepatitis B Virus Infection. Front. Med. 2021, 8, 816474. [Google Scholar] [CrossRef]
- Alvarez, R.A.; Maestre, A.M.; Law, K.; Durham, N.D.; Barria, M.I.; Ishii-Watabe, A.; Tada, M.; Kapoor, M.; Hotta, M.T.; Rodriguez-Caprio, G.; et al. Enhanced FCGR2A and FCGR3A signaling by HIV viremic controller IgG. JCI Insight 2017, 2, e88226. [Google Scholar] [CrossRef]
- Alagarasu, K.; Bachal, R.V.; Damle, I.; Shah, P.S.; Cecilia, D. Association of FCGR2A p.R131H and CCL2 c.-2518 A>G gene variants with thrombocytopenia in patients with dengue virus infection. Hum. Immunol. 2015, 76, 819–822. [Google Scholar] [CrossRef]
- Martínez-Betancur, V.; Martínez-Gutierrez, M. Proteomic profile of human monocytic cells infected with dengue virus. Asian Pac. J. Trop. Bio. 2016, 6, 914–923. [Google Scholar] [CrossRef]
- Saad, Z.M.; Fouad, Y.; Ali, L.H.; Hassanin, T.M. Clinical Significance of Annexin A4 as a Biomarker in the Early Diagnosis of Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Prieto, A.; Rubic, I.; Gonzalez-Sanchez, J.C.; Kules, J.; Martinez-Subiela, S.; Ceron, J.J.; Bernal, E.; Torres-Cantero, A.; Vicente-Romero, M.R.; Mrljak, V.; et al. Saliva changes in composition associated to COVID-19: A preliminary study. Sci. Rep. 2022, 12, 10879. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kakizaki, M.; Shimizu, T.; Carreras, J.; Chiba, T.; Chamoto, K.; Kagawa, T.; Aoki, T.; Nakamura, N.; Ando, K.; et al. PD-L1 is induced on the hepatocyte surface via CKLF-like MARVEL transmembrane domain-containing protein 6 up-regulation by the anti-HBV drug Entecavir. Int. Immunol. 2020, 32, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Coombs, K.M.; Berard, A.; Xu, W.; Krokhin, O.; Meng, X.; Cortens, J.P.; Kobasa, D.; Wilkins, J.; Brown, E.G. Quantitative proteomic analyses of influenza virus-infected cultured human lung cells. J. Virol. 2010, 84, 10888–10906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, W.; Mo, X.; Zhao, H.; Zheng, H.; Ke, C.; Zheng, W.; Tu, Y.; Zhang, Y. Erratum: Differential expressed genes in ECV304 Endothelial-like Cells infected with Human Cytomegalovirus. Afr. Health Sci. 2013, 13, 864–879. [Google Scholar] [CrossRef][Green Version]
- Kang, S.D.; Chatterjee, S.; Alam, S.; Salzberg, A.C.; Milici, J.; van der Burg, S.H.; Meyers, C. Effect of Productive Human Papillomavirus 16 Infection on Global Gene Expression in Cervical Epithelium. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Alfaro, E.; Diaz-Garcia, E.; Garcia-Tovar, S.; Zamarron, E.; Mangas, A.; Galera, R.; Lopez-Collazo, E.; Garcia-Rio, F.; Cubillos-Zapata, C. Upregulated Proteasome Subunits in COVID-19 Patients: A Link with Hypoxemia, Lymphopenia and Inflammation. Biomolecules 2022, 12, 442. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Yu, C.; Zhang, C.; Tang, Q.; Huang, H.; Zhao, Z. Hepatitis C virus induces oxidation and degradation of apolipoprotein B to enhance lipid accumulation and promote viral production. PLoS Pathog. 2021, 17, e1009889. [Google Scholar] [CrossRef]
- Dong, S.; Kong, N.; Wang, C.; Li, Y.; Sun, D.; Qin, W.; Zhai, H.; Zhai, X.; Yang, X.; Ye, C.; et al. FUBP3 Degrades the Porcine Epidemic Diarrhea Virus Nucleocapsid Protein and Induces the Production of Type I Interferon. J. Virol. 2022, 96, e0061822. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.J.; Lin, C.W.; Lai, C.C.; Lan, Y.C.; Lai, C.H.; Hung, C.H.; Hsueh, K.C.; Lin, T.H.; Chang, H.C.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Tong, W.; Chen, Y.M. FUSE binding protein FUBP3 is a potent regulator in Japanese encephalitis virus infection. Virol. J. 2021, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Montespan, C.; Marvin, S.A.; Austin, S.; Burrage, A.M.; Roger, B.; Rayne, F.; Faure, M.; Campell, E.M.; Schneider, C.; Reimer, R.; et al. Multi-layered control of Galectin-8 mediated autophagy during adenovirus cell entry through a conserved PPxY motif in the viral capsid. PLoS Pathog. 2017, 13, e1006217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, P.; He, D.C.; Wang, H.; He, H. RACK1 degrades MAVS to promote bovine ephemeral fever virus replication via upregulating E3 ubiquitin ligase STUB1. Vet. Microbiol. 2021, 257, 109096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Q.; Li, X.; Zhao, D.; Liu, Y.; Shen, Q.; Yang, M.; Wang, C.; Li, N.; Cao, X. Cytoplasmic STAT4 Promotes Antiviral Type I IFN Production by Blocking CHIP-Mediated Degradation of RIG-I. J. Immunol. 2016, 196, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, S.; Zhu, C.; Wang, C.; Yu, N.; Lin, Z.; Gan, J.; Guo, Y.; Huang, X.; He, Y.; et al. STUB1 is targeted by the SUMO-interacting motif of EBNA1 to maintain Epstein-Barr Virus latency. PLoS Pathog. 2020, 16, e1008447. [Google Scholar] [CrossRef]
- Pericle, F.; Pinto, L.A.; Hicks, S.; Kirken, R.A.; Sconocchia, G.; Rusnak, J.; Dolan, M.J.; Shearer, G.M.; Segal, D.M. HIV-1 infection induces a selective reduction in STAT5 protein expression. J. Immunol. 1998, 160, 28–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.X.; Mao, Q.Z.; Zhuo, J.C.; Huang, H.J.; Lu, J.B.; Zhang, C.X.; Li, J.M.; Chen, J.P.; Lu, G. The JAK-STAT pathway promotes persistent viral infection by activating apoptosis in insect vectors. PLoS Pathog. 2023, 19, e1011266. [Google Scholar] [CrossRef]
- Aydemir, M.N.; Aydemir, H.B.; Korkmaz, E.M.; Budak, M.; Cekin, N.; Pinarbasi, E. Computationally predicted SARS-CoV-2 encoded microRNAs target NFKB, JAK/STAT and TGFB signaling pathways. Gene Rep. 2021, 22, 101012. [Google Scholar] [CrossRef]
- Prost, S.; Le Dantec, M.; Auge, S.; Le Grand, R.; Derdouch, S.; Auregan, G.; Deglon, N.; Relouzat, F.; Aubertin, A.M.; Maillere, B.; et al. Human and simian immunodeficiency viruses deregulate early hematopoiesis through a Nef/PPARgamma/STAT5 signaling pathway in macaques. J. Clin. Investig. 2008, 118, 1765–1775. [Google Scholar] [CrossRef]
- Diaz-Salazar, C.; Bou-Puerto, R.; Mujal, A.M.; Lau, C.M.; von Hoesslin, M.; Zehn, D.; Sun, J.C. Cell-intrinsic adrenergic signaling controls the adaptive NK cell response to viral infection. J. Exp. Med. 2020, 217, e20190549. [Google Scholar] [CrossRef]
- Wieduwild, E.; Girard-Madoux, M.J.; Quatrini, L.; Laprie, C.; Chasson, L.; Rossignol, R.; Bernat, C.; Guia, S.; Ugolini, S. beta2-adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J. Exp. Med. 2020, 217, e20190554. [Google Scholar] [CrossRef]
- Chaipan, C.; Steffen, I.; Tsegaye, T.S.; Bertram, S.; Glowacka, I.; Kato, Y.; Schmokel, J.; Munch, J.; Simmons, G.; Gerardy-Schahn, R.; et al. Incorporation of podoplanin into HIV released from HEK-293T cells, but not PBMC, is required for efficient binding to the attachment factor CLEC-2. Retrovirology 2010, 7, 47. [Google Scholar] [CrossRef]
- Amsden, H.; Kourko, O.; Roth, M.; Gee, K. Antiviral Activities of Interleukin-27: A Partner for Interferons? Front. Immunol. 2022, 13, 902853. [Google Scholar] [CrossRef]
- Harker, J.A.; Wong, K.A.; Dallari, S.; Bao, P.; Dolgoter, A.; Jo, Y.; Wehrens, E.J.; Macal, M.; Zuniga, E.I. Interleukin-27R Signaling Mediates Early Viral Containment and Impacts Innate and Adaptive Immunity after Chronic Lymphocytic Choriomeningitis Virus Infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Bertram, E.M.; Tafuri, A.; Shahinian, A.; Chan, V.S.; Hunziker, L.; Recher, M.; Ohashi, P.S.; Mak, T.W.; Watts, T.H. Role of ICOS versus CD28 in antiviral immunity. Eur. J. Immunol. 2002, 32, 3376–3385. [Google Scholar] [CrossRef]
- Yeh, M.M.; Boukhar, S.; Roberts, B.; Dasgupta, N.; Daoud, S.S. Genomic variants link to hepatitis C racial disparities. Oncotarget 2017, 8, 59455–59475. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Zhang, Y.; Gu, J.; Wang, Y.; Yan, Y.; Pan, D.; Sun, Z. Cerebrospinal fluid proteomics in meningitis patients with reactivated varicella zoster virus. Immun. Inflamm. Dis. 2023, 11, e1038. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, X.Y.; Zhao, C.; Sun, S.H.; Meng, Z.F.; Zhang, J.M.; Xu, J.Q.; Xie, Y.H.; Yuan, Z.H.; Wen, Y.M. Transcriptional analysis of immune-related genes in dendritic cells from hepatitis B surface antigen (HBsAg)-positive transgenic mice and regulation of Fc gamma receptor IIB by HBsAg-anti-HBs complex. J. Med. Virol. 2011, 83, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Haslbauer, J.D.; Savic Prince, S.; Stalder, A.K.; Matter, M.S.; Zinner, C.P.; Jahn, K.; Obermann, E.; Hanke, J.; Leuzinger, K.; Hirsch, H.H.; et al. Differential Gene Expression of SARS-CoV-2 positive Bronchoalveolar Lavages: A Case Series. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2023, 10, 000532057. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Wietgrefe, S.W.; Shang, L.; Reilly, C.S.; Southern, P.J.; Perkey, K.E.; Duan, L.; Kohler, H.; Muller, S.; Robinson, J.; et al. Live simian immunodeficiency virus vaccine correlate of protection: Immune complex-inhibitory Fc receptor interactions that reduce target cell availability. J. Immunol. 2014, 193, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Weisberger, J.; Cornfield, D.; Gorczyca, W.; Liu, Z. Down-regulation of pan-T-cell antigens, particularly CD7, in acute infectious mononucleosis. Am. J. Clin. Pathol. 2003, 120, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.I.; Balamuth, F.B.; Ugen, K.E.; Williams, W.V.; Weiner, D.B. Identification of CD7 glycoprotein as an accessory molecule in HIV-1-mediated syncytium formation and cellfree infection. J. Immunol. 1994, 152, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Akl, H.; Badran, B.M.; Zein, N.E.; Bex, F.; Sotiriou, C.; Willard-Gallo, K.E.; Burny, A.; Martiat, P. HTLV-I infection of WE17/10 CD4+ cell line leads to progressive alteration of Ca2+ influx that eventually results in loss of CD7 expression and activation of an antiapoptotic pathway involving AKT and BAD which paves the way for malignant transformation. Leukemia 2007, 21, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Tai, Y.; Hua, G.; Yang, X.; Chen, J.; Li, J.; Deng, X. Melanocytes in black-boned chicken have immune contribution under infectious bursal disease virus infection. Poult. Sci. 2021, 100, 101498. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, Z.; Liu, X.; Zhou, L.; Ding, X.; Hou, Y. Role of SARS-CoV-2 nucleocapsid protein in affecting immune cells and insights on its molecular mechanisms. Exp. Ther. Med. 2023, 26, 504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.; Lei, X.; Xie, Z.; Qi, L.; Wang, H.; Xiao, X.; Xiao, J.; Zheng, Y.; Dong, C.; et al. Targeting lysophospholipid acid receptor 1 and ROCK kinases promotes antiviral innate immunity. Sci. Adv. 2021, 7, eabb5933. [Google Scholar] [CrossRef]
- Krishnan, M.N.; Ng, A.; Sukumaran, B.; Gilfoy, F.D.; Uchil, P.D.; Sultana, H.; Brass, A.L.; Adametz, R.; Tsui, M.; Qian, F.; et al. RNA interference screen for human genes associated with West Nile virus infection. Nature 2008, 455, 242–245. [Google Scholar] [CrossRef]
- Li, C.W.; Jheng, B.R.; Chen, B.S. Investigating genetic-and-epigenetic networks, and the cellular mechanisms occurring in Epstein-Barr virus-infected human B lymphocytes via big data mining and genome-wide two-sided NGS data identification. PLoS ONE 2018, 13, e0202537. [Google Scholar] [CrossRef]
- Haberl, E.M.; Feder, S.; Pohl, R.; Rein-Fischboeck, L.; Durholz, K.; Eichelberger, L.; Wanninger, J.; Weiss, T.S.; Buechler, C. Chemerin Is Induced in Non-Alcoholic Fatty Liver Disease and Hepatitis B-Related Hepatocellular Carcinoma. Cancers 2020, 12, 2967. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Edinger, A.L.; Stordeur, P.; Rucker, J.; Verhasselt, V.; Sharron, M.; Govaerts, C.; Mollereau, C.; Vassart, G.; Doms, R.W.; et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol. 1998, 28, 1689–1700. [Google Scholar] [CrossRef]
- Cayabyab, M.; Hinuma, S.; Farzan, M.; Choe, H.; Fukusumi, S.; Kitada, C.; Nishizawa, N.; Hosoya, M.; Nishimura, O.; Messele, T.; et al. Apelin, the natural ligand of the orphan seven-transmembrane receptor APJ, inhibits human immunodeficiency virus type 1 entry. J. Virol. 2000, 74, 11972–11976. [Google Scholar] [CrossRef] [PubMed]
- Nong, W.; Ma, L.; Lan, B.; Liu, N.; Yang, H.; Lao, X.; Deng, Q.; Huang, Z. Comprehensive Identification of Bridge Genes to Explain the Progression from Chronic Hepatitis B Virus Infection to Hepatocellular Carcinoma. J. Inflamm. Res. 2021, 14, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Muresan, S.; Weber, S.N.; Lammert, F.; Krawczyk, M. Acute Pancreatitis in the Setting of Hepatitis E Virus (Genotype 3) Infection and Compound CLDN2-PRSS1 Risk Variants. Pancreas 2020, 49, e91–e93. [Google Scholar] [CrossRef] [PubMed]
- Delva, J.L.; Daled, S.; Van Waesberghe, C.; Almey, R.; Jansens, R.J.J.; Deforce, D.; Dhaenens, M.; Favoreel, H.W. Proteomic Comparison of Three Wild-Type Pseudorabies Virus Strains and the Attenuated Bartha Strain Reveals Reduced Incorporation of Several Tegument Proteins in Bartha Virions. J. Virol. 2022, 96, e0115822. [Google Scholar] [CrossRef]
- Tsai, C.H.; Wu, A.C.; Chiang, B.L.; Yang, Y.H.; Hung, S.P.; Su, M.W.; Chang, Y.J.; Lee, Y.L. CEACAM3 decreases asthma exacerbations and modulates respiratory syncytial virus latent infection in children. Thorax 2020, 75, 725–734. [Google Scholar] [CrossRef]
- de Almeida Chuffa, L.G.; Freire, P.P.; Dos Santos Souza, J.; de Mello, M.C.; de Oliveira Neto, M.; Carvalho, R.F. Aging whole blood transcriptome reveals candidate genes for SARS-CoV-2-related vascular and immune alterations. J. Mol. Med. 2022, 100, 285–301. [Google Scholar] [CrossRef]
- Wu, S.; Yang, S.; Ou, M.; Chen, J.; Huang, J.; Xiong, D.; Sun, W.; Xiao, L. Transcriptome Analysis Reveals the Role of Cellular Calcium Disorder in Varicella Zoster Virus-Induced Post-Herpetic Neuralgia. Front. Mol. Neurosci. 2021, 14, 665931. [Google Scholar] [CrossRef]
- Isken, O.; Postel, A.; Bruhn, B.; Lattwein, E.; Becher, P.; Tautz, N. CRISPR/Cas9-Mediated Knockout of DNAJC14 Verifies This Chaperone as a Pivotal Host Factor for RNA Replication of Pestiviruses. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Yi, Z.; Sperzel, L.; Nurnberger, C.; Bredenbeek, P.J.; Lubick, K.J.; Best, S.M.; Stoyanov, C.T.; Law, L.M.; Yuan, Z.; Rice, C.M.; et al. Identification and characterization of the host protein DNAJC14 as a broadly active flavivirus replication modulator. PLoS Pathog. 2011, 7, e1001255. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.L.; de Souza, G.A.P.; de Souza Terceti, M.; de Castro, R.F.G.; de Mello Silva, B.; Novaes, R.D.; Malaquias, L.C.C.; Coelho, L.F.L. Integrative transcriptome analysis of SARS-CoV-2 human-infected cells combined with deep learning algorithms identifies two potential cellular targets for the treatment of coronavirus disease. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2023, 54, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, L.P.; Kambara, H.; Chen, Y.A.; Nishimura, Y.; Moriishi, K.; Okamoto, T.; Morita, E.; Abe, T.; Mori, Y.; Matsuura, Y.; et al. Understanding the biological context of NS5A-host interactions in HCV infection: A network-based approach. J. Proteome Res. 2013, 12, 2537–2551. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, I.K.; Lee, J.Y.; Tabata, K.; Romero-Brey, I.; Paul, D.; Schult, P.; Lohmann, V.; Kaderali, L.; Bartenschlager, R. Hepatitis C Virus Replication Depends on Endosomal Cholesterol Homeostasis. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Paust, S.; Gill, H.S.; Wang, B.Z.; Flynn, M.P.; Moseman, E.A.; Senman, B.; Szczepanik, M.; Telenti, A.; Askenase, P.W.; Compans, R.W.; et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010, 11, 1127–1135. [Google Scholar] [CrossRef]
- Liao, F.; Alkhatib, G.; Peden, K.W.; Sharma, G.; Berger, E.A.; Farber, J.M. STRL33, A novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J. Exp. Med. 1997, 185, 2015–2023. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; Florido, M.; Lin, L.C.W.; Quan, D.; Armitage, E.; Stifter, S.A.; Stambas, J.; Britton, W.J. CXCR6-Deficiency Improves the Control of Pulmonary Mycobacterium tuberculosis and Influenza Infection Independent of T-Lymphocyte Recruitment to the Lungs. Front. Immunol. 2019, 10, 339. [Google Scholar] [CrossRef]
- Pirozyan, M.R.; Nguyen, N.; Cameron, B.; Luciani, F.; Bull, R.A.; Zekry, A.; Lloyd, A.R. Chemokine-Regulated Recruitment of Antigen-Specific T-Cell Subpopulations to the Liver in Acute and Chronic Hepatitis C Infection. J. Infect. Dis. 2019, 219, 1430–1438. [Google Scholar] [CrossRef]
- Teran, L.M.; Ruggeberg, S.; Santiago, J.; Fuentes-Arenas, F.; Hernandez, J.L.; Montes-Vizuet, A.R.; Xinping, L.; Franz, T. Immune response to seasonal influenza A virus infection: A proteomic approach. Arch. Med. Res. 2012, 43, 464–469. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, Z.N.; Jiang, Y.J.; Fu, Y.J.; Shang, H. Transcriptional insights into the CD8(+) T cell response in mono-HIV and HCV infection. J. Transl. Med. 2020, 18, 96. [Google Scholar] [CrossRef]
- Santana, B.B.; Queiroz, M.A.F.; Cerveira, R.A.; Rodrigues, C.M.; da Silva Graca Amoras, E.; da Costa, C.A.; de Sousa, M.S.; Ishak, R.; Goulart, L.R.; Vallinoto, A.C.R. Low Annexin A1 level in HTLV-1 infected patients is a potential biomarker for the clinical progression and diagnosis of HAM/TSP. BMC Infect. Dis. 2021, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Shaath, H.; Vishnubalaji, R.; Elkord, E.; Alajez, N.M. Single-Cell Transcriptome Analysis Highlights a Role for Neutrophils and Inflammatory Macrophages in the Pathogenesis of Severe COVID-19. Cells 2020, 9, 2374. [Google Scholar] [CrossRef] [PubMed]
- Duhalde Vega, M.; Olivera, D.; Gastao Davanzo, G.; Bertullo, M.; Noya, V.; Fabiano de Souza, G.; Primon Muraro, S.; Castro, I.; Arevalo, A.P.; Crispo, M.; et al. PD-1/PD-L1 blockade abrogates a dysfunctional innate-adaptive immune axis in critical beta-coronavirus disease. Sci. Adv. 2022, 8, eabn6545. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Zhang, Y.; Tan, B.; Liu, T.T.; Wang, Y.Y.; Shu, H.B. The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation. J. Immunol. 2010, 184, 6249–6255. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Yin, H.; Wang, F.; Liu, Z.; Luan, X.; Sun, L.; Liu, W.; Shang, Y. Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity. PLoS Pathog. 2022, 18, e1010544. [Google Scholar] [CrossRef]
- Chen, Z.L.; Yin, Z.J.; Qiu, T.Y.; Chen, J.; Liu, J.; Zhang, X.Y.; Xu, J.Q. Revealing the characteristics of ZIKV infection through tissue-specific transcriptome sequencing analysis. BMC Genom. 2022, 23, 697. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sen, E.; Basu, A. Pyruvate dehydrogenase kinase 1 promotes neuronal apoptosis upon Japanese encephalitis virus infection. IBRO Neurosci. Rep. 2022, 13, 410–419. [Google Scholar] [CrossRef]
- Jung, G.S.; Jeon, J.H.; Choi, Y.K.; Jang, S.Y.; Park, S.Y.; Kim, S.W.; Byun, J.K.; Kim, M.K.; Lee, S.; Shin, E.C.; et al. Pyruvate dehydrogenase kinase regulates hepatitis C virus replication. Sci. Rep. 2016, 6, 30846. [Google Scholar] [CrossRef]
- Dawood, R.M.; El-Meguid, M.A.; Ibrahim, M.K.; Bader El Din, N.G.; Barakat, A.; El-Wakeel, K.; Alla, M.; Wu, G.Y.; El Awady, M.K. Dysregulation of fibrosis related genes in HCV induced liver disease. Gene 2018, 664, 58–69. [Google Scholar] [CrossRef]
- Schmidt, N.; Lareau, C.A.; Keshishian, H.; Ganskih, S.; Schneider, C.; Hennig, T.; Melanson, R.; Werner, S.; Wei, Y.; Zimmer, M.; et al. The SARS-CoV-2 RNA-protein interactome in infected human cells. Nat. Microbiol. 2021, 6, 339–353. [Google Scholar] [CrossRef]
- Villalba, M.; Fredericksen, F.; Otth, C.; Olavarria, V. Transcriptomic analysis of responses to cytopathic bovine viral diarrhea virus-1 (BVDV-1) infection in MDBK cells. Mol. Immunol. 2016, 71, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.; de la Monte, S.; Kim, M.; Herrmann, M.; Tanaka, S.; Von Dem Bussche, A.; Kew, M.C.; Trepo, C.; Wands, J.R. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 2004, 127, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Deribe, Y.L.; Skanland, S.S.; Stieglitz, B.; Grabbe, C.; Franz-Wachtel, M.; van Wijk, S.J.; Goswami, P.; Nagy, V.; Terzic, J.; et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature 2011, 471, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Fang, L.; Ding, Z.; Wang, D.; Hao, W.; Gao, L.; Ke, W.; Chen, H.; Xiao, S. Porcine Reproductive and Respiratory Syndrome Virus nsp1alpha Inhibits NF-kappaB Activation by Targeting the Linear Ubiquitin Chain Assembly Complex. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef] [PubMed]
- McLaren, P.J.; Gawanbacht, A.; Pyndiah, N.; Krapp, C.; Hotter, D.; Kluge, S.F.; Gotz, N.; Heilmann, J.; Mack, K.; Sauter, D.; et al. Identification of potential HIV restriction factors by combining evolutionary genomic signatures with functional analyses. Retrovirology 2015, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Kotelkin, A.; Prikhod’ko, E.A.; Cohen, J.I.; Collins, P.L.; Bukreyev, A. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 2003, 77, 9156–9172. [Google Scholar] [CrossRef]
- Ebrahimpour Gorji, A.; Roudbari, Z.; Ebrahimpour Gorji, F.; Sadeghi, B. Computational study of zebrafish immune-targeted microarray data for prediction of preventive drug candidates. Vet. Res. Forum Int. Q. J. 2021, 12, 87–93. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Li, Z.; Rao, F.; Xu, H.; Lu, J.; Ma, X.; Zhang, M.; Xie, Z. Comprehensive analyses identify potential biomarkers for encephalitis in HIV infection. Sci. Rep. 2023, 13, 18418. [Google Scholar] [CrossRef]
- Drozdzik, M.; Lapczuk-Romanska, J.; Wenzel, C.; Skalski, L.; Szelag-Pieniek, S.; Post, M.; Syczewska, M.; Kurzawski, M.; Oswald, S. Protein Abundance of Drug Transporters in Human Hepatitis C Livers. Int. J. Mol. Sci. 2022, 23, 7947. [Google Scholar] [CrossRef]
- Airo, A.M.; Felix-Lopez, A.; Mancinelli, V.; Evseev, D.; Lopez-Orozco, J.; Shire, K.; Paszkowski, P.; Frappier, L.; Magor, K.E.; Hobman, T.C. Flavivirus Capsid Proteins Inhibit the Interferon Response. Viruses 2022, 14, 968. [Google Scholar] [CrossRef] [PubMed]
- Ammour, Y.; Susova, O.; Krasnov, G.; Nikolaeva, E.; Varachev, V.; Schetinina, Y.; Gavrilova, M.; Mitrofanov, A.; Poletaeva, A.; Bekyashev, A.; et al. Transcriptome Analysis of Human Glioblastoma Cells Susceptible to Infection with the Leningrad-16 Vaccine Strain of Measles Virus. Viruses 2022, 14, 2433. [Google Scholar] [CrossRef] [PubMed]
- Molinero, M.; Gomez, S.; Benitez, I.D.; Vengoechea, J.J.; Gonzalez, J.; Polanco, D.; Gort-Paniello, C.; Moncusi-Moix, A.; Garcia-Hidalgo, M.C.; Perez-Pons, M.; et al. Multiplex protein profiling of bronchial aspirates reveals disease-, mortality- and respiratory sequelae-associated signatures in critically ill patients with ARDS secondary to SARS-CoV-2 infection. Front. Immunol. 2022, 13, 942443. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.Q.; Goergen, K.M.; Grill, D.E.; Haralambieva, I.H.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Virus-specific and shared gene expression signatures in immune cells after vaccination in response to influenza and vaccinia stimulation. Front. Immunol. 2023, 14, 1168784. [Google Scholar] [CrossRef] [PubMed]
- Vrazas, V.; Moustafa, S.; Makridakis, M.; Karakasiliotis, I.; Vlahou, A.; Mavromara, P.; Katsani, K.R. A Proteomic Approach to Study the Biological Role of Hepatitis C Virus Protein Core+1/ARFP. Viruses 2022, 14, 1694. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.P.; Bradel-Tretheway, B.; Piehowski, P.D.; Brewer, H.M.; Lee, B.N.R.; Usher, N.T.; Zamora, J.L.R.; Ortega, V.; Contreras, E.M.; Teuton, J.R.; et al. Nipah Virus-Like Particle Egress Is Modulated by Cytoskeletal and Vesicular Trafficking Pathways: A Validated Particle Proteomics Analysis. mSystems 2019, 4, e00194-19. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Cao, L.; Wu, X.M.; Chang, M.X. The function of zebrafish gpbar1 in antiviral response and lipid metabolism. Dev. Comp. Immunol. 2021, 116, 103955. [Google Scholar] [CrossRef]
- Smith, T.; Rohaim, M.A.; Munir, M. Mapping molecular gene signatures mediated by SARS-COV-2 and large-scale and genome-wide transcriptomics comparative analysis among respiratory viruses of medical importance. Mol. Cell. Probes 2022, 64, 101820. [Google Scholar] [CrossRef]
- Xiong, Q.; Huang, H.; Wang, N.; Chen, R.; Chen, N.; Han, H.; Wang, Q.; Siwko, S.; Liu, M.; Qian, M.; et al. Metabolite-Sensing G Protein Coupled Receptor TGR5 Protects Host From Viral Infection Through Amplifying Type I Interferon Responses. Front. Immunol. 2018, 9, 2289. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Zhang, Y.; Li, Y.; Lu, L. Grass carp reovirus NS26 interacts with cellular lipopolysaccharide-induced tumor necrosis factor-alpha factor, LITAF. Virus Genes 2016, 52, 789–796. [Google Scholar] [CrossRef]
- Eaton, H.E.; Ferreira Lacerda, A.; Desrochers, G.; Metcalf, J.; Angers, A.; Brunetti, C.R. Cellular LITAF interacts with frog virus 3 75L protein and alters its subcellular localization. J. Virol. 2013, 87, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Kuchipudi, S.V.; Tellabati, M.; Sebastian, S.; Londt, B.Z.; Jansen, C.; Vervelde, L.; Brookes, S.M.; Brown, I.H.; Dunham, S.P.; Chang, K.C. Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses. Vet. Res. 2014, 45, 118. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.G.; Zhu, C.L.; Cheng, D.Z.; Xie, Y.; Liu, F.; Zhou, X. Enchanced levels of apolipoprotein M during HBV infection feedback suppresses HBV replication. Lipids Health Dis. 2011, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yao, W.; Huang, J.; Xiao, J.; Chen, W.; Hu, L.; Mai, R.; Liang, M.; Chen, D.; Jiang, N.; et al. Apolipoprotein M, identified as a novel hepatitis C virus (HCV) particle associated protein, contributes to HCV assembly and interacts with E2 protein. Antivir. Res. 2020, 177, 104756. [Google Scholar] [CrossRef]
- Gonzalez-Dunia, D.; Watanabe, M.; Syan, S.; Mallory, M.; Masliah, E.; De La Torre, J.C. Synaptic pathology in Borna disease virus persistent infection. J. Virol. 2000, 74, 3441–3448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Afkhami-Goli, A.; Liu, S.H.; Zhu, Y.; Antony, J.M.; Arab, H.; Power, C. Dual lentivirus infection potentiates neuroinflammation and neurodegeneration: Viral copassage enhances neurovirulence. J. Neurovirology 2009, 15, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, R.; Li Puma, D.D.; Ripoli, C.; Marcocci, M.E.; De Chiara, G.; Garaci, E.; Palamara, A.T.; Grassi, C. Herpes Simplex Virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-beta protein accumulation. Sci. Rep. 2015, 5, 15444. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.; Guise, A.J.; Nakayama, T.; Schlaffner, C.N.; Meziani, A.; Kumar, M.; Cheng, L.; Vaughan, D.J.; Kodani, A.; Van Haren, S.; et al. Integrative systems biology characterizes immune-mediated neurodevelopmental changes in murine Zika virus microcephaly. iScience 2023, 26, 106909. [Google Scholar] [CrossRef]
- Ma-Lauer, Y.; Carbajo-Lozoya, J.; Hein, M.Y.; Muller, M.A.; Deng, W.; Lei, J.; Meyer, B.; Kusov, Y.; von Brunn, B.; Bairad, D.R.; et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc. Natl. Acad. Sci. USA 2016, 113, E5192–E5201. [Google Scholar] [CrossRef]
- Chen, H.; Gao, X.; Zhao, S.; Bao, C.; Ming, X.; Qian, Y.; Zhou, Y.; Jung, Y.S. Pirh2 restricts influenza A virus replication by modulating short-chain ubiquitination of its nucleoprotein. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22537. [Google Scholar] [CrossRef]
- Opriessnig, T.; Karuppannan, A.K.; Halbur, P.G.; Calvert, J.G.; Nitzel, G.P.; Matzinger, S.R.; Meng, X.J. Porcine circovirus type 2a or 2b based experimental vaccines provide protection against PCV2d/porcine parvovirus 2 co-challenge. Vaccine 2020, 38, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cortay, J.C.; Logan, I.R.; Sapountzi, V.; Robson, C.N.; Gerlier, D. Inhibition of ubiquitination and stabilization of human ubiquitin E3 ligase PIRH2 by measles virus phosphoprotein. J. Virol. 2005, 79, 11824–11836. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Lo, K.W.; Wei, W.; Tsao, S.W.; Chung, G.T.Y.; Ibrahim, M.H.; Dawson, C.W.; Murray, P.G.; Paterson, I.C.; Yap, L.F. Oncogenic S1P signalling in EBV-associated nasopharyngeal carcinoma activates AKT and promotes cell migration through S1P receptor 3. J. Pathol. 2017, 242, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.; Poole, E.; Krishna, B.; Sellart, I.; Wills, M.R.; Murphy, E.; Sinclair, J. The Expression of Human Cytomegalovirus MicroRNA MiR-UL148D during Latent Infection in Primary Myeloid Cells Inhibits Activin A-triggered Secretion of IL-6. Sci. Rep. 2016, 6, 31205. [Google Scholar] [CrossRef] [PubMed]
- Pryce, R.; Azarm, K.; Rissanen, I.; Harlos, K.; Bowden, T.A.; Lee, B. A key region of molecular specificity orchestrates unique ephrin-B1 utilization by Cedar virus. Life Sci. Alliance 2020, 3, e201900578. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, S.; Liu, M.; Wang, S.; Bi, Z.; Fan, W.; Shi, Z.; Song, S.; Yan, L. Hsp70 Inhibits the Replication of Fowl Adenovirus Serotype 4 by Suppressing Viral Hexon with the Assistance of DnaJC7. J. Virol. 2022, 96, e0080722. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, T.; Liu, S.; Zhu, Y. Porcine circovirus type 2 exploits cap to inhibit PKR activation through interaction with Hsp40. Vet. Microbiol. 2021, 252, 108929. [Google Scholar] [CrossRef]
- He, J.; Zheng, Y.W.; Lin, Y.F.; Mi, S.; Qin, X.W.; Weng, S.P.; He, J.G.; Guo, C.J. Caveolae Restrict Tiger Frog Virus Release in HepG2 cells and Caveolae-Associated Proteins Incorporated into Virus Particles. Sci. Rep. 2016, 6, 21663. [Google Scholar] [CrossRef]
- Kipper, S.; Hamad, S.; Caly, L.; Avrahami, D.; Bacharach, E.; Jans, D.A.; Gerber, D.; Bajorek, M. New host factors important for respiratory syncytial virus (RSV) replication revealed by a novel microfluidics screen for interactors of matrix (M) protein. Mol. Cell. Proteom. MCP 2015, 14, 532–543. [Google Scholar] [CrossRef]
- Kim, J.Y.; Wang, L.; Lee, J.; Ou, J.J. Hepatitis C Virus Induces the Localization of Lipid Rafts to Autophagosomes for Its RNA Replication. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Waris, G.; Huh, K.W.; Siddiqui, A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol. Cell. Biol. 2001, 21, 7721–7730. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Wu, C.H.; Chien, K.Y.; Lai, C.H.; Li, G.J.; Liu, Y.Y.; Lin, G.; Ho, H.Y. Enteroviral 2B Interacts with VDAC3 to Regulate Reactive Oxygen Species Generation That Is Essential to Viral Replication. Viruses 2022, 14, 1717. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, R.V.; Su, T.; Trimble, L.A.; Lieberman, J.; Ardman, B. Enhanced susceptibility to human immunodeficiency virus infection in CD4+ T lymphocytes genetically deficient in CD43. AIDS Res. Hum. Retroviruses 1995, 11, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, S.W.; Wright, D.G. Characterization of influenza A virus binding sites on human neutrophils. J. Immunol. 1994, 152, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, T.; Satoh, T.; Somboonthum, P.; Kawaguchi, Y.; Mori, Y.; Arase, H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. USA 2010, 107, 866–871. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, L.H.; Sadiq, S.A.; Lederman, S.; Latov, N. The gp120 glycoprotein of HIV-1 binds to sulfatide and to the myelin associated glycoprotein. J. Neurosci. Res. 1992, 33, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, J.; del Campo, J.A.; Rojas, L.; Garcia-Lozano, R.J.; Buti, M.; Sola, R.; Forns, X.; Moreno-Otero, R.; Andrade, R.; Diago, M.; et al. Fine-mapping butyrophilin family genes revealed several polymorphisms influencing viral genotype selection in hepatitis C infection. Genes Immun. 2015, 16, 297–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teoh, K.T.; Siu, Y.L.; Chan, W.L.; Schluter, M.A.; Liu, C.J.; Peiris, J.S.; Bruzzone, R.; Margolis, B.; Nal, B. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol. Biol. Cell 2010, 21, 3838–3852. [Google Scholar] [CrossRef]
- He, J.; Yang, L.; Chang, P.; Yang, S.; Wang, Y.; Lin, S.; Tang, Q.; Zhang, Y. Zika Virus Induces Degradation of the Numb Protein Required through Embryonic Neurogenesis. Viruses 2023, 15, 1258. [Google Scholar] [CrossRef]
- Liu, D.; Cui, L.; Wang, Y.; Yang, G.; He, J.; Hao, R.; Fan, C.; Qu, M.; Liu, Z.; Wang, M.; et al. Hepatitis B e antigen and its precursors promote the progress of hepatocellular carcinoma by interacting with NUMB and decreasing p53 activity. Hepatology 2016, 64, 390–404. [Google Scholar] [CrossRef]
- Nadella, M.V.; Shu, S.T.; Dirksen, W.P.; Thudi, N.K.; Nadella, K.S.; Fernandez, S.A.; Lairmore, M.D.; Green, P.L.; Rosol, T.J. Expression of parathyroid hormone-related protein during immortalization of human peripheral blood mononuclear cells by HTLV-1: Implications for transformation. Retrovirology 2008, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Sander, W.J.; O’Neill, H.G.; Pohl, C.H. Prostaglandin E(2) As a Modulator of Viral Infections. Front. Physiol. 2017, 8, 89. [Google Scholar] [CrossRef]
- Kong, L.; Aoyagi, H.; Yang, Z.; Ouyang, T.; Matsuda, M.; Fujimoto, A.; Watashi, K.; Suzuki, R.; Arita, M.; Yamagoe, S.; et al. Surfeit 4 Contributes to the Replication of Hepatitis C Virus Using Double-Membrane Vesicles. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Yao, H.; Cai, Y.; Liao, K.; Seth, P.; Buch, S. HIV-1 Tat disrupts CX3CL1-CX3CR1 axis in microglia via the NF-kappaBYY1 pathway. Curr. HIV Res. 2014, 12, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Kimura, O.; Tanaka, Y.; Ninomiya, M.; Iwata, T.; Kogure, T.; Inoue, J.; Sugiyama, M.; Morosawa, T.; Fujisaka, Y.; et al. Differential Expression of CX3CL1 in Hepatitis B Virus-Replicating Hepatoma Cells Can Affect the Migration Activity of CX3CR1+ Immune Cells. J. Virol. 2015, 89, 7016–7027. [Google Scholar] [CrossRef] [PubMed]
- Todt, D.; Friesland, M.; Moeller, N.; Praditya, D.; Kinast, V.; Bruggemann, Y.; Knegendorf, L.; Burkard, T.; Steinmann, J.; Burm, R.; et al. Robust hepatitis E virus infection and transcriptional response in human hepatocytes. Proc. Natl. Acad. Sci. USA 2020, 117, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, A.; Woziwodzka, A.; Rybicka, M.; Bielawski, K.P.; Sikorska, K. Polymorphisms Related to Iron Homeostasis Associate with Liver Disease in Chronic Hepatitis C. Viruses 2023, 15, 1710. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Hossain, M.M.; Sata, T.N.; Yadav, A.K.; Zadran, S.; Sah, A.K.; Nayak, B.; Shalimar; Venugopal, S.K. Hepatitis B Virus X Protein Inhibits the Expression of Barrier To Autointegration factor1 via Upregulating miR-203 Expression in Hepatic Cells. Microbiol. Spectr. 2023, 11, e0123522. [Google Scholar] [CrossRef]
- Wiebe, M.S.; Traktman, P. Poxviral B1 kinase overcomes barrier to autointegration factor, a host defense against virus replication. Cell Host Microbe 2007, 1, 187–197. [Google Scholar] [CrossRef]
- Jamin, A.; Thunuguntla, P.; Wicklund, A.; Jones, C.; Wiebe, M.S. Barrier to auto integration factor becomes dephosphorylated during HSV-1 Infection and Can Act as a host defense by impairing viral DNA replication and gene expression. PLoS ONE 2014, 9, e100511. [Google Scholar] [CrossRef]
- Kundlacz, C.; Pourcelot, M.; Fablet, A.; Amaral Da Silva Moraes, R.; Leger, T.; Morlet, B.; Viarouge, C.; Sailleau, C.; Turpaud, M.; Gorlier, A.; et al. Novel Function of Bluetongue Virus NS3 Protein in Regulation of the MAPK/ERK Signaling Pathway. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Lasithiotaki, I.; Antoniou, K.M.; Derdas, S.P.; Sarchianaki, E.; Symvoulakis, E.K.; Psaraki, A.; Spandidos, D.A.; Stathopoulos, E.N.; Siafakas, N.M.; Sourvinos, G. The presence of Merkel cell polyomavirus is associated with deregulated expression of BRAF and Bcl-2 genes in non-small cell lung cancer. Int. J. Cancer 2013, 133, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Bakshi, S.; Lytras, S.; Zakaria, M.K.; Swingler, S.; Worrell, J.C.; Herder, V.; Hargrave, K.E.; Varjak, M.; Cameron-Ruiz, N.; et al. BTN3A3 evasion promotes the zoonotic potential of influenza A viruses. Nature 2023, 619, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Alagarasu, K.; Patil, P.S.; Shil, P.; Seervi, M.; Kakade, M.B.; Tillu, H.; Salunke, A. In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides 2017, 92, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cooper, C.L.; Wang, G.; Morwitzer, M.J.; Kota, K.; Tran, J.P.; Bradfute, S.B.; Liu, Y.; Shao, J.; Zhang, A.K.; et al. Engineered Human Cathelicidin Antimicrobial Peptides Inhibit Ebola Virus Infection. iScience 2020, 23, 100999. [Google Scholar] [CrossRef] [PubMed]
- LeMessurier, K.S.; Lin, Y.; McCullers, J.A.; Samarasinghe, A.E. Antimicrobial peptides alter early immune response to influenza A virus infection in C57BL/6 mice. Antivir. Res. 2016, 133, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Enyindah-Asonye, G.; Nwankwo, A.; Rahman, M.A.; Hunegnaw, R.; Hogge, C.; Helmold Hait, S.; Ko, E.J.; Hoang, T.; Robert-Guroff, M. Overexpression of CD6 and PD-1 Identifies Dysfunctional CD8(+) T-Cells During Chronic SIV Infection of Rhesus Macaques. Front. Immunol. 2019, 10, 3005. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, J.; Zhang, Z.; Yang, Y.; Zhou, Q.; Wu, T.; Chen, T.; Wu, Z.; Zhang, P.; Cui, J.; et al. NLRC5 restricts dengue virus infection by promoting the autophagic degradation of viral NS3 through E3 ligase CUL2 (cullin 2). Autophagy 2023, 19, 1332–1347. [Google Scholar] [CrossRef]
- Hyeon, S.; Lee, M.K.; Kim, Y.E.; Lee, G.M.; Ahn, J.H. Degradation of SAMHD1 Restriction Factor Through Cullin-Ring E3 Ligase Complexes During Human Cytomegalovirus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 391. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Li, Z.; Liu, X.; Liu, G.; Harris, R.S.; Yu, X.F. Cellular requirements for bovine immunodeficiency virus Vif-mediated inactivation of bovine APOBEC3 proteins. J. Virol. 2014, 88, 12528–12540. [Google Scholar] [CrossRef]
- Pawlica, P.; Yario, T.A.; White, S.; Wang, J.; Moss, W.N.; Hui, P.; Vinetz, J.M.; Steitz, J.A. SARS-CoV-2 expresses a microRNA-like small RNA able to selectively repress host genes. Proc. Natl. Acad. Sci. USA 2021, 118, e2116668118. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.; Hooper, I.; Lundberg, L.; Shafagati, N.; Johnson, A.; Senina, S.; de la Fuente, C.; Hoover, L.I.; Fredricksen, B.L.; Dinman, J.; et al. Small molecule inhibitors of Ago2 decrease Venezuelan equine encephalitis virus replication. Antivir. Res. 2014, 112, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, S.; Li, C.; Shi, J.; Peng, Z.; Liu, C.; Han, H.; Ma, Y.; Zheng, L.; Xu, S.; et al. CRISPR/Cas9-Mediated Knockout of the Dicer and Ago2 Genes in BHK-21 Cell Promoted Seneca Virus A Replication and Enhanced Autophagy. Front. Cell. Infect. Microbiol. 2022, 12, 865744. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, X.; Yi, C.; Zhang, D.; Lin, X.; Sun, X.; Chen, H.; Jin, M. AGO2 Negatively Regulates Type I Interferon Signaling Pathway by Competition Binding IRF3 with CBP/p300. Front. Cell. Infect. Microbiol. 2017, 7, 195. [Google Scholar] [CrossRef]
