The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model
Abstract
:1. Introduction
1.1. Distinct Features of Different CNS Cell Types as Sensors and Responders to Infection
1.2. The mCoV Encephalomyelitis Model
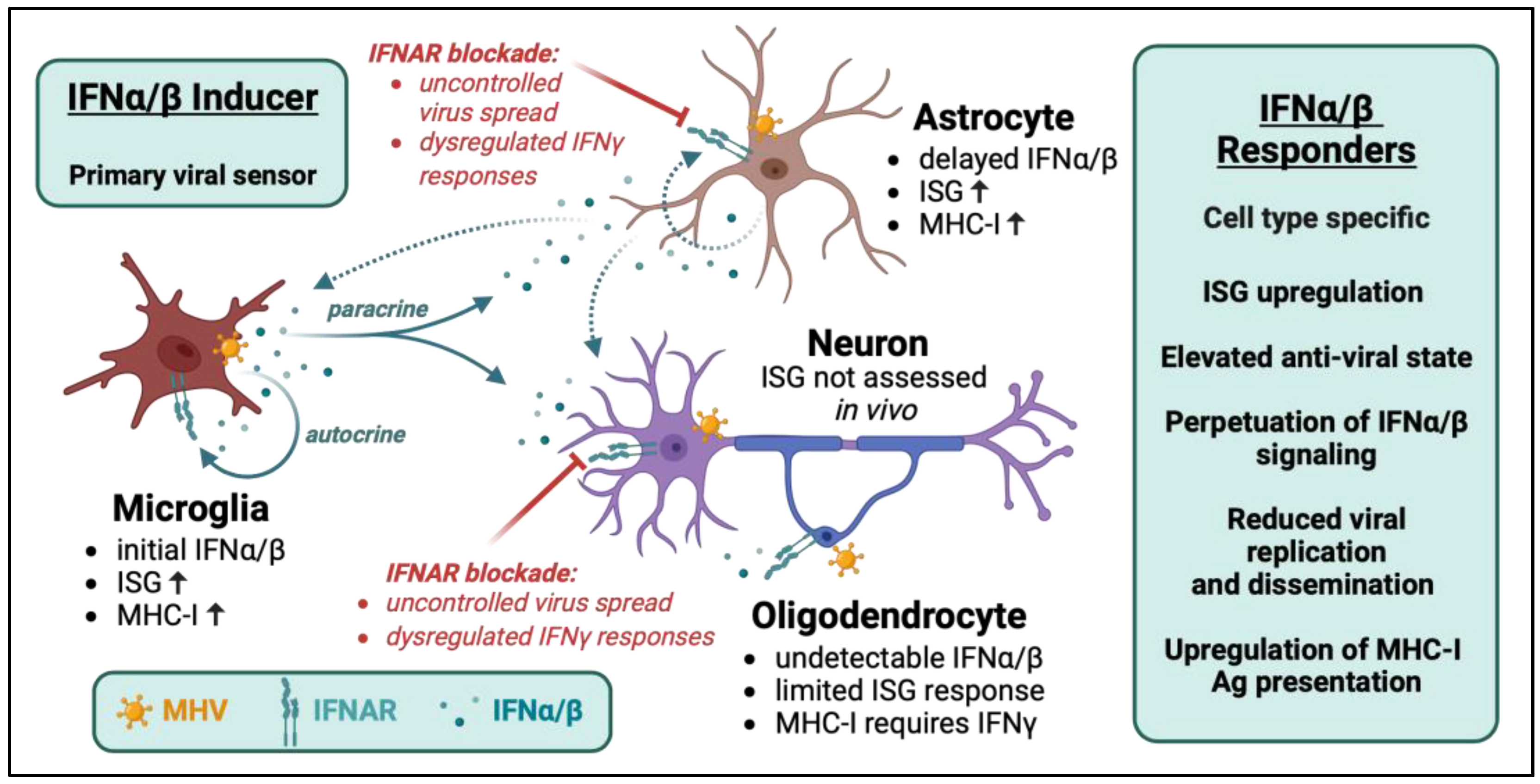
2. Interplay between IFNα/β Inducer and Responder Cells in Controlling Viral Dissemination
3. Crosstalk between IFNα/β and IFNγ
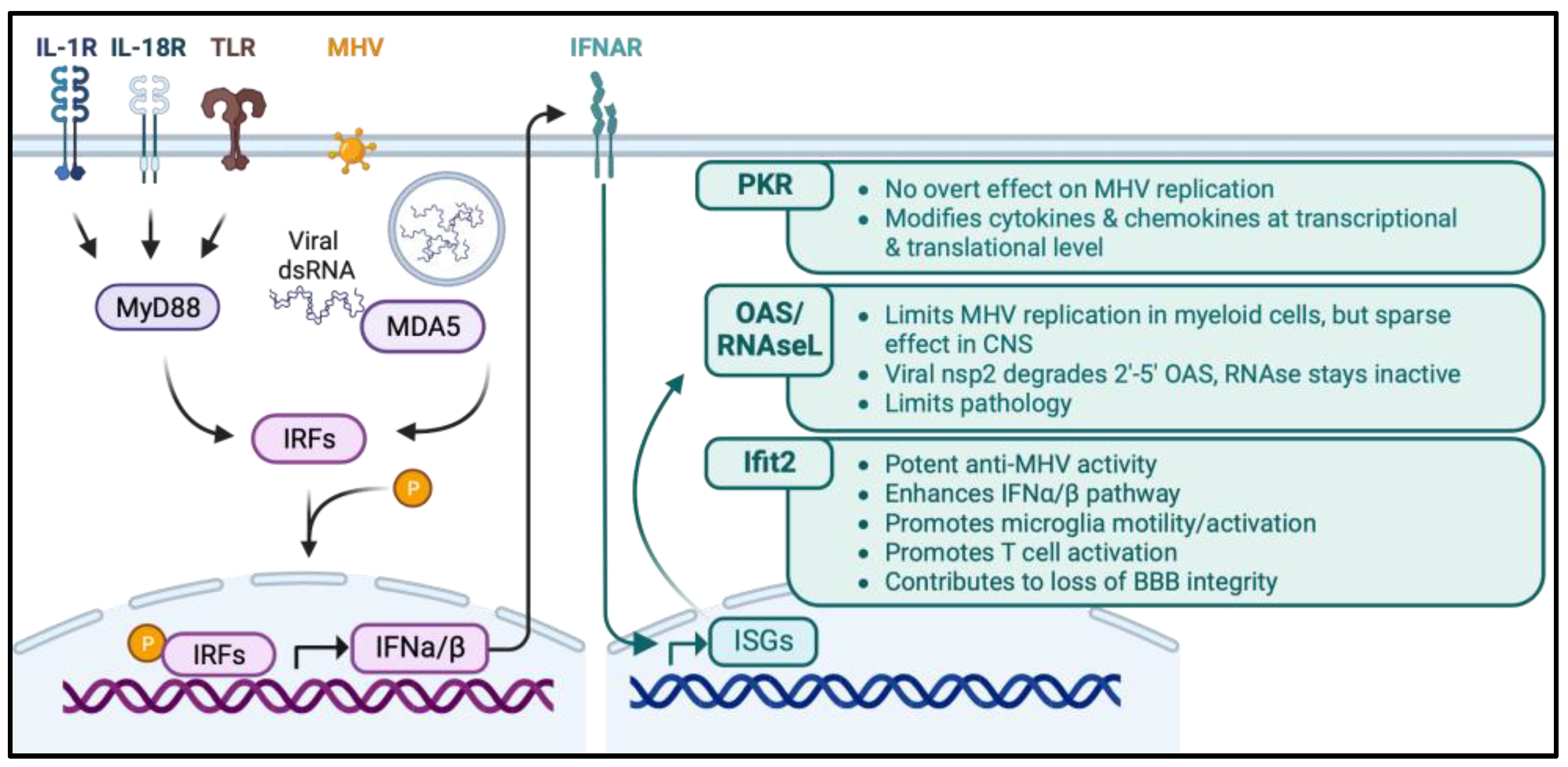
4. Anti-Viral and Immune Modulatory Roles of Select Innate Molecules
4.1. MDA5 and MyD88
4.2. PKR
4.3. OAS/RNase L
4.4. IFIT2
5. Participation of Cells Promoting T Cell Access to the Parenchyma
5.1. Meningeal Stromal Cell Activation in Promoting Protective CD8 T Cell Immunity
5.2. Role of Myeloid Cells in Regulating T Cell Parenchymal Access and Function
6. Conclusions and Gaps in Knowledge
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhatbar, C.; Prinz, M. The roles of microglia in viral encephalitis: From sensome to therapeutic targeting. Cell. Mol. Immunol. 2021, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.L. Acute Viral Encephalitis. N. Engl. J. Med. 2018, 379, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.A. Emergent Viral Infections of the CNS. J. Neuropathol. Exp. Neurol. 2020, 79, 823–842. [Google Scholar] [CrossRef]
- Klein, R.S.; Garber, C.; Funk, K.E.; Salimi, H.; Soung, A.; Kanmogne, M.; Manivasagam, S.; Agner, S.; Cain, M. Neuroinflammation During RNA Viral Infections. Annu. Rev. Immunol. 2019, 37, 73–95. [Google Scholar] [CrossRef]
- Swanson, P.A., 2nd; McGavern, D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015, 11, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Mesev, E.V.; LeDesma, R.A.; Ploss, A. Decoding type I and III interferon signalling during viral infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef]
- Singh, H.; Koury, J.; Kaul, M. Innate Immune Sensing of Viruses and Its Consequences for the Central Nervous System. Viruses 2021, 13, 170. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.P.; Wearsch, P.A.; Canaday, D.H.; Meyerson, H.J.; Liu, Y.C.; Wang, Y.; Boom, W.H.; Harding, C.V. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J. Immunol. 2012, 188, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Duong, E.; Fessenden, T.B.; Lutz, E.; Dinter, T.; Yim, L.; Blatt, S.; Bhutkar, A.; Wittrup, K.D.; Spranger, S. Type I interferon activates MHC class I-dressed CD11b(+) conventional dendritic cells to promote protective anti-tumor CD8(+) T cell immunity. Immunity 2022, 55, 308–323. [Google Scholar] [CrossRef]
- Kessing, C.F.; Tyor, W.R. Interferon-α induces neurotoxicity through activation of the type I receptor and the GluN2A subunit of the NMDA receptor. J. Interferon Cytokine Res. 2015, 35, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406. [Google Scholar] [CrossRef]
- Paul, S.; Ricour, C.; Sommereyns, C.; Sorgeloos, F.; Michiels, T. Type I interferon response in the central nervous system. Biochimie 2007, 89, 770–778. [Google Scholar] [CrossRef]
- Zegenhagen, L.; Kurhade, C.; Koniszewski, N.; Overby, A.K.; Kroger, A. Brain heterogeneity leads to differential innate immune responses and modulates pathogenesis of viral infections. Cytokine Growth Factor Rev. 2016, 30, 95–101. [Google Scholar] [CrossRef]
- Cavanaugh, S.E.; Holmgren, A.M.; Rall, G.F. Homeostatic interferon expression in neurons is sufficient for early control of viral infection. J. Neuroimmunol. 2015, 279, 11–19. [Google Scholar] [CrossRef]
- Chhatbar, C.; Detje, C.N.; Grabski, E.; Borst, K.; Spanier, J.; Ghita, L.; Elliott, D.A.; Jordao, M.J.C.; Mueller, N.; Sutton, J.; et al. Type I Interferon Receptor Signaling of Neurons and Astrocytes Regulates Microglia Activation during Viral Encephalitis. Cell Rep. 2018, 25, 118–129.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Sorgeloos, F.; Kreit, M.; Hermant, P.; Lardinois, C.; Michiels, T. Antiviral type I and type III interferon responses in the central nervous system. Viruses 2013, 5, 834–857. [Google Scholar] [CrossRef]
- Miller, K.D.; Schnell, M.J.; Rall, G.F. Keeping it in check: Chronic viral infection and antiviral immunity in the brain. Nat. Rev. Neurosci. 2016, 17, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Bergmann, C.C. Intercellular Communication Is Key for Protective IFNα/β Signaling During Viral Central Nervous System Infection. Viral Immunol. 2019, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Blank, T.; Prinz, M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia 2013, 61, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kapil, P.; Butchi, N.B.; Stohlman, S.A.; Bergmann, C.C. Oligodendroglia are limited in type I interferon induction and responsiveness in vivo. Glia 2012, 60, 1555–1566. [Google Scholar] [CrossRef]
- Viengkhou, B.; Hofer, M.J. Breaking down the cellular responses to type I interferon neurotoxicity in the brain. Front. Immunol. 2023, 14, 1110593. [Google Scholar] [CrossRef]
- Alsohime, F.; Martin-Fernandez, M.; Temsah, M.H.; Alabdulhafid, M.; Le Voyer, T.; Alghamdi, M.; Qiu, X.; Alotaibi, N.; Alkahtani, A.; Buta, S.; et al. JAK Inhibitor Therapy in a Child with Inherited USP18 Deficiency. N. Engl. J. Med. 2020, 382, 256–265. [Google Scholar] [CrossRef]
- Bastard, P.; Manry, J.; Chen, J.; Rosain, J.; Seeleuthner, Y.; AbuZaitun, O.; Lorenzo, L.; Khan, T.; Hasek, M.; Hernandez, N.; et al. Herpes simplex encephalitis in a patient with a distinctive form of inherited IFNAR1 deficiency. J. Clin. Investig. 2021, 131, e139980. [Google Scholar] [CrossRef]
- Baruch, K.; Deczkowska, A.; David, E.; Castellano, J.M.; Miller, O.; Kertser, A.; Berkutzki, T.; Barnett-Itzhaki, Z.; Bezalel, D.; Wyss-Coray, T.; et al. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 2014, 346, 89–93. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Bennett, F.C.; Bennett, M.L. Microglia in antiviral immunity of the brain and spinal cord. Semin. Immunol. 2022, 60, 101650. [Google Scholar] [CrossRef]
- Manglani, M.; McGavern, D.B. New advances in CNS immunity against viral infection. Curr. Opin. Virol. 2018, 28, 116–126. [Google Scholar] [CrossRef]
- Bergmann, C.C.; Lane, T.E.; Stohlman, S.A. Coronavirus infection of the central nervous system: Host-virus stand-off. Nat. Rev. Microbiol. 2006, 4, 121–132. [Google Scholar] [CrossRef]
- Bender, S.J.; Weiss, S.R. Pathogenesis of murine coronavirus in the central nervous system. J. Neuroimmune Pharmacol. 2010, 5, 336–354. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Wheeler, D.L. Neurotropic Coronavirus Infections. In Neurotropic Viral Infections: Volume 1: Neurotropic RNA Viruses; Springer: Cham, Switzerland, 2016; pp. 115–148. [Google Scholar] [CrossRef]
- Templeton, S.P.; Perlman, S. Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM. Immunol. Res. 2007, 39, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Stohlman, S.A.; Hinton, D.R. Viral Induced Demyelination. Brain Pathol. 2001, 11, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.O.; Trousdale, M.D.; El-Zaatari, F.A.; Stohlman, S.A.; Weiner, L.P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 1986, 58, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J.; Scheen, E.; Seo, S.H.; Koval, M.; Weiss, S.R. Enhanced green fluorescent protein expression may be used to monitor murine coronavirus spread in vitro and in the mouse central nervous system. J. Neurovirol. 2002, 8, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J.; Fu, L.; Tsai, J.C.; Weiss, S.R.; Lavi, E. Demyelination determinants map to the spike glycoprotein gene of coronavirus mouse hepatitis virus. J. Virol. 2000, 74, 9206–9213. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Zhang, Q.; Anthony, S.M.; Zhou, Y.; Zou, X.; Cassell, M.; Perlman, S. Oligodendrocytes that survive acute coronavirus infection induce prolonged inflammatory responses in the CNS. Proc. Natl. Acad. Sci. USA 2020, 117, 15902–15910. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Perlman, S. Viral expression of CCL2 is sufficient to induce demyelination in RAG1-/- mice infected with a neurotropic coronavirus. J. Virol. 2005, 79, 7113–7120. [Google Scholar] [CrossRef]
- Coley, S.E.; Lavi, E.; Sawicki, S.G.; Fu, L.; Schelle, B.; Karl, N.; Siddell, S.G.; Thiel, V. Recombinant mouse hepatitis virus strain A59 from cloned, full-length cDNA replicates to high titers in vitro and is fully pathogenic in vivo. J. Virol. 2005, 79, 3097–3106. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Barragan, L.; Züst, R.; Weber, F.; Spiegel, M.; Lang, K.S.; Akira, S.; Thiel, V.; Ludewig, B. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood 2007, 109, 1131–1137. [Google Scholar] [CrossRef]
- Ireland, D.D.; Stohlman, S.A.; Hinton, D.R.; Atkinson, R.; Bergmann, C.C. Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J. Virol. 2008, 82, 300–310. [Google Scholar] [CrossRef]
- Hwang, M.; Bergmann, C.C. Neuronal Ablation of Alpha/Beta Interferon (IFN-α/β) Signaling Exacerbates Central Nervous System Viral Dissemination and Impairs IFN-γ Responsiveness in Microglia/Macrophages. J. Virol. 2020, 94, e00422-20. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Bergmann, C.C. Alpha/Beta Interferon (IFN-α/β) Signaling in Astrocytes Mediates Protection against Viral Encephalomyelitis and Regulates IFN-γ-Dependent Responses. J. Virol. 2018, 92, e01901-17. [Google Scholar] [CrossRef] [PubMed]
- Phares, T.W.; Marques, C.P.; Stohlman, S.A.; Hinton, D.R.; Bergmann, C.C. Factors supporting intrathecal humoral responses following viral encephalomyelitis. J. Virol. 2011, 85, 2589–2598. [Google Scholar] [CrossRef]
- Cervantes-Barragán, L.; Kalinke, U.; Züst, R.; König, M.; Reizis, B.; López-Macías, C.; Thiel, V.; Ludewig, B. Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J. Immunol. 2009, 182, 1099–1106. [Google Scholar] [CrossRef]
- Malone, K.E.; Stohlman, S.A.; Ramakrishna, C.; Macklin, W.; Bergmann, C.C. Induction of class I antigen processing components in oligodendroglia and microglia during viral encephalomyelitis. Glia 2008, 56, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Butchi, N.B.; Hinton, D.R.; Stohlman, S.A.; Kapil, P.; Fensterl, V.; Sen, G.C.; Bergmann, C.C. Ifit2 deficiency results in uncontrolled neurotropic coronavirus replication and enhanced encephalitis via impaired alpha/beta interferon induction in macrophages. J. Virol. 2014, 88, 1051–1064. [Google Scholar] [CrossRef]
- Roth-Cross, J.K.; Bender, S.J.; Weiss, S.R. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J. Virol. 2008, 82, 9829–9838. [Google Scholar] [CrossRef] [PubMed]
- Phares, T.W.; Ramakrishna, C.; Parra, G.I.; Epstein, A.; Chen, L.; Atkinson, R.; Stohlman, S.A.; Bergmann, C.C. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. J. Immunol. 2009, 182, 5430–5438. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.M.; Weiss, S.R. Murine Coronavirus Cell Type Dependent Interaction with the Type I Interferon Response. Viruses 2009, 1, 689–712. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, C.; Kallfass, C.; Lienenklaus, S.; Spanier, J.; Kalinke, U.; Rieder, M.; Conzelmann, K.K.; Michiels, T.; Staeheli, P. Abortively Infected Astrocytes Appear To Represent the Main Source of Interferon Beta in the Virus-Infected Brain. J. Virol. 2015, 90, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Gottlieb, D.; Diamond, M.S. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 2003, 77, 13203–13213. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Michaelsen-Preusse, K.; Finsterbusch, K.; Stegemann-Koniszewski, S.; Bruder, D.; Grashoff, M.; Korte, M.; Koster, M.; Kalinke, U.; Hauser, H.; et al. Interferon regulatory factor-1 protects from fatal neurotropic infection with vesicular stomatitis virus by specific inhibition of viral replication in neurons. PLoS Pathog. 2014, 10, e1003999. [Google Scholar] [CrossRef]
- Rosato, P.C.; Leib, D.A. Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis. PLoS Pathog. 2015, 11, e1005028. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Harschnitz, O.; Studer, L.; Casanova, J.L. Neuron-intrinsic immunity to viruses in mice and humans. Curr. Opin. Immunol. 2021, 72, 309–317. [Google Scholar] [CrossRef]
- Cho, H.; Proll, S.C.; Szretter, K.J.; Katze, M.G.; Gale, M., Jr.; Diamond, M.S. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat. Med. 2013, 19, 458–464. [Google Scholar] [CrossRef]
- Ishibashi, D.; Homma, T.; Nakagaki, T.; Fuse, T.; Sano, K.; Satoh, K.; Mori, T.; Atarashi, R.; Nishida, N. Type I interferon protects neurons from prions in in vivo models. Brain 2019, 142, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Detje, C.N.; Meyer, T.; Schmidt, H.; Kreuz, D.; Rose, J.K.; Bechmann, I.; Prinz, M.; Kalinke, U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J. Immunol. 2009, 182, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Delhaye, S.; Paul, S.; Blakqori, G.; Minet, M.; Weber, F.; Staeheli, P.; Michiels, T. Neurons produce type I interferon during viral encephalitis. Proc. Natl. Acad. Sci. USA 2006, 103, 7835–7840. [Google Scholar] [CrossRef]
- Savarin, C.; Bergmann, C.C. Fine Tuning the Cytokine Storm by IFN and IL-10 Following Neurotropic Coronavirus Encephalomyelitis. Front. Immunol. 2018, 9, 3022. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Swiecki, M.; Cella, M.; Alber, G.; Schreiber, R.D.; Gilfillan, S.; Colonna, M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe 2012, 11, 631–642. [Google Scholar] [CrossRef]
- Fitzgerald-Bocarsly, P.; Feng, D. The role of type I interferon production by dendritic cells in host defense. Biochimie 2007, 89, 843–855. [Google Scholar] [CrossRef]
- Neumann, H.; Cavalié, A.; Jenne, D.E.; Wekerle, H. Induction of MHC class I genes in neurons. Science 1995, 269, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, B.D.S.; Grund, E.M.; Standiford, M.M.; Mirchia, K.; Westphal, M.S.; Muschler, E.S.; Howe, C.L. CD8+ T cells recognizing a neuron-restricted antigen injure axons in a model of multiple sclerosis. J. Clin. Investig. 2023, 133, e162788. [Google Scholar] [CrossRef]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89 Pt 1, 1–47. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Niyongere, S.A.; Lee, S.J.; Baker, B.J.; Benveniste, E.N. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J. Immunol. 2008, 181, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Phares, T.W.; Stohlman, S.A.; Hinton, D.R.; Atkinson, R.; Bergmann, C.C. Enhanced antiviral T cell function in the absence of B7-H1 is insufficient to prevent persistence but exacerbates axonal bystander damage during viral encephalomyelitis. J. Immunol. 2010, 185, 5607–5618. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Kitaura, M.; Kato, T.; Watanabe, Y.; Kawade, Y.; Muramatsu, S. Contrasting effect of alpha/beta- and gamma-interferons on expression of macrophage Ia antigens. J. Exp. Med. 1986, 163, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, M.; Humann, J.; Penheiter, K.; Andreasen, K.; Lenz, L.L. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J. Exp. Med. 2010, 207, 327–337. [Google Scholar] [CrossRef]
- Yoshida, R.; Murray, H.W.; Nathan, C.F. Agonist and antagonist effects of interferon alpha and beta on activation of human macrophages. Two classes of interferon gamma receptors and blockade of the high-affinity sites by interferon alpha or beta. J. Exp. Med. 1988, 167, 1171–1185. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef]
- Chen, S.; Liao, Z.; Xu, P. Mitochondrial control of innate immune responses. Front. Immunol. 2023, 14, 1166214. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189.e15. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Arend, W.P.; Palmer, G.; Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008, 223, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Butchi, N.; Kapil, P.; Puntambekar, S.; Stohlman, S.A.; Hinton, D.R.; Bergmann, C.C. Myd88 Initiates Early Innate Immune Responses and Promotes CD4 T Cells during Coronavirus Encephalomyelitis. J. Virol. 2015, 89, 9299–9312. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Zalinger, Z.B.; Elliott, R.; Weiss, S.R. Role of the inflammasome-related cytokines Il-1 and Il-18 during infection with murine coronavirus. J. Neurovirol. 2017, 23, 845–854. [Google Scholar] [CrossRef]
- Garcia, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of protein kinase PKR in cell biology: From antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2006, 70, 1032–1060. [Google Scholar] [CrossRef]
- Kapil, P.; Stohlman, S.A.; Hinton, D.R.; Bergmann, C.C. PKR mediated regulation of inflammation and IL-10 during viral encephalomyelitis. J. Neuroimmunol. 2014, 270, 1–12. [Google Scholar] [CrossRef]
- Zhao, L.; Jha, B.K.; Wu, A.; Elliott, R.; Ziebuhr, J.; Gorbalenya, A.E.; Silverman, R.H.; Weiss, S.R. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe 2012, 11, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Gusho, E.; Baskar, D.; Banerjee, S. New advances in our understanding of the “unique” RNase L in host pathogen interaction and immune signaling. Cytokine 2020, 133, 153847. [Google Scholar] [CrossRef] [PubMed]
- Drappier, M.; Sorgeloos, F.; Michiels, T. The OAS/RNaseL pathway and its inhibition by viruses. Virologie 2014, 18, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Malathi, K.; Dong, B.; Gale, M., Jr.; Silverman, R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 2007, 448, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Bisbal, C.; Martinand, C.; Silhol, M.; Lebleu, B.; Salehzada, T. Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2-5A pathway. J. Biol. Chem. 1995, 270, 13308–13317. [Google Scholar] [CrossRef]
- Ireland, D.D.; Stohlman, S.A.; Hinton, D.R.; Kapil, P.; Silverman, R.H.; Atkinson, R.A.; Bergmann, C.C. RNase L mediated protection from virus induced demyelination. PLoS Pathog. 2009, 5, e1000602. [Google Scholar] [CrossRef] [PubMed]
- Roth-Cross, J.K.; Stokes, H.; Chang, G.; Chua, M.M.; Thiel, V.; Weiss, S.R.; Gorbalenya, A.E.; Siddell, S.G. Organ-specific attenuation of murine hepatitis virus strain A59 by replacement of catalytic residues in the putative viral cyclic phosphodiesterase ns2. J. Virol. 2009, 83, 3743–3753. [Google Scholar] [CrossRef]
- Zhao, L.; Birdwell, L.D.; Wu, A.; Elliott, R.; Rose, K.M.; Phillips, J.M.; Li, Y.; Grinspan, J.; Silverman, R.H.; Weiss, S.R. Cell-type-specific activation of the oligoadenylate synthetase-RNase L pathway by a murine coronavirus. J. Virol. 2013, 87, 8408–8418. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Sen, G.C. Interferon-induced Ifit proteins: Their role in viral pathogenesis. J. Virol. 2015, 89, 2462–2468. [Google Scholar] [CrossRef]
- Fensterl, V.; Wetzel, J.L.; Ramachandran, S.; Ogino, T.; Stohlman, S.A.; Bergmann, C.C.; Diamond, M.S.; Virgin, H.W.; Sen, G.C. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog. 2012, 8, e1002712. [Google Scholar] [CrossRef]
- Das Sarma, J.; Burrows, A.; Rayman, P.; Hwang, M.H.; Kundu, S.; Sharma, N.; Bergmann, C.; Sen, G.C. Ifit2 deficiency restricts microglial activation and leukocyte migration following murine coronavirus (m-CoV) CNS infection. PLoS Pathog. 2020, 16, e1009034. [Google Scholar] [CrossRef]
- Sharma, M.; Chakravarty, D.; Hussain, A.; Zalavadia, A.; Burrows, A.; Rayman, P.; Sharma, N.; Kenyon, L.C.; Bergmann, C.; Sen, G.C.; et al. Ifit2 restricts murine coronavirus spread to the spinal cord white matter and its associated myelin pathology. J. Virol. 2023, 97, e0074923. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shrestha, B.; Sen, G.C.; Diamond, M.S. A role for Ifit2 in restricting West Nile virus infection in the brain. J. Virol. 2013, 87, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Sugumar, P.; Bhandari, P.; Rangarajan, P.N. Identification of Japanese encephalitis virus-inducible genes in mouse brain and characterization of GARG39/IFIT2 as a microtubule-associated protein. J. Gen. Virol. 2006, 87 Pt 11, 3285–3289. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Fensterl, V.; Lawrence, T.M.; Hudacek, A.W.; Sen, G.C.; Schnell, M.J. Ifit2 Is a Restriction Factor in Rabies Virus Pathogenicity. J. Virol. 2017, 91(17), e00889-17. [Google Scholar] [CrossRef]
- Fensterl, V.; Wetzel, J.L.; Sen, G.C. Interferon-induced protein Ifit2 protects mice from infection of the peripheral nervous system by vesicular stomatitis virus. J. Virol. 2014, 88, 10303–10311. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, S.; Xu, F.; Kong, Y.; Wan, L.; Zhang, Y.; Zhang, Z. Inhibition of Proteasome Activity Induces Aggregation of IFIT2 in the Centrosome and Enhances IFIT2-Induced Cell Apoptosis. Int. J. Biol. Sci. 2017, 13, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhai, W.; Zheng, X.; Xie, Q.; Zhou, Q.; Tao, M.; Zhu, Y.; Wu, C.; Jiang, J. Decreased IFIT2 Expression Promotes Gastric Cancer Progression and Predicts Poor Prognosis of the Patients. Cell Physiol. Biochem. 2018, 45, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Regmi, P.; Liu, C.J.; Lo, J.F.; Lee, T.C. IFIT2 Depletion Promotes Cancer Stem Cell-like Phenotypes in Oral Cancer. Biomedicines 2023, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Pidugu, V.K.; Pidugu, H.B.; Wu, M.M.; Liu, C.J.; Lee, T.C. Emerging Functions of Human IFIT Proteins in Cancer. Front. Mol. Biosci. 2019, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Rai, N.K.; Burrows, A.; Kim, S.; Tripathi, A.; Weinberg, S.E.; Dutta, R.; Sen, G.C.; Min, B. IFN-Induced Protein with Tetratricopeptide Repeats 2 Limits Autoimmune Inflammation by Regulating Myeloid Cell Activation and Metabolic Activity. J. Immunol. 2023, 210, 721–731. [Google Scholar] [CrossRef]
- Weigel, M.; Wang, L.; Fu, M.M. Microtubule organization and dynamics in oligodendrocytes, astrocytes, and microglia. Dev. Neurobiol. 2021, 81, 310–320. [Google Scholar] [CrossRef]
- Hosking, M.P.; Lane, T.E. The role of chemokines during viral infection of the CNS. PLoS Pathog. 2010, 6, e1000937. [Google Scholar] [CrossRef]
- Lane, T.E.; Hardison, J.L.; Walsh, K.B. Functional diversity of chemokines and chemokine receptors in response to viral infection of the central nervous system. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 303, pp. 1–27. [Google Scholar] [CrossRef]
