Effect of a Second Pregnancy on the HPV Serology in Mothers Followed Up in the Finnish Family HPV Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Serology
2.3. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruni, L.; Diaz, M.; Castellsague, X.; Ferrer, E.; Bosch, F.X.; de Sanjose, S. Cervical Human Papillomavirus Prevalence in 5 Continents: Meta-Analysis of 1 Million Women with Normal Cytological Findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The Estimated Lifetime Probability of Acquiring Human Papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Sarkola, M.E.; Grénman, S.E.; Rintala, M.A.M.; Syrjänen, K.J.; Syrjänen, S.M. Effect of Second Pregnancy on Maternal Carriage and Outcome of High-Risk Human Papillomavirus (HPV). Gynecol. Obstet. Investig. 2009, 67, 208–216. [Google Scholar] [CrossRef]
- Morrison, E.A.B.; Gammon, M.D.; Goldberg, G.L.; Vermund, S.H.; Burk, R.D. Pregnancy and Cervical Infection with Human Papillomaviruses. Int. J. Gynecol. Obstet. 1996, 54, 125–130. [Google Scholar] [CrossRef]
- Hernández-Girón, C.; Smith, J.S.; Lorincz, A.; Lazcano, E.; Hernández-Ávila, M.; Salmerón, J. High-Risk Human Papillomavirus Detection and Related Risk Factors among Pregnant and Nonpregnant Women in Mexico. Sex. Transm. Dis. 2005, 32, 613–618. [Google Scholar] [CrossRef]
- Pandey, D.; Solleti, V.; Jain, G.; Das, A.; Shama Prasada, K.; Acharya, S.; Satyamoorthy, K. Human Papillomavirus (HPV) Infection in Early Pregnancy: Prevalence and Implications. Infect. Dis. Obstet. Gynecol. 2019, 2019, 4376902. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, L.; Sun, Y.; Wang, Z. The Prevalence and Risk of Human Papillomavirus Infection in Pregnant Women. Epidemiol. Infect. 2014, 142, 1567–1578. [Google Scholar] [CrossRef]
- Carter, J.J.; Koutsky, L.A.; Hughes, J.P.; Lee, S.K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. Comparison of Human Papillomavirus Types 16, 18, and 6 Capsid Antibody Responses Following Incident Infection. J. Infect. Dis. 2000, 181, 1911–1919. [Google Scholar] [CrossRef]
- Beachler, D.C.; Jenkins, G.; Safaeian, M.; Kreimer, A.R.; Wentzensen, N. Natural Acquired Immunity Against Subsequent Genital Human Papillomavirus Infection: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2016, 213, 1444. [Google Scholar] [CrossRef]
- Rintala, M.A.M.; Grénman, S.E.; Puranen, M.H.; Isolauri, E.; Ekblad, U.; Kero, P.O.; Syrjänen, S.M. Transmission of High-Risk Human Papillomavirus (HPV) between Parents and Infant: A Prospective Study of HPV in Families in Finland. J. Clin. Microbiol. 2005, 43, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.; Waterboer, T.; Sarkola, M.; Michael, K.; Rintala, M.; Syrjänen, K.; Grenman, S.; Pawlita, M. Dynamics of Human Papillomavirus Serology in Women Followed up for 36 Months after Pregnancy. J. General. Virol. 2009, 90, 1515–1526. [Google Scholar] [CrossRef]
- Louvanto, K.; Rintala, M.A.; Syrjänen, K.J.; Grénman, S.E.; Syrjänen, S.M. Genotype-Specific Persistence of Genital Human Papillomavirus (HPV) Infections in Women Followed for 6 Years in the Finnish Family HPV Study. J. Infect. Dis. 2010, 202, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Waterboer, T.; Sehr, P.; Michael, K.M.; Franceschi, S.; Nieland, J.D.; Joos, T.O.; Templin, M.F.; Pawlita, M. Multiplex Human Papillomavirus Serology Based on in Situ-Purified Glutathione S-Transferase Fusion Proteins. Clin. Chem. 2005, 51, 1845–1853. [Google Scholar] [CrossRef]
- Chelimo, C.; Wouldes, T.A.; Cameron, L.D.; Elwood, J.M. Risk Factors for and Prevention of Human Papillomaviruses (HPV), Genital Warts and Cervical Cancer. J. Infect. 2013, 66, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, J.; Chu, B.; Zhang, X.; Ru, X.; Chen, Y.; Chen, Y.; Cheng, X. Characteristics and Related Factors of High-Risk Human Papillomavirus Infection in Pregnant Women. Med. Sci. Monit. 2021, 27, e929100. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Lima, J.; Cambridge, G.; Vilas-Boas, A.; Martins, C.; Borrego, L.M.; Leandro, M. Serum Markers of B-Cell Activation in Pregnancy during Late Gestation, Delivery, and the Postpartum Period. Am. J. Reprod. Immunol. 2019, 81, e13090. [Google Scholar] [CrossRef]
- Zgura, F.A.; Bratila, E.; Vladareanu, S.; Zgura, A. Transplacental Transmission of Human Papillomavirus. Maedica 2015, 10, 159. [Google Scholar]
- Sethi, S.; Muller, M.; Schneider, A.; Blettner, M.; Smith, E.; Turek, L.; Wahrendorf, J.; Gissmann, L.; Chang-Claude, J. Serologic Response to the E4, E6, and E7 Proteins of Human Papillomavirus Type 16 in Pregnant Women. Am. J. Obstet. Gynecol. 1998, 178, 360–364. [Google Scholar] [CrossRef]
- Pirttilä, T.; Syrjänen, S.; Louvanto, K.; Loimaranta, V. Longitudinal Dynamics of HPV16 Antibodies in Saliva and Serum among Pregnant Women. Viruses 2022, 14, 2567. [Google Scholar] [CrossRef]
- Linthorst, J.; Welkers, M.R.A.; Sistermans, E.A. Clinically Relevant DNA Viruses in Pregnancy. Prenat. Diagn. 2023, 43, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Iams, J.D.; Porter, K.; Glaser, R. Epstein-Barr Virus Reactivation during Pregnancy and Postpartum: Effects of Race and Racial Discrimination. Brain Behav. Immun. 2012, 26, 1280. [Google Scholar] [CrossRef]
- Andrievskaya, I.A.; Zhukovets, I.V.; Dovzhikova, I.V.; Ishutina, N.A.; Petrova, K.K. The Effect of HSV-1 Seropositivity on the Course of Pregnancy, Childbirth and the Condition of Newborns. Microorganisms 2022, 10, 176. [Google Scholar] [CrossRef]
- Davis, N.L.; King, C.C.; Kourtis, A.P. Cytomegalovirus Infection in Pregnancy. Birth Defects Res. 2017, 109, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Unger, E.R.; Kemp, T.J.; Pinto, L.A. The Second HPV Serology Meeting: Progress and Challenges in Standardization of Human Papillomavirus Serology Assays. Vaccine 2023, 41, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.A.; Li, Y.; Porras, C.; Pawlita, M.; Ghosh, A.; Rodriguez, A.C.; Schiffman, M.; Wacholder, S.; Kemp, T.J.; Gonzalez, P.; et al. Glutathione S-Transferase L1 Multiplex Serology as a Measure of Cumulative Infection with Human Papillomavirus. BMC Infect. Dis. 2014, 14, 120. [Google Scholar] [CrossRef]
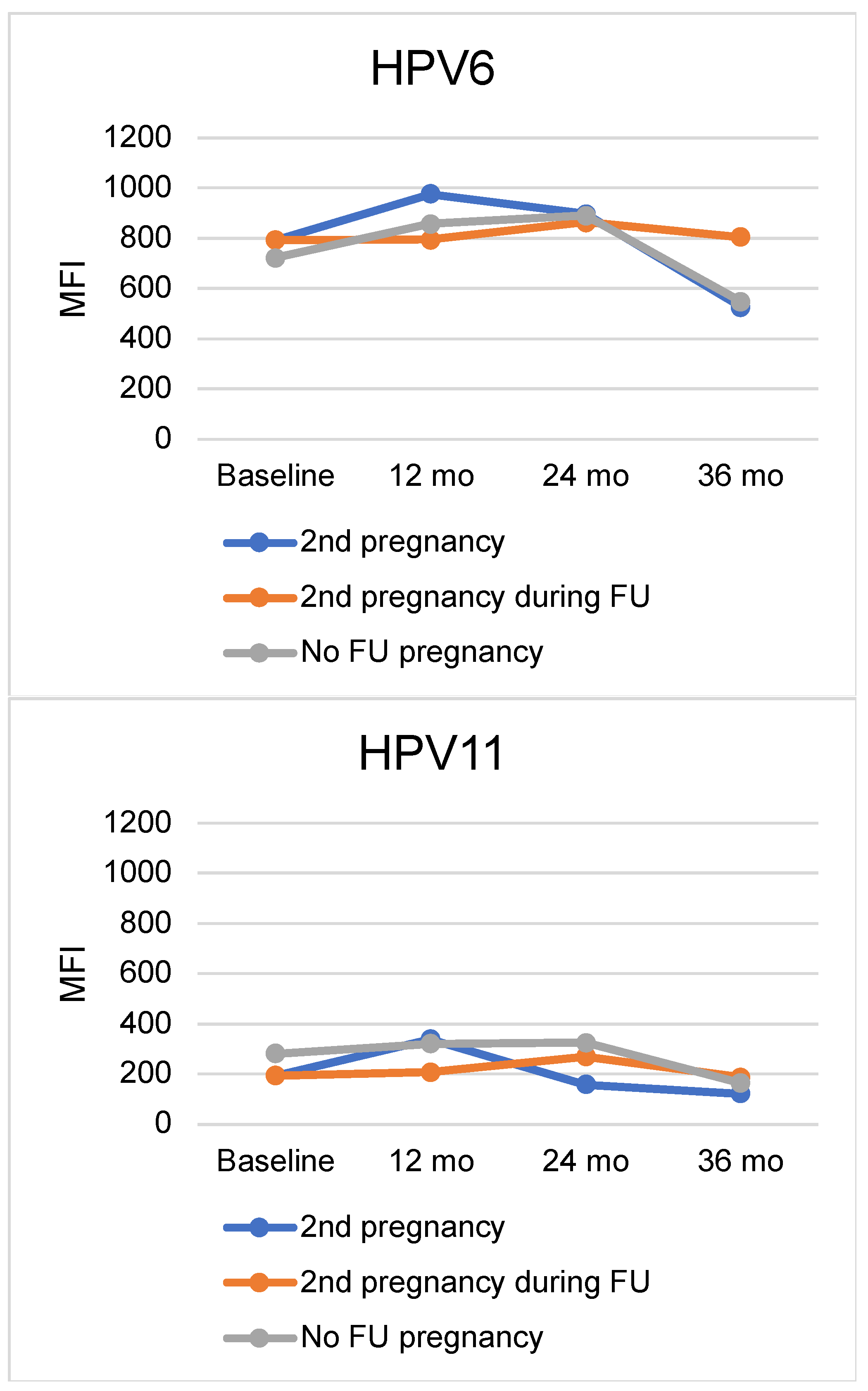
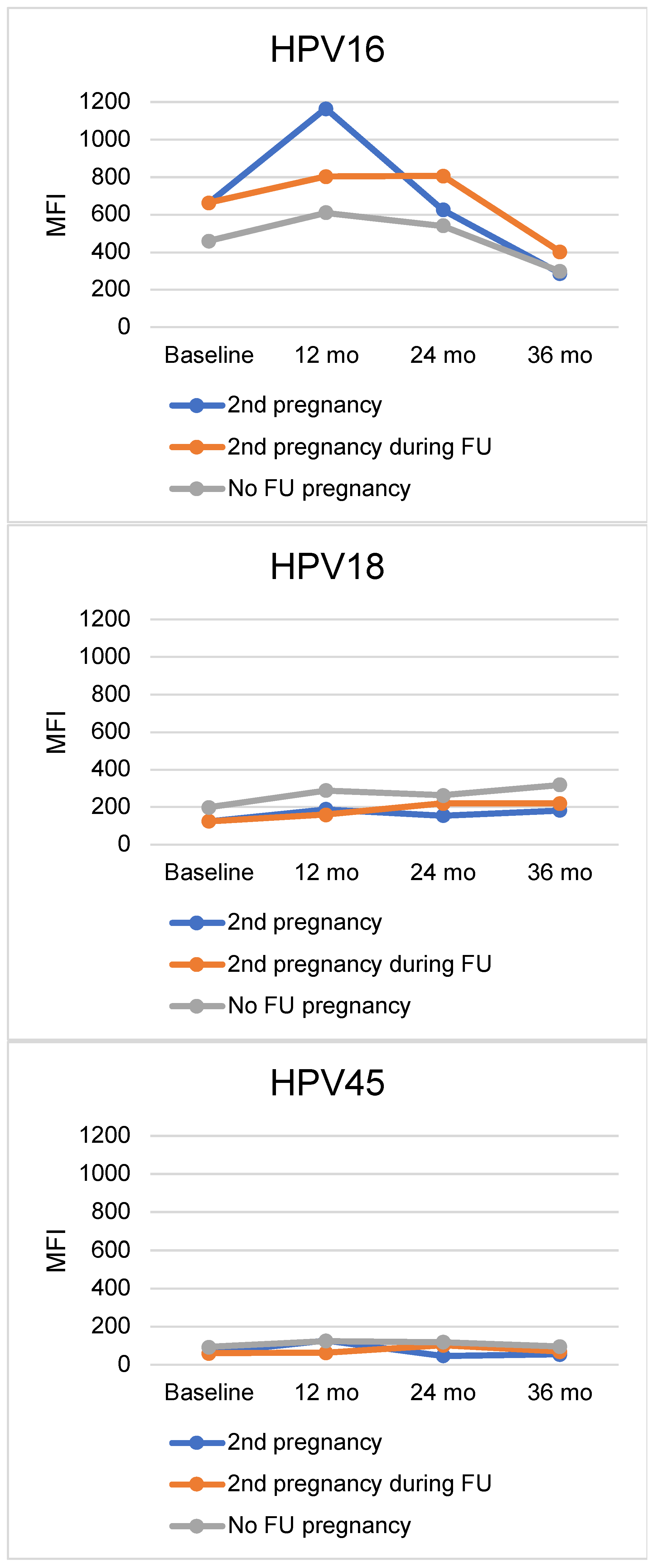
| Baseline | 12 Months | 24 Months | 36 Months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | ||
| n (%) | n (%) | n (%) | n (%) | ||||||
| 2nd Pregnancy at 12 mo (n = 27) | |||||||||
| HPV6 | Seropositive | 17 (63.0) | 162 (54.0) | 18 (66.7) | 178 (69.3) | 19 (73.1) | 159 (67.4) | 14 (51.9) | 126 (53.8) |
| Seronegative | 10 (37.0) | 138 (46.0) | 9 (33.3) | 79 (30.7) | 7 (26.9) | 77 (32.6) | 13 (48.1) | 108 (46.2) | |
| HPV11 | Seropositive | 8 (29.6) | 62 (20.7) | 9 (33.3) | 69 (26.8) | 7 (26.9) | 55 (23.3) | 6 (22.2) | 33 (14.1) |
| Seronegative | 19 (70.4) | 238 (79.3) | 18 (66.7) | 188 (73.2) | 19 (73.1) | 181 (76.7) | 21 (77.8) | 201 (85.9) | |
| HPV16 | Seropositive | 10 (37.0) | 99 (33.0) | 12 (44.4) | 104 (40.5) | 10 (38.5) | 78 (33.1) | 8 (29.6) | 66 (28.2) |
| Seronegative | 17 (63.0) | 201 (67.0) | 15 (55.6) | 153 (59.5) | 16 (61.5) | 158 (66.9) | 19 (70.4) | 168 (71.8) | |
| HPV18 | Seropositive | 5 (18.5) | 61 (20.3) | 5 (18.5) | 70 (27.2) | 7 (26.9) | 52 (22.0) | 8 (29.6) | 53 (22.6) |
| Seronegative | 22 (81.5) | 239 (79.7) | 22 (81.5) | 187 (72.8) | 19 (73.1) | 184 (78.0) | 19 (70.4) | 181 (77.4) | |
| HPV45 | Seropositive | 3 (11.1) | 28 (9.3) | 4 (14.8) | 28 (10.9) | 5 (19.2) | 19 (8.1) | 2 (7.4) | 18 (7.7) |
| Seronegative | 24 (88.9) | 272 (90.7) | 23 (85.2) | 229 (89.1) | 21 (80.8) | 217 (91.9) | 25 (92.6) | 216 (92.3) | |
| 2nd pregnancy at 24 mo (n = 43) | |||||||||
| HPV6 | Seropositive | 15 (34.9) a | 164 (57.7) a | 26 (60.5) | 170 (70.5) | 23 (54.8) | 155 (70.5) | 16 (38.1) b | 124 (56.6) b |
| Seronegative | 28 (65.1) a | 120 (42.3)a | 17 (39.5) | 71 (29.5) | 19 (45.2) | 65 (29.5) | 26 (61.9) b | 95 (43.4) b | |
| HPV11 | Seropositive | 2 (4.7) c | 68 (23.9) c | 6 (14.0) d | 72 (29.9) d | 3 (7.1) e | 59 (26.8) e | 3 (7.1) | 36 (16.4) |
| Seronegative | 41 (95.3) c | 216 (76.1)c | 37 (86.0) d | 169 (70.1) d | 39 (92.9) e | 161 (73.2) e | 39 (92.9) | 183 (83.6) | |
| HPV16 | Seropositive | 10 (23.3) | 99 (34.9) | 14 (32.6) | 102 (42.3) | 10 (23.8) | 78 (35.5) | 9 (21.4) | 65 (29.7) |