- Devadas, K.; Biswas, S.; Haleyurgirisetty, M.; Wood, O.; Ragupathy, V.; Lee, S.; Hewlett, I. Analysis of Host Gene Expression Profile in HIV-1 and HIV-2 Infected T-Cells. PLoS ONE 2016, 11, e0147421. [Google Scholar] [CrossRef]
- Conn, K.L.; Hendzel, M.J.; Schang, L.M. The differential mobilization of histones H3.1 and H3.3 by herpes simplex virus 1 relates histone dynamics to the assembly of viral chromatin. PLoS Pathog. 2013, 9, e1003695. [Google Scholar] [CrossRef]
- Ambagala, A.P.; Bosma, T.; Ali, M.A.; Poustovoitov, M.; Chen, J.J.; Gershon, M.D.; Adams, P.D.; Cohen, J.I. Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J. Virol. 2009, 83, 200–209. [Google Scholar] [CrossRef]
- Hsieh, I.N.; White, M.; Hoeksema, M.; Deluna, X.; Hartshorn, K. Histone H4 potentiates neutrophil inflammatory responses to influenza A virus: Down-modulation by H4 binding to C-reactive protein and Surfactant protein D. PLoS ONE 2021, 16, e0247605. [Google Scholar] [CrossRef]
- Nishitsuji, H.; Ujino, S.; Harada, K.; Shimotohno, K. TIP60 Complex Inhibits Hepatitis B Virus Transcription. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Truong, A.D.; Tran, H.T.T.; Nguyen, H.T.; Chu, N.T.; Hong, Y.H.; Lillehoj, H.S.; Dang, H.V.; Song, K.D. Molecular and functional characterization of chicken interleukin 1 receptor 2 (chIL-1R2). Poult. Sci. 2023, 102, 102399. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Sun, R.; Li, S.; Chen, L.; Zhan, Y.; Xie, M.; Yang, J.; Wang, Y.; Zhu, A.; et al. Longitudinal virological changes and underlying pathogenesis in hospitalized COVID-19 patients in Guangzhou, China. Sci. China. Life Sci. 2021, 64, 2129–2143. [Google Scholar] [CrossRef]
- Cho, Y.B.; Hong, S.; Kang, K.W.; Kang, J.H.; Lee, S.M.; Seo, Y.J. Selective and ATP-competitive kinesin KIF18A inhibitor suppresses the replication of influenza A virus. J. Cell. Mol. Med. 2020, 24, 5463–5475. [Google Scholar] [CrossRef]
- Sorgho, P.A.; Martinson, J.J.; Djigma, F.W.; Yonli, A.T.; Nagalo, B.M.; Compaore, T.R.; Obiri-Yeboah, D.; Diarra, B.; Sombie, H.K.; Zongo, A.W.; et al. Insights into the Interplay between KIR Gene Frequencies and Chronic HBV Infection in Burkina Faso. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018060. [Google Scholar] [CrossRef]
- Podhorzer, A.; Dirchwolf, M.; Machicote, A.; Belen, S.; Montal, S.; Paz, S.; Fainboim, H.; Podesta, L.G.; Fainboim, L. The Clinical Features of Patients with Chronic Hepatitis C Virus Infections Are Associated with Killer Cell Immunoglobulin-Like Receptor Genes and Their Expression on the Surface of Natural Killer Cells. Front. Immunol. 2017, 8, 1912. [Google Scholar] [CrossRef]
- Aoukaty, A.; Lee, I.F.; Wu, J.; Tan, R. Chronic active Epstein-Barr virus infection associated with low expression of leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) on natural killer cells. J. Clin. Immunol. 2003, 23, 141–145. [Google Scholar] [CrossRef]
- Kumawat, K.; Geerdink, R.J.; Hennus, M.P.; Roda, M.A.; van Ark, I.; Leusink-Muis, T.; Folkerts, G.; van Oort-Jansen, A.; Mazharian, A.; Watson, S.P.; et al. LAIR-1 Limits Neutrophilic Airway Inflammation. Front. Immunol. 2019, 10, 842. [Google Scholar] [CrossRef]
- Martinez-Torres, F.J.; Wagner, S.; Haas, J.; Kehm, R.; Sellner, J.; Hacke, W.; Meyding-Lamade, U. Increased presence of matrix metalloproteinases 2 and 9 in short- and long-term experimental herpes simplex virus encephalitis. Neurosci. Lett. 2004, 368, 274–278. [Google Scholar] [CrossRef]
- Ahmed, F.; Husain, M. Human N-Alpha-Acetyltransferase 60 Promotes Influenza A Virus Infection by Dampening the Interferon Alpha Signaling. Front. Immunol. 2021, 12, 771792. [Google Scholar] [CrossRef]
- Alpuche-Lazcano, S.P.; Saliba, J.; Costa, V.V.; Campolina-Silva, G.H.; Marim, F.M.; Ribeiro, L.S.; Blank, V.; Mouland, A.J.; Teixeira, M.M.; Gatignol, A. Profound downregulation of neural transcription factor Npas4 and Nr4a family in fetal mice neurons infected with Zika virus. PLoS Neglected Trop. Dis. 2021, 15, e0009425. [Google Scholar] [CrossRef]
- He, Z.; Jing, S.; Yang, T.; Chen, J.; Huang, F.; Zhang, W.; Peng, Z.; Liu, B.; Ma, X.; Wu, L.; et al. PIWIL4 Maintains HIV-1 Latency by Enforcing Epigenetically Suppressive Modifications on the 5’ Long Terminal Repeat. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Zenner, H.L.; Yoshimura, S.; Barr, F.A.; Crump, C.M. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J. Virol. 2011, 85, 8012–8021. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.Y.; Yun, H.S.; Lee, H.J.; Lee, M.H.; Jung, S.E.; Kim, J.W.; Jeon, J.P.; Shin, Y.K.; Rhee, H.S.; Kimm, K.C.; et al. Identification of stathmin 1 expression induced by Epstein-Barr virus in human B lymphocytes. Cell Prolif. 2007, 40, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Thompson, C.D.; Schowalter, R.M.; Lowy, D.R.; Schiller, J.T. Identification of a role for the trans-Golgi network in human papillomavirus 16 pseudovirus infection. J. Virol. 2013, 87, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.L.; Mavrakis, M.; McDonald, N.J.; Yilla, M.; Sheng, J.; Bellini, W.J.; Zhao, L.; Le Doux, J.M.; Shaw, M.W.; Luo, C.C.; et al. Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus. J. Virol. 2005, 79, 11742–11751. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Xie, R.; Yao, Y.; Yu, M.; Chang, F.; Xing, L.; Zhang, Y.; Liu, Y.; Wang, S.; Farooque, M.; et al. MiR-125b Suppression Inhibits Apoptosis and Negatively Regulates Sema4D in Avian Leukosis Virus-Transformed Cells. Viruses 2019, 11, 728. [Google Scholar] [CrossRef]
- Eriksson, E.M.; Milush, J.M.; Ho, E.L.; Batista, M.D.; Holditch, S.J.; Keh, C.E.; Norris, P.J.; Keating, S.M.; Deeks, S.G.; Hunt, P.W.; et al. Expansion of CD8+ T cells lacking Sema4D/CD100 during HIV-1 infection identifies a subset of T cells with decreased functional capacity. Blood 2012, 119, 745–755. [Google Scholar] [CrossRef]
- He, Y.; Guo, Y.; Fan, C.; Lei, Y.; Zhou, Y.; Zhang, M.; Ye, C.; Ji, G.; Ma, L.; Lian, J.; et al. Interferon-alpha-Enhanced CD100/Plexin-B1/B2 Interactions Promote Natural Killer Cell Functions in Patients with Chronic Hepatitis C Virus Infection. Front. Immunol. 2017, 8, 1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Q.; Zheng, M.; Wen, J.; Li, Q.; Zhao, G. Viral-Host Interactome Analysis Reveals Chicken STAU2 Interacts With Non-structural Protein 1 and Promotes the Replication of H5N1 Avian Influenza Virus. Front. Immunol. 2021, 12, 590679. [Google Scholar] [CrossRef]
- Yan, N.; Regalado-Magdos, A.D.; Stiggelbout, B.; Lee-Kirsch, M.A.; Lieberman, J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 2010, 11, 1005–1013. [Google Scholar] [CrossRef]
- Yeh, D.W.; Zhao, X.; Siddique, H.R.; Zheng, M.; Choi, H.Y.; Machida, T.; Narayanan, P.; Kou, Y.; Punj, V.; Tahara, S.M.; et al. MSI2 promotes translation of multiple IRES-containing oncogenes and virus to induce self-renewal of tumor initiating stem-like cells. Cell Death Discov. 2023, 9, 141. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, H.; Kalsoom, F.; Sajjad, M.A.; Lee, H.W.; Lim, J.H.; Jung, J.; Chwae, Y.J.; Park, S.; Shin, H.J.; et al. Ca(2+)/Calmodulin-Dependent Protein Kinase II Inhibits Hepatitis B Virus Replication from cccDNA via AMPK Activation and AKT/mTOR Suppression. Microorganisms 2022, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Haolong, C.; Du, N.; Hongchao, T.; Yang, Y.; Wei, Z.; Hua, Z.; Wenliang, Z.; Lei, S.; Po, T. Enterovirus 71 VP1 activates calmodulin-dependent protein kinase II and results in the rearrangement of vimentin in human astrocyte cells. PLoS ONE 2013, 8, e73900. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, S.; Windsor, M.; Nagata, K.I.; Inagaki, M.; Wileman, T. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J. Virol. 2005, 79, 11766–11775. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, Z.; Wu, Z.; Liu, C.; Yu, M.; Wen, M.; Zhang, L.; Wang, X. DDAH2 suppresses RLR-MAVS-mediated innate antiviral immunity by stimulating nitric oxide-activated, Drp1-induced mitochondrial fission. Sci. Signal. 2021, 14, eabc7931. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, Q.; Jia, J.; Cong, P.; Mo, D.; Yu, X.; Qin, L.; Li, A.; Niu, Y.; Zhu, K.; et al. Proteome changes of lungs artificially infected with H-PRRSV and N-PRRSV by two-dimensional fluorescence difference gel electrophoresis. Virol. J. 2010, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guan, X.; Lv, J.; Li, X.; Wang, Y.; Li, L. Limb-bud and Heart (LBH) functions as a tumor suppressor of nasopharyngeal carcinoma by inducing G1/S cell cycle arrest. Sci. Rep. 2015, 5, 7626. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; McPherson, A.J.; Jones, R.B.; Kawamura, K.S.; Lin, G.H.; Lang, P.A.; Ambagala, T.; Pellegrini, M.; Calzascia, T.; Aidarus, N.; et al. Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J. Exp. Med. 2012, 209, 77–91. [Google Scholar] [CrossRef]
- Siegler, G.; Meyer, B.; Dawson, C.; Brachtel, E.; Lennerz, J.; Koch, C.; Kremmer, E.; Niedobitek, E.; Gonnella, R.; Pilch, B.Z.; et al. Expression of tumor necrosis factor receptor-associated factor 1 in nasopharyngeal carcinoma: Possible upregulation by Epstein-Barr virus latent membrane protein 1. Int. J. Cancer 2004, 112, 265–272. [Google Scholar] [CrossRef]
- Schneider, M.A.; Spoden, G.A.; Florin, L.; Lambert, C. Identification of the dynein light chains required for human papillomavirus infection. Cell. Microbiol. 2011, 13, 32–46. [Google Scholar] [CrossRef]
- Yu, T.; Ding, Y.; Zhang, Y.; Liu, Y.; Li, Y.; Lei, J.; Zhou, J.; Song, S.; Hu, B. Circular RNA GATAD2A promotes H1N1 replication through inhibiting autophagy. Vet. Microbiol. 2019, 231, 238–245. [Google Scholar] [CrossRef]
- Cruz, J.L.; Sola, I.; Becares, M.; Alberca, B.; Plana, J.; Enjuanes, L.; Zuniga, S. Coronavirus gene 7 counteracts host defenses and modulates virus virulence. PLoS Pathog. 2011, 7, e1002090. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, W.; Zhang, W.; Li, J.; Yang, G.; Zhao, S.; Zheng, Y. Cap Is the Protease of the Porcine Circovirus 2. Viruses 2022, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, X.; Qin, C.; Huang, K.; Sun, X.; Zhao, L.; Jin, M. Avian Chaperonin Containing TCP1 Subunit 5 Supports Influenza A Virus Replication by Interacting With Viral Nucleoprotein, PB1, and PB2 Proteins. Front. Microbiol. 2020, 11, 538355. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, S.; Guarino, A.M.; Giaquinto, L.; Polishchuk, E.V.; Santoro, M.; Di Tullio, G.; Wilson, C.; Panariello, F.; Soares, V.C.; Dias, S.S.G.; et al. The role of NSP6 in the biogenesis of the SARS-CoV-2 replication organelle. Nature 2022, 606, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Mohl, B.P.; Bartlett, C.; Mankouri, J.; Harris, M. Early events in the generation of autophagosomes are required for the formation of membrane structures involved in hepatitis C virus genome replication. J. Gen. Virol. 2016, 97, 680–693. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, J.; Hao, Y.; Yang, X.; Shi, X.; Zhang, D.; Zhao, D.; Yan, W.; Bie, X.; Chen, L.; et al. DDX20: A Multifunctional Complex Protein. Molecules 2023, 28, 7198. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.; Yu, X.M.; Odorico, S.; Jain, V.; Marin, A.; Ma, S.; Kenney, S.; Chen, H. The Epstein-Barr virus EBNA2 protein induces a subset of NOTCH target genes in thyroid cancer cell lines but fails to suppress proliferation. Surgery 2017, 161, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, X.; Chen, X.; Cao, Q.; Zhang, X.; Zhou, Y.; Li, W.; Hong, L.; Xie, H.; Liu, X.; et al. Multiple novel hepatocellular carcinoma signature genes are commonly controlled by the master pluripotency factor OCT4. Cell. Oncol. 2020, 43, 279–295. [Google Scholar] [CrossRef]
- Ashrafi, F.; Ghezeldasht, S.A.; Ghobadi, M.Z. Identification of joint gene players implicated in the pathogenesis of HTLV-1 and BLV through a comprehensive system biology analysis. Microb. Pathog. 2021, 160, 105153. [Google Scholar] [CrossRef]
- Li, S.; Li, R.; Ahmad, I.; Liu, X.; Johnson, S.F.; Sun, L.; Zheng, Y.H. Cul3-KLHL20 E3 ubiquitin ligase plays a key role in the arms race between HIV-1 Nef and host SERINC5 restriction. Nat. Commun. 2022, 13, 2242. [Google Scholar] [CrossRef]
- Bedadala, G.R.; Pinnoji, R.C.; Hsia, S.C. Early growth response gene 1 (Egr-1) regulates HSV-1 ICP4 and ICP22 gene expression. Cell Res. 2007, 17, 546–555. [Google Scholar] [CrossRef]
- Cai, Y.; Xia, J.; Wang, N.; Zhou, H. Identification of prognostic alternative splicing signatures in hepatitis B or/and C viruses related hepatocellular carcinoma. Genomics 2020, 112, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Fino, K.K.; Yang, L.; Silveyra, P.; Hu, S.; Umstead, T.M.; DiAngelo, S.; Halstead, E.S.; Cooper, T.K.; Abraham, T.; Takahashi, Y.; et al. SH3GLB2/endophilin B2 regulates lung homeostasis and recovery from severe influenza A virus infection. Sci. Rep. 2017, 7, 7262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Y.Y.; Wang, H.Y.; Wang, J.F.; He, X.J. The IFN-gamma-induced immunoproteasome is suppressed in highly pathogenic porcine reproductive and respiratory syndrome virus-infected alveolar macrophages. Vet. Immunol. Immunopathol. 2020, 226, 110069. [Google Scholar] [CrossRef]
- Chavali, P.L.; Stojic, L.; Meredith, L.W.; Joseph, N.; Nahorski, M.S.; Sanford, T.J.; Sweeney, T.R.; Krishna, B.A.; Hosmillo, M.; Firth, A.E.; et al. Neurodevelopmental protein Musashi-1 interacts with the Zika genome and promotes viral replication. Science 2017, 357, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Mangeat, B.; Gers-Huber, G.; Lehmann, M.; Zufferey, M.; Luban, J.; Piguet, V. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009, 5, e1000574. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, M.; Lau, S.; Samuel, C.E.; Hornung, V.; Weber, F. NSs Virulence Factor of Rift Valley Fever Virus Engages the F-Box Proteins FBXW11 and beta-TRCP1 To Degrade the Antiviral Protein Kinase PKR. J. Virol. 2016, 90, 6140–6147. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zeng, Z.; Gong, Z.; Zhang, W.; Li, X.; He, B.; Song, Y.; Li, Q.; Zeng, Y.; Liao, Q.; et al. EBV-miR-BART10-3p facilitates epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma by targeting BTRC. Oncotarget 2015, 6, 41766–41782. [Google Scholar] [CrossRef]
- Margottin, F.; Bour, S.P.; Durand, H.; Selig, L.; Benichou, S.; Richard, V.; Thomas, D.; Strebel, K.; Benarous, R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1998, 1, 565–574. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, R.; Saha, S.; Agrahari, A.K.; Shukla, S.; Singh, O.N.; Berry, U.; Anurag; Maiti, T.K.; Asthana, S.; et al. RNA-Protein Interactome at the Hepatitis E Virus Internal Ribosome Entry Site. Microbiol. Spectr. 2023, 11, e0282722. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, J.; Li, L.; Li, X.; Tufan, N.L.; Wu, M.C.; Wang, H.Y.; Arbuthnot, P.; Kew, M.; Feitelson, M.A. Human S15a expression is upregulated by hepatitis B virus X protein. Mol. Carcinog. 2004, 40, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Qu, S.; Liu, G.; Liu, L.; Yu, Y.; Ding, G.; Zhao, Y.; Li, Y.; Xie, Y.; Zhang, J.; et al. Comparative Transcriptomic Analysis of Primary Duck Hepatocytes Provides Insight into Differential Susceptibility to DHBV Infection. PLoS ONE 2016, 11, e0149702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfaller, C.K.; George, C.X.; Samuel, C.E. Adenosine Deaminases Acting on RNA (ADARs) and Viral Infections. Annu. Rev. Virol. 2021, 8, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.T.; Schon, K.; Ebbecke, T.; Goethe, R.; Ruland, J.; Baumgartner, W.; Becker, S.C.; Lepenies, B. The CARD9-Associated C-Type Lectin, Mincle, Recognizes La Crosse Virus (LACV) but Plays a Limited Role in Early Antiviral Responses against LACV. Viruses 2019, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Poeck, H.; Bscheider, M.; Gross, O.; Finger, K.; Roth, S.; Rebsamen, M.; Hannesschlager, N.; Schlee, M.; Rothenfusser, S.; Barchet, W.; et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat. Immunol. 2010, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, X.; Yu, Y.; Li, Z.; Xie, Y. CARD9 mediates T cell inflammatory response in Coxsackievirus B3-induced acute myocarditis. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2020, 49, 107261. [Google Scholar] [CrossRef]
- Peters, G.A.; Khoo, D.; Mohr, I.; Sen, G.C. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 2002, 76, 11054–11064. [Google Scholar] [CrossRef]
- Hume, A.; Muhlberger, E. Marburg Virus Viral Protein 35 Inhibits Protein Kinase R Activation in a Cell Type-Specific Manner. J. Infect. Dis. 2018, 218, S403–S408. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Liao, G.R.; Sen, G.C.; Lin, F.Y.; Hsu, W.L. Regulation of PACT-Mediated Protein Kinase Activation by the OV20.0 Protein of Orf Virus. J. Virol. 2015, 89, 11619–11629. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, M.; Zhu, J.; Li, X.; Luo, T.R.; Liang, J. Host Desmin Interacts with RABV Matrix Protein and Facilitates Virus Propagation. Viruses 2023, 15, 434. [Google Scholar] [CrossRef]
- Nedellec, P.; Vicart, P.; Laurent-Winter, C.; Martinat, C.; Prevost, M.C.; Brahic, M. Interaction of Theiler’s virus with intermediate filaments of infected cells. J. Virol. 1998, 72, 9553–9560. [Google Scholar] [CrossRef] [PubMed]
- Shoeman, R.L.; Sachse, C.; Honer, B.; Mothes, E.; Kaufmann, M.; Traub, P. Cleavage of human and mouse cytoskeletal and sarcomeric proteins by human immunodeficiency virus type 1 protease. Actin, desmin, myosin, and tropomyosin. Am. J. Pathol. 1993, 142, 221–230. [Google Scholar] [PubMed]
- Dang, W.; Yin, Y.; Wang, Y.; Wang, W.; Su, J.; Sprengers, D.; van der Laan, L.J.W.; Felczak, K.; Pankiewicz, K.W.; Chang, K.O.; et al. Inhibition of Calcineurin or IMP Dehydrogenase Exerts Moderate to Potent Antiviral Activity against Norovirus Replication. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Na Rangsee, N.; Yanatatsaneejit, P.; Pisitkun, T.; Somparn, P.; Jintaridth, P.; Topanurak, S. Host proteome linked to HPV E7-mediated specific gene hypermethylation in cancer pathways. Infect. Agents Cancer 2020, 15, 7. [Google Scholar] [CrossRef]
- Russell, T.; Samolej, J.; Hollinshead, M.; Smith, G.L.; Kite, J.; Elliott, G. Novel Role for ESCRT-III Component CHMP4C in the Integrity of the Endocytic Network Utilized for Herpes Simplex Virus Envelopment. mBio 2021, 12. [Google Scholar] [CrossRef]
- von Schwedler, U.K.; Stuchell, M.; Muller, B.; Ward, D.M.; Chung, H.Y.; Morita, E.; Wang, H.E.; Davis, T.; He, G.P.; Cimbora, D.M.; et al. The protein network of HIV budding. Cell 2003, 114, 701–713. [Google Scholar] [CrossRef]
- Tabata, K.; Arimoto, M.; Arakawa, M.; Nara, A.; Saito, K.; Omori, H.; Arai, A.; Ishikawa, T.; Konishi, E.; Suzuki, R.; et al. Unique Requirement for ESCRT Factors in Flavivirus Particle Formation on the Endoplasmic Reticulum. Cell Rep. 2016, 16, 2339–2347. [Google Scholar] [CrossRef]


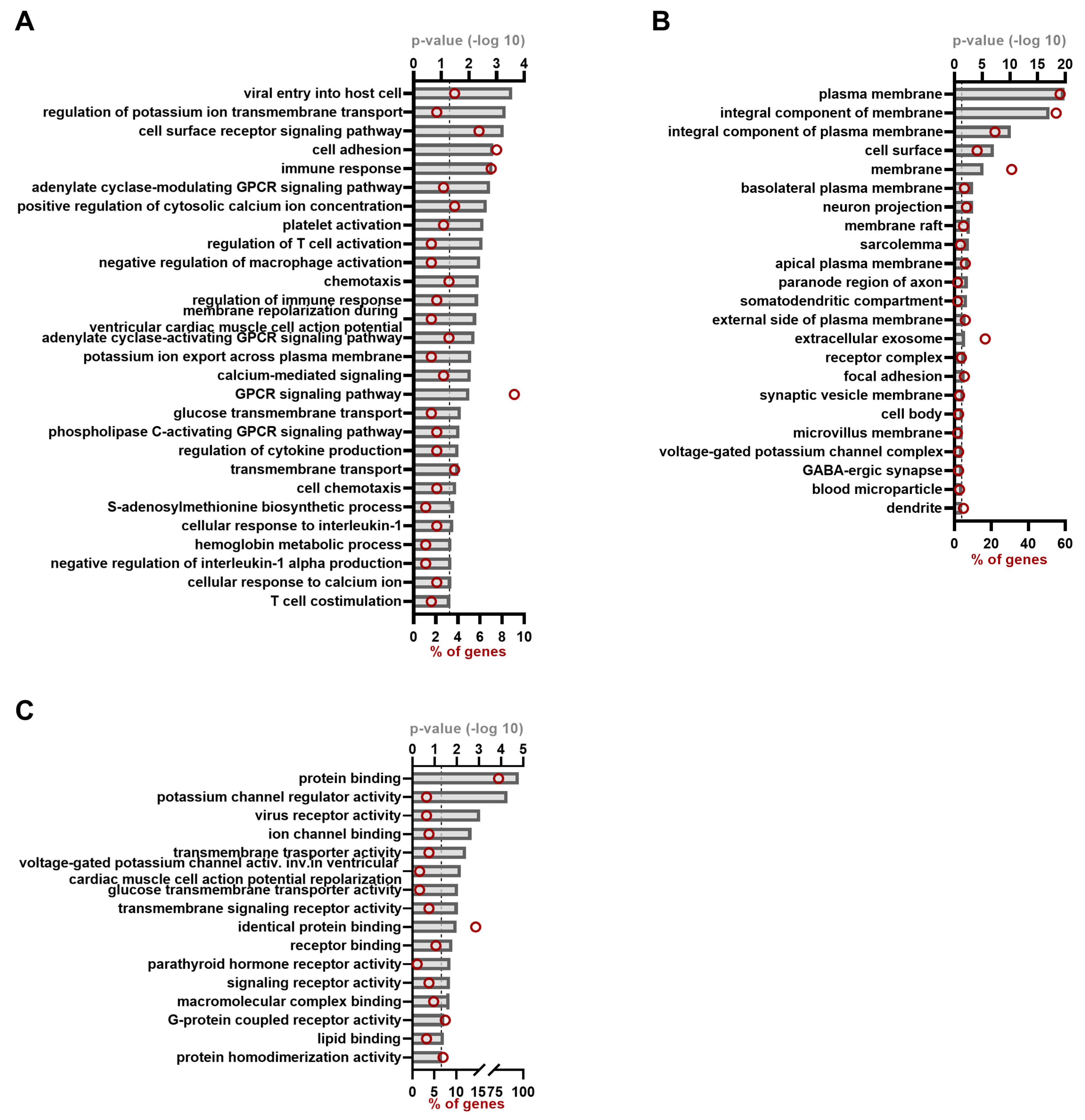

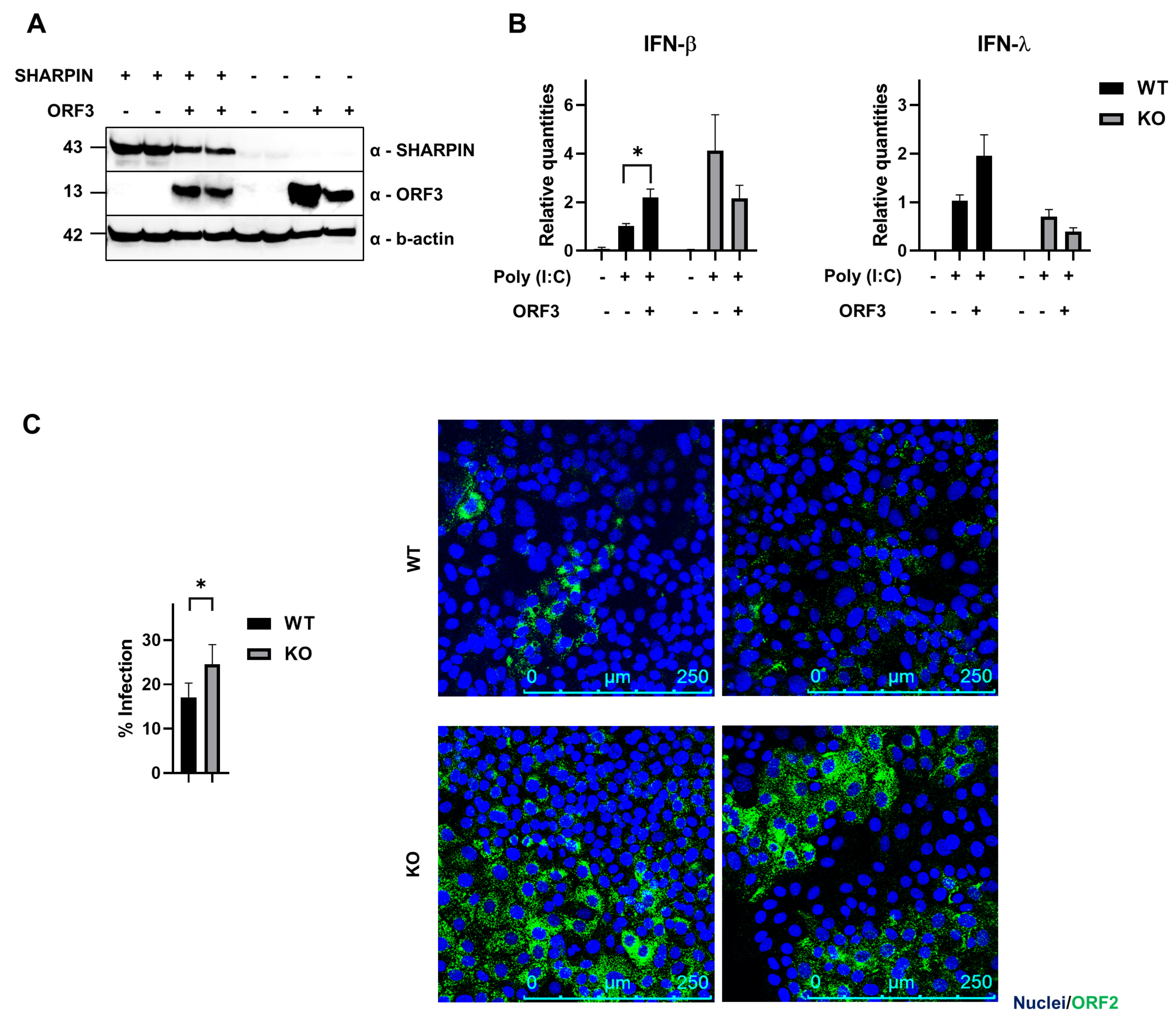
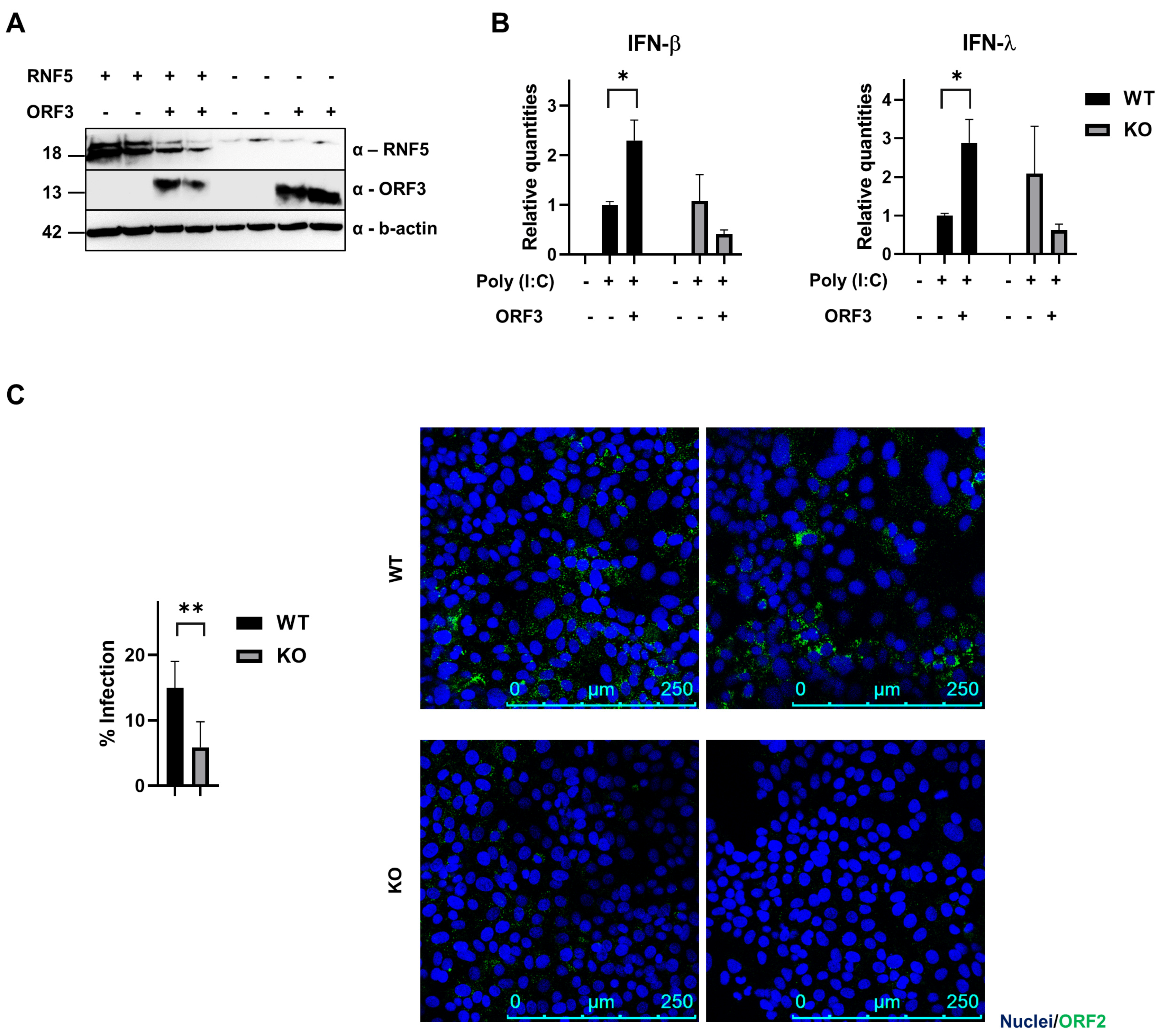
| Primer Name | Sequence (5′ → 3′) |
|---|---|
| Gt-1 ORF2 MAPPIT FW | gtcgacgagctccggatccatgcgccctcggcctattttg |
| Gt-1 ORF2 MAPPIT RV | gcggccgccaaataaactataactcccgagttttacccaccttcatc |
| Gt-1 ORF2 KISS FW | caattgaccatgcgccctcggcctattttg |
| Gt-1 ORF2 KISS RV | gcggccgctaactcccgagttttacccaccttcatcttaaggcgctg |
| Gt-1 ORF3 MAPPIT FW | gtcgacgagctccggatccatgaataacatgtcttttgctgcgcccatg |
| Gt-1 ORF3 MAPPIT RV | gcggccgcggagcgaccgcggttagc |
| Gt-1 ORF3 KISS FW | caattgaccatgaataacatgtcttttgctgcgcccatgggttc |
| Gt-1 ORF3 KISS RV | gcggccgcgcggcgcggccccagctg |
| Gt-1 ORF4 MAPPIT FW | gtcgacgagctccggatccatgttgcgcggacagcaaatc |
| Gt-1 ORF4 MAPPIT RV | gcggccgcttagctcacatacatccgcagggcag |
| Gt-1 ORF4 KISS FW | caattgaccatgttgcgcggacagcaaatc |
| Gt-1 ORF4 KISS RV | gcggccgcgctcacatacatccgcagggcag |
| Gt-3 ORF2 MAPPIT FW | gtcgacgagctccggatccaccatgtgccctagggttg |
| Gt-3 ORF2 MAPPIT RV | gcggccgcttaagactcccgggttttgcctacc |
| Gt-3 ORF2 KISS FW | caattgaccatgtgccctagggttgttc |
| Gt-3 ORF2 KISS RV | gcggccgcagactcccgggttttgcctacc |
| Gt-3 ORF3 MAPPIT FW | gtcgacgagctccggatccaccatgggatcaccatgtgccctagg |
| Gt-3 ORF3 MAPPIT RV | gcggccgctcaacggcgcagccccagc |
| Gt-3 ORF3 KISS FW | caattgaccatgggatcaccatgtgccctagggttg |
| Gt-3 ORF3 KISS RV | gcggccgcacggcgcagccccagctg |
| Gene Targeted | Oligo Name | Sequence (5′ → 3′) |
|---|---|---|
| SHARPIN | SHARPIN_ FW | CACCGCCTAGTCCGAGGTGCCACCG |
| SHARPIN_ RV | AAACCGGTGGCACCTCGGACTAGGC | |
| RNF5 | RNF5_ FW | CACCGAAGCCCCCGGTATCACCAAA |
| RNF5_ RV | AAACTTTGGTGATACCGGGGGCTTC |
| Antibody | Reference | |
|---|---|---|
| Primary antibodies | SHARPIN | MAB8100, R&D systems, Mineapolis, MN, USA |
| RNF5 | PA5-31793, Invitrogen, Waltham, MA, USA | |
| ORF3 | Bs-0212R, Bioss, Woburn, MA, USA | |
| β-ACTIN | BA3R, Invitrogen, Waltham, MA, USA | |
| Secondary antibodies | Sheep anti-mouse HRP-linked | NA931, Cytiva, Amersham, UK |
| Donkey anti-rabbit HRP-linked | NA934, Cytiva, Amersham, UK | |
| Gene Name | Assay ID |
|---|---|
| IFNB1 | Hs01077958_s1 |
| IFNL2/3 | Hs04193049_gH |
| HPRT1 | Hs99999909_m1 |
| RPL30 | Hs00265497_m1 |
| CYC1 | Hs00357717_m1 |
| YWHAZ | Hs01122445_g1 |
| ATP5B | Hs00969569_m1 |
| Construct | Entrez_ID | Value PIB | Value BP | BP/PIB | BP/BIP | Lowest Value (Min BP/PIB and BP/BIP) | SCORE |
|---|---|---|---|---|---|---|---|
| Gt-1 ORF3 MAPPIT | SHARPIN | 1.17 | 82.09 | 69.9 | 61.2 | 61.2 | + |
| Gt-1 ORF3 KISS | SHARPIN | 1857 | 796,444 | 429 | 42.2 | 42.2 | + |
| Gt-3 ORF3 KISS | SHARPIN | 1857 | 187,953 | 101.2 | 25.4 | 25.4 | + |
| Gt-1 ORF3 KISS | RNF5 | 3114 | 250,917 | 80.6 | 33.8 | 33.8 | + |
| Gt-3 ORF3 KISS | RNF5 | 3114 | 365,420 | 117.4 | 19.4 | 19.4 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corneillie, L.; Lemmens, I.; Weening, K.; De Meyer, A.; Van Houtte, F.; Tavernier, J.; Meuleman, P. Virus–Host Protein Interaction Network of the Hepatitis E Virus ORF2-4 by Mammalian Two-Hybrid Assays. Viruses 2023, 15, 2412. https://doi.org/10.3390/v15122412
Corneillie L, Lemmens I, Weening K, De Meyer A, Van Houtte F, Tavernier J, Meuleman P. Virus–Host Protein Interaction Network of the Hepatitis E Virus ORF2-4 by Mammalian Two-Hybrid Assays. Viruses. 2023; 15(12):2412. https://doi.org/10.3390/v15122412
Chicago/Turabian StyleCorneillie, Laura, Irma Lemmens, Karin Weening, Amse De Meyer, Freya Van Houtte, Jan Tavernier, and Philip Meuleman. 2023. "Virus–Host Protein Interaction Network of the Hepatitis E Virus ORF2-4 by Mammalian Two-Hybrid Assays" Viruses 15, no. 12: 2412. https://doi.org/10.3390/v15122412
APA StyleCorneillie, L., Lemmens, I., Weening, K., De Meyer, A., Van Houtte, F., Tavernier, J., & Meuleman, P. (2023). Virus–Host Protein Interaction Network of the Hepatitis E Virus ORF2-4 by Mammalian Two-Hybrid Assays. Viruses, 15(12), 2412. https://doi.org/10.3390/v15122412




