In Ovo Vaccination with Recombinant Herpes Virus of the Turkey-Laryngotracheitis Vaccine Adjuvanted with CpG-Oligonucleotide Provides Protection against a Viral Challenge in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
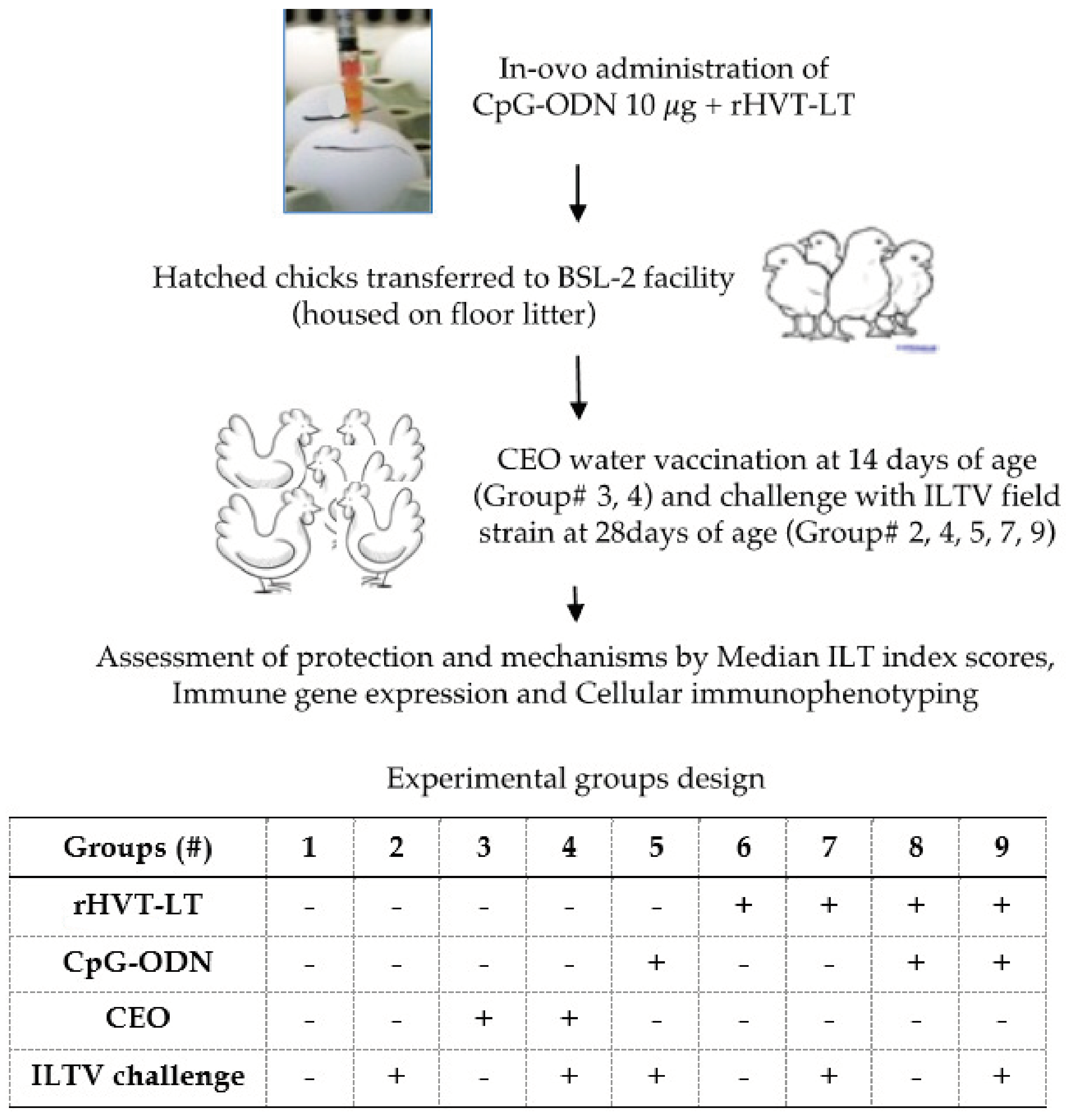
2.1. Animals and Experimental Design
2.2. Vaccines, Adjuvant, and Virus
2.3. Immune Gene Expression
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
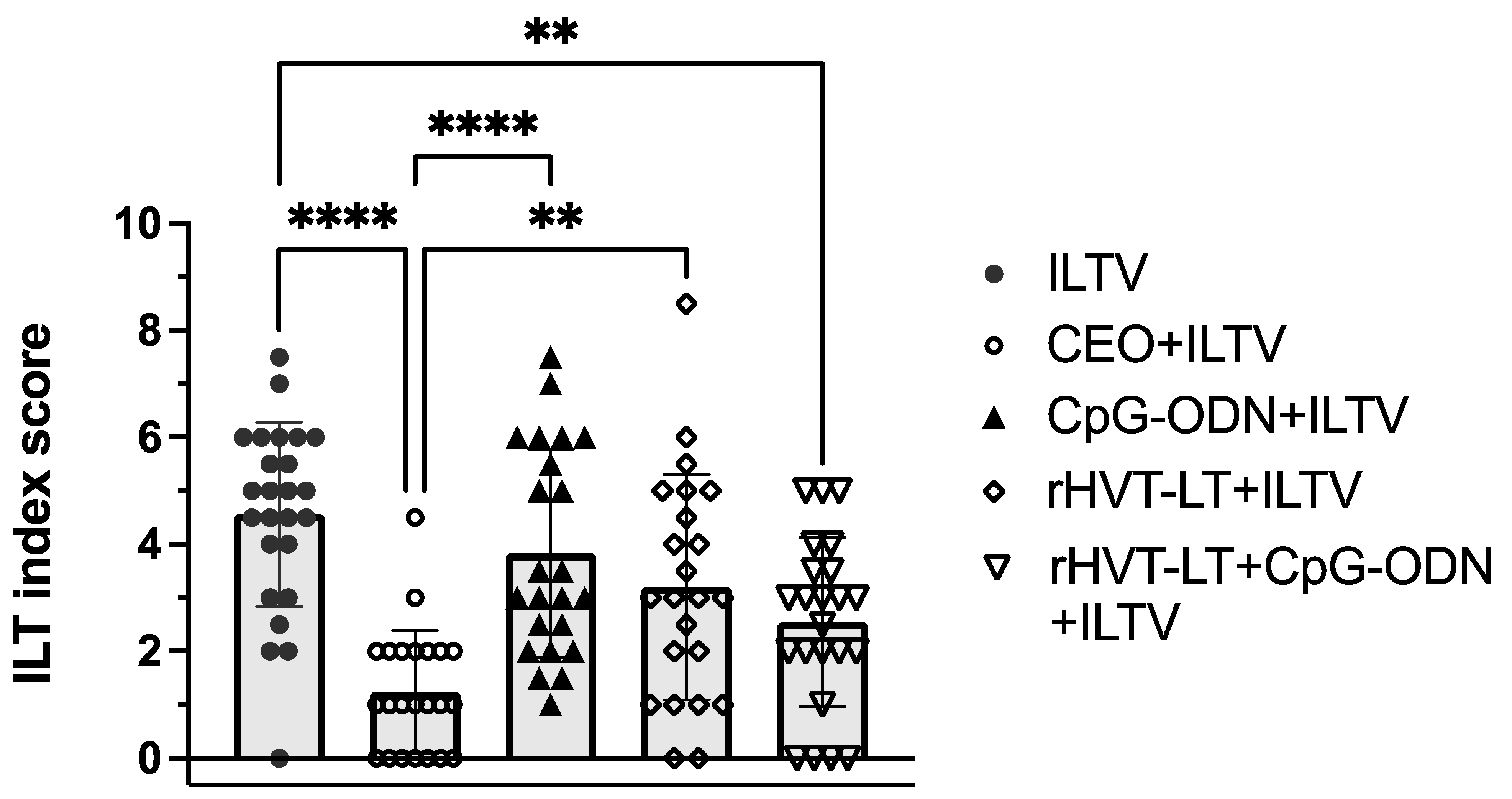
3.1. Protection against ILTV Challenge
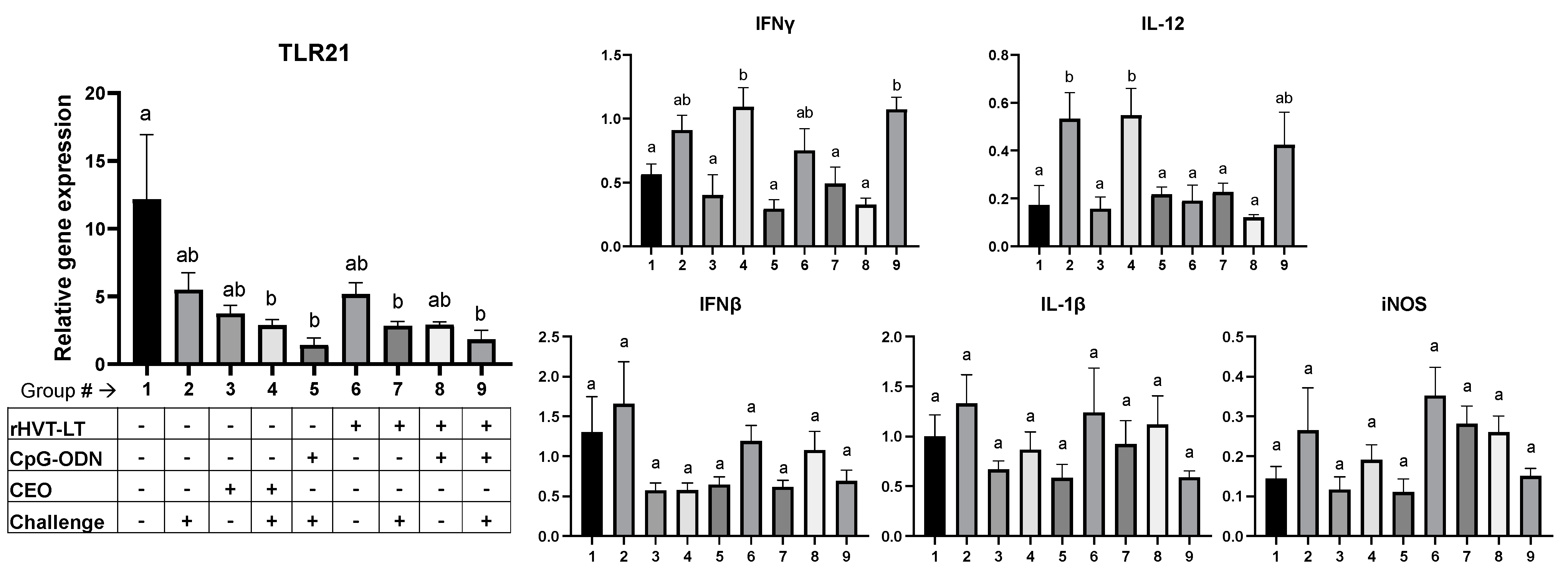
3.2. Immune Gene Expression
3.3. Immunophenotyping
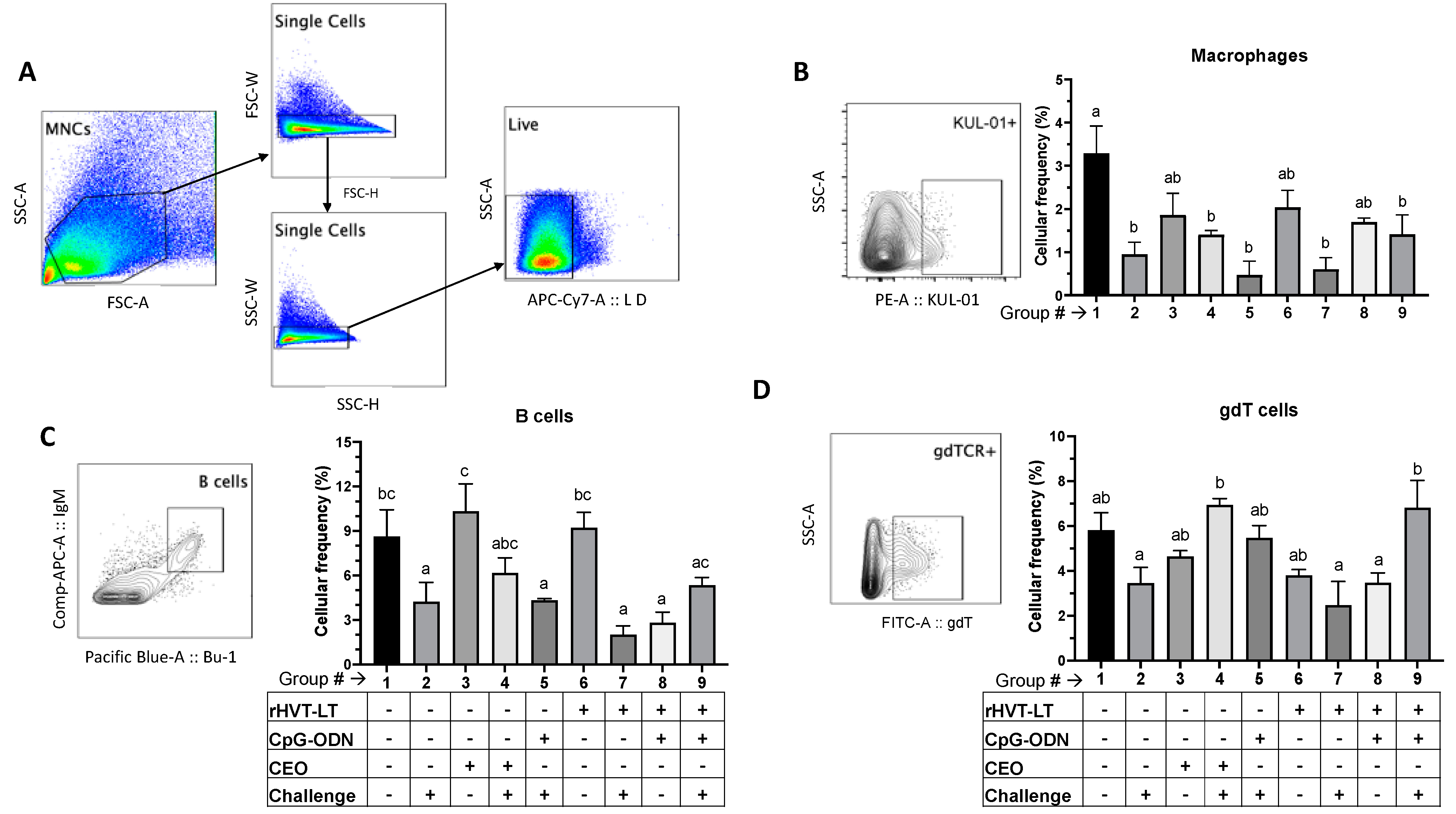