| Seronegative | 33 (76.7) | 185 (65.1) | 29 (67.4) | 139 (57.7) | 32 (76.2) | 142 (64.5) | 33 (78.6) | 154 (70.3) | |
| HPV18 | Seropositive | 4 (9.3) | 62 (21.8) | 4 (9.3) f | 71 (29.5) f | 6 (14.3) | 53 (24.1) | 3 (7.1) g | 58 (26.5) g |
| Seronegative | 39 (90.7) | 222 (78.2) | 39 (90.7) f | 170 (70.5) f | 36 (85.7) | 167 (75.9) | 39 (92.9) g | 161 (73.5) g | |
| HPV45 | Seropositive | 1 (2.3) | 30 (10.6) | 1 (2.3) h | 31 (12.9) h | 1 (2.4) | 23 (10.5) | 2 (4.8) | 18 (8.2) |
| Seronegative | 42 (97.7) | 254 (89.4) | 42 (97.7) h | 210 (87.1) h | 41 (97.6) | 197 (89.5) | 40 (95.2) | 201 (91.8) | |
| 2nd pregnancy at 36 mo (n = 19) | |||||||||
| HPV6 | Seropositive | 8 (42.1) | 171 (55.5) | 12 (63.2) | 184 (69.4) | 13 (68.4) | 165 (67.9) | 14 (70.0) | 126 (52.3) |
| Seronegative | 11 (57.9) | 137 (44.5) | 7 (36.8) | 81 (30.6) | 6 (31.6) | 78 (32.1) | 6 (30.0) | 115 (47.7) | |
| HPV11 | Seropositive | 3 (15.8) | 67 (21.8) | 6 (31.6) | 72 (27.2) | 9 (47.4) i | 53 (21.8) i | 3 (15.0) | 36 (14.9) |
| Seronegative | 16 (84.2) | 241 (78.2) | 13 (68.4) | 193 (72.8) | 10 (52.6) i | 190 (78.2) i | 17 (85.0) | 205 (85.1) | |
| HPV16 | Seropositive | 8 (42.1) | 101 (32.8) | 11 (57.9) | 105 (39.6) | 11 (57.9) j | 77 (31.7) j | 8 (40.0) | 66 (27.4) |
| Seronegative | 11 (57.9) | 207 (67.2) | 8 (42.1) | 160 (60.4) | 8 (42.1) j | 166 (68.3) j | 12 (60.0) | 175 (72.6) | |
| HPV18 | Seropositive | 1 (5.3) | 65 (21.1) | 4 (21.1) | 71 (26.8) | 3 (15.8) | 56 (23.0) | 3 (15.0) | 58 (24.1) |
| Seronegative | 18 (94.7) | 243 (78.9) | 15 (78.9) | 194 (73.2) | 16 (84.2) | 187 (77.0) | 17 (85.0) | 183 (75.9) | |
| HPV45 | Seropositive | 1 (5.3) | 30 (9.7) | 3 (15.8) | 29 (10.9) | 3 (15.8) | 21 (8.6) | 2 (10.0) | 18 (7.5) |
| Seronegative | 18 (94.7) | 278 (90.3) | 16 (84.2) | 236 (89.1) | 16 (84.2) | 222 (91.4) | 18 (90.0) | 223 (92.5) | |
| Variable | 2nd Pregnancy | No 2nd Pregnancy | Significance |
|---|---|---|---|
| n (%) | |||
| Marital status | p = 0.045 * | ||
| Single | 2 (2.3) | 18 (8.9) | |
| Other (unmarried couple, married, divorced) | 84 (97.7) | 184 (91.1) | |
| Number of deliveries | p = 0.002 * | ||
| 0 | 1 (1.2) | 1 (0.5) | |
| 1 | 75 (87.2) | 138 (68.7) | |
| 2 | 8 (9.3) | 54 (26.9) | |
| 3 | 2 (2.3) | 5 (2.5) | |
| 4 | 0 (0.0) | 3 (1.5) | |
| Age at first intercourse | p = 0.179 * | ||
| ≤13 | 3 (3.5) | 4 (2.0) | |
| 14–16 | 44 (51.2) | 117 (57.9) | |
| 17–19 | 32 (37.2) | 75 (37.1) | |
| ≥20 | 7 (8.1) | 6 (3.0) | |
| Number of lifetime sexual partners | p = 0.038 | ||
| 1–2 | 28 (32.9) | 43 (21.3) | |
| 3–5 | 27 (31.8) | 64 (31.7) | |
| 6–10 | 20 (23.5) | 45 (22.3) | |
| >10 | 10 (11.8) | 50 (24.8) | |
| Number of sexual partners by the age of 20 | p = 0.262 * | ||
| 0–2 | 44 (51.2) | 80 (39.6) | |
| 3–5 | 24 (27.9) | 74 (36.6) | |
| 6–10 | 14 (16.3) | 32 (15.8) | |
| >10 | 4 (4.7) | 16 (7.9) | |
| Frequency of intercourse, n/month | p = 0.103 * | ||
| 0–1 | 0 (0.0) | 7 (3.5) | |
| 2–4 | 27 (31.4) | 59 (29.2) | |
| 5–10 | 53 (61.6) | 108 (53.5) | |
| >10 | 6 (7.0) | 28 (13.9) | |
| Oral sex | p = 0.217 | ||
| Regular | 7 (8.1) | 28 (13.9) | |
| Occasionally | 64 (74.4) | 130 (64.4) | |
| Never | 15 (17.4) | 44 (21.8) | |
| Anal sex | p = 0.179 * | ||
| Regular | 2 (2.3) | 1 (0.5) | |
| Occasionally | 12 (14.0) | 40 (19.8) | |
| Never | 72 (83.7) | 161 (79.7) | |
| Age at onset of oral contraceptive | p = 0.544 * | ||
| Never used | 7 (8.1) | 17 (8.5) | |
| ≤13 years | 0 (0.0) | 3 (1.5) | |
| 14–16 years | 36 (41.9) | 81 (40.3) | |
| 17–19 years | 31 (36.0) | 83 (41.3) | |
| ≥20 years | 12 (14.0) | 17 (8.5) | |
| Contraception methods used previously | p = 0.032 | ||
| Condom | 33 (36.7) | 65 (27.2) | |
| Oral contraceptive | 7 (7.8) | 6 (2.5) | |
| Intrauterine device | 17 (18.9) | 44 (18.4) | |
| None | 33 (36.7) | 124 (51.9) | |
| Smoking habits | p = 0.661 * | ||
| Not smoker | 47 (54.7) | 96 (47.8) | |
| 1–10 cigarettes per day | 23 (26.7) | 61 (30.3) | |
| 11–20 cigarettes per day | 14 (16.3) | 41 (20.4) | |
| >20 cigarettes per day | 2 (2.3) | 3 (1.5) | |
| Pack years of smoking | p = 0.685 | ||
| Lower tertile (<2.5) | 14 (38.9) | 31 (33.0) | |
| Median tertile (<6.0) | 10 (27.8) | 34 (36.2) | |
| Upper tertile (>6.0) | 12 (33.3) | 29 (30.9) | |
| Alcohol use | p = 0.154 | ||
| Yes | 73 (85.9) | 185 (91.6) | |
| No | 12 (14.1) | 17 (8.4) | |
| History of STDs | p = 0.038 | ||
| STD history | 24 (26.7) | 39 (16.3) | |
| No STDs | 66 (73.3) | 200 (83.7) | |
| History of genital warts | p = 0.521 | ||
| Yes | 22 (25.6%) | 58 (29.3) | |
| No | 64 (74.4) | 140 (70.7) | |
| Age at diagnosis of genital warts | p = 0.903 * | ||
| Never | 64 (74.4) | 142 (71.7) | |
| <20 years | 9 (10.5) | 27 (13.6) | |
| 20–24 years | 10 (11.6) | 21 (10.6) | |
| >25 years | 3 (3.5) | 8 (4.0) | |
| Treatment of genital warts | p = 0.840 * | ||
| No treatment | 12 (40.0) | 29 (37.2) | |
| Topical treatment | 6 (20.0) | 25 (32.1) | |
| Electrocautery | 1 (3.3) | 3 (3.8) | |
| Cryotherapy | 1 (3.3) | 2 (2.6) | |
| Laser therapy | 4 (13.3) | 8 (10.3) | |
| Surgery | 0 (0.0) | 1 (1.3) | |
| Several treatments | 6 (20.0) | 10 (12.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suominen, H.; Suominen, N.; Syrjänen, K.; Waterboer, T.; Grénman, S.; Syrjänen, S.; Louvanto, K. Effect of a Second Pregnancy on the HPV Serology in Mothers Followed Up in the Finnish Family HPV Study. Viruses 2023, 15, 2109. https://doi.org/10.3390/v15102109
Suominen H, Suominen N, Syrjänen K, Waterboer T, Grénman S, Syrjänen S, Louvanto K. Effect of a Second Pregnancy on the HPV Serology in Mothers Followed Up in the Finnish Family HPV Study. Viruses. 2023; 15(10):2109. https://doi.org/10.3390/v15102109
Chicago/Turabian StyleSuominen, Helmi, Nelli Suominen, Kari Syrjänen, Tim Waterboer, Seija Grénman, Stina Syrjänen, and Karolina Louvanto. 2023. "Effect of a Second Pregnancy on the HPV Serology in Mothers Followed Up in the Finnish Family HPV Study" Viruses 15, no. 10: 2109. https://doi.org/10.3390/v15102109
APA StyleSuominen, H., Suominen, N., Syrjänen, K., Waterboer, T., Grénman, S., Syrjänen, S., & Louvanto, K. (2023). Effect of a Second Pregnancy on the HPV Serology in Mothers Followed Up in the Finnish Family HPV Study. Viruses, 15(10), 2109. https://doi.org/10.3390/v15102109