- Hosking, M.P.; Lane, T.E. ELR(+) chemokine signaling in host defense and disease in a viral model of central nervous system disease. Front. Cell Neurosci. 2014, 8, 165. [Google Scholar] [CrossRef]
- Skinner, D.; Marro, B.S.; Lane, T.E. Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease. Viral Immunol. 2019, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Troili, F.; Cipollini, V.; Moci, M.; Morena, E.; Palotai, M.; Rinaldi, V.; Romano, C.; Ristori, G.; Giubilei, F.; Salvetti, M.; et al. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad Between the Immune, Vascular and Nervous System. Front. Neuroanat. 2020, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Ampie, L.; McGavern, D.B. Immunological defense of CNS barriers against infections. Immunity 2022, 55, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Negron, A.; Stüve, O.; Forsthuber, T.G. Ectopic Lymphoid Follicles in Multiple Sclerosis: Centers for Disease Control? Front. Neurol. 2020, 11, 607766. [Google Scholar] [CrossRef] [PubMed]
- Mitsdoerffer, M.; Peters, A. Tertiary Lymphoid Organs in Central Nervous System Autoimmunity. Front. Immunol. 2016, 7, 451. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Kakizaki, M.; Ikehara, Y.; Togayachi, A. Formation of fibroblastic reticular network in the brain after infection with neurovirulent murine coronavirus. Neuropathology 2016, 36, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Cupovic, J.; Onder, L.; Gil-Cruz, C.; Weiler, E.; Caviezel-Firner, S.; Perez-Shibayama, C.; Rulicke, T.; Bechmann, I.; Ludewig, B. Central Nervous System Stromal Cells Control Local CD8(+) T Cell Responses during Virus-Induced Neuroinflammation. Immunity 2016, 44, 622–633. [Google Scholar] [CrossRef]
- Comerford, I.; Harata-Lee, Y.; Bunting, M.D.; Gregor, C.; Kara, E.E.; McColl, S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013, 24, 269–283. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.; Zhou, X.; Kosla, J.; Adili, A.; Garcia Beccaria, M.; Kotsiliti, E.; Pfister, D.; Johlke, A.L.; Sinha, A.; Sankowski, R.; et al. Age-Related Gliosis Promotes Central Nervous System Lymphoma through CCL19-Mediated Tumor Cell Retention. Cancer Cell 2019, 36, 250–267.e259. [Google Scholar] [CrossRef] [PubMed]
- Columba-Cabezas, S.; Serafini, B.; Ambrosini, E.; Aloisi, F. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: Implications for the maintenance of chronic neuroinflammation. Brain Pathol. 2003, 13, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Nicola, D.; Pallas-Bazarra, N.; Valle-Argos, B.; Nieto-Sampedro, M. CCR7 is expressed in astrocytes and upregulated after an inflammatory injury. J. Neuroimmunol. 2010, 227, 87–92. [Google Scholar] [CrossRef]
- Alt, C.; Laschinger, M.; Engelhardt, B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2002, 32, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Alanko, J.; Uçar, M.C.; Canigova, N.; Stopp, J.; Schwarz, J.; Merrin, J.; Hannezo, E.; Sixt, M. CCR7 acts as both a sensor and a sink for CCL19 to coordinate collective leukocyte migration. Sci. Immunol. 2023, 8, eadc9584. [Google Scholar] [CrossRef]
- Waltl, I.; Kalinke, U. Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci. 2022, 45, 158–170. [Google Scholar] [CrossRef]
- Cowan, M.N.; Sethi, I.; Harris, T.H. Microglia in CNS infections: Insights from Toxoplasma gondii and other pathogens. Trends Parasitol. 2022, 38, 217–229. [Google Scholar] [CrossRef]
- Savarin, C.; Dutta, R.; Bergmann, C.C. Distinct Gene Profiles of Bone Marrow-Derived Macrophages and Microglia During Neurotropic Coronavirus-Induced Demyelination. Front. Immunol. 2018, 9, 1325. [Google Scholar] [CrossRef]
- Syage, A.R.; Ekiz, H.A.; Skinner, D.D.; Stone, C.; O’Connell, R.M.; Lane, T.E. Single-Cell RNA Sequencing Reveals the Diversity of the Immunological Landscape following Central Nervous System Infection by a Murine Coronavirus. J. Virol. 2020, 94, e01295-20. [Google Scholar] [CrossRef]
- Lei, F.; Cui, N.; Zhou, C.; Chodosh, J.; Vavvas, D.G.; Paschalis, E.I. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc. Natl. Acad. Sci. USA 2020, 117, 23336–23338. [Google Scholar] [CrossRef]
- Funk, K.E.; Klein, R.S. CSF1R antagonism limits local restimulation of antiviral CD8(+) T cells during viral encephalitis. J. Neuroinflamm. 2019, 16, 22. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Sariol, A.; Meyerholz, D.K.; Perlman, S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Investig. 2018, 128, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Mangale, V.; Syage, A.R.; Ekiz, H.A.; Skinner, D.D.; Cheng, Y.; Stone, C.L.; Brown, R.M.; O’Connell, R.M.; Green, K.N.; Lane, T.E. Microglia influence host defense, disease, and repair following murine coronavirus infection of the central nervous system. Glia 2020, 68, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Savarin, C.; Stohlman, S.A.; Atkinson, R.; Ransohoff, R.M.; Bergmann, C.C. Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis. J. Virol. 2010, 84, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.P.; Kuziel, W.A.; Lane, T.E. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J. Immunol. 2001, 167, 4585–4592. [Google Scholar] [CrossRef]
- Held, K.S.; Chen, B.P.; Kuziel, W.A.; Rollins, B.J.; Lane, T.E. Differential roles of CCL2 and CCR2 in host defense to coronavirus infection. Virology 2004, 329, 251–260. [Google Scholar] [CrossRef]
- Zhou, J.; Marten, N.W.; Bergmann, C.C.; Macklin, W.B.; Hinton, D.R.; Stohlman, S.A. Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis. J. Virol. 2005, 79, 4764–4773. [Google Scholar] [CrossRef]
- Sengupta, S.; Addya, S.; Biswas, D.; Banerjee, P.; Sarma, J.D. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in murine beta-coronavirus-induced neuroinflammation. Virology 2022, 566, 122–135. [Google Scholar] [CrossRef]
- Savarin, C.; Bergmann, C.C.; Hinton, D.R.; Stohlman, S.A. MMP-independent role of TIMP-1 at the blood brain barrier during viral encephalomyelitis. ASN Neuro 2013, 5, e00127. [Google Scholar] [CrossRef]
- De Masi, R.; Orlando, S.; Bagordo, F.; Grassi, T. IFP35 Is a Relevant Factor in Innate Immunity, Multiple Sclerosis, and Other Chronic Inflammatory Diseases: A Review. Biology 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, N.; Cheng, Q.; Deng, F.; Liu, M.; Zhu, A.; Min, Y.Q.; Zhu, D.; Huang, W.; Feng, X.; et al. IFP35 as a promising biomarker and therapeutic target for the syndromes induced by SARS-CoV-2 or influenza virus. Cell Rep. 2021, 37, 110126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boylan, B.T.; Hwang, M.; Bergmann, C.C. The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model. Viruses 2023, 15, 2400. https://doi.org/10.3390/v15122400
Boylan BT, Hwang M, Bergmann CC. The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model. Viruses. 2023; 15(12):2400. https://doi.org/10.3390/v15122400
Chicago/Turabian StyleBoylan, Brendan T., Mihyun Hwang, and Cornelia C. Bergmann. 2023. "The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model" Viruses 15, no. 12: 2400. https://doi.org/10.3390/v15122400
APA StyleBoylan, B. T., Hwang, M., & Bergmann, C. C. (2023). The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model. Viruses, 15(12), 2400. https://doi.org/10.3390/v15122400