3.3.1. Macrophage, B Cell, and γδ T Cell Responses
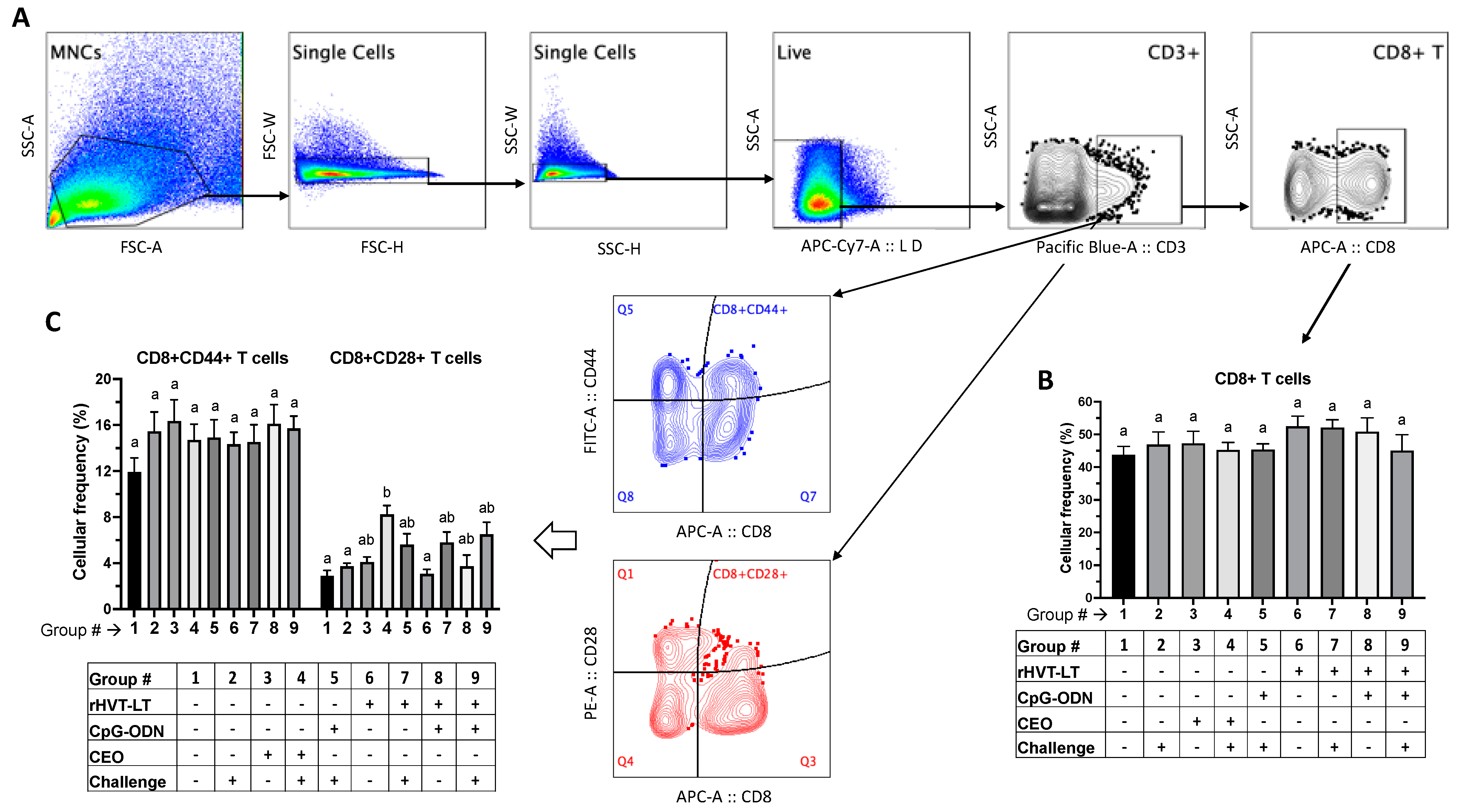
3.3.2. T Cell Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dufour-Zavala, L. Epizootiology of Infectious Laryngotracheitis and Presentation of an Industry Control Program. Avian Dis. 2008, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- García, M. Current and Future Vaccines and Vaccination Strategies against Infectious Laryngotracheitis (ILT) Respiratory Disease of Poultry. Vet. Microbiol. 2017, 206, 157–162. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Zavala, G. Commercial Vaccines and Vaccination Strategies Against Infectious Laryngotracheitis: What We Have Learned and Knowledge Gaps That Remain. Avian Dis. 2019, 63, 325–334. [Google Scholar] [CrossRef]
- Guy, J.S.; Barnes, H.J.; Smith, L. Increased Virulence of Modified-Live Infectious Laryngotracheitis Vaccine Virus Following Bird-to-Bird Passage. Avian Dis. 1991, 35, 348–355. [Google Scholar] [CrossRef]
- Guy, J.S.; Barnes, H.J.; Smith, L.G. Rapid Diagnosis of Infectious Laryngotracheitis Using a Monoclonal Antibody-Based Immunoperoxidase Procedure. Avian Pathol. 1992, 21, 77–86. [Google Scholar] [CrossRef]
- Hilbink, F.W.; Oei, H.L.; van Roozelaar, D.J. Virulence of Five Live Vaccines against Avian Infectious Laryngotracheitis and Their Immunogenicity and Spread after Eyedrop or Spray Application. Vet. Q. 1987, 9, 215–225. [Google Scholar] [CrossRef]
- Rodríguez-Avila, A.; Oldoni, I.; Riblet, S.; García, M. Replication and Transmission of Live Attenuated Infectious Laryngotracheitis Virus (ILTV) Vaccines. Avian Dis. 2007, 51, 905–911. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Cortes, A.L.; Guy, J.S.; Turpin, E.; Williams, C. Replication of Recombinant Herpesvirus of Turkey Expressing Genes of Infectious Laryngotracheitis Virus in Specific Pathogen Free and Broiler Chickens Following in ovo and Subcutaneous Vaccination. Avian Pathol. 2011, 40, 395–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gimeno, I.M.; Cortes, A.L.; Faiz, N.M.; Hernandez-Ortiz, B.A.; Guy, J.S.; Hunt, H.D.; Silva, R.F. Evaluation of the Protection Efficacy of a Serotype 1 Marek’s Disease Virus-Vectored Bivalent Vaccine Against Infectious Laryngotracheitis and Marek’s Disease. Avian Dis. 2015, 59, 255–262. [Google Scholar] [CrossRef]
- Maekawa, D.; Riblet, S.M.; Newman, L.; Koopman, R.; Barbosa, T.; García, M. Evaluation of Vaccination against Infectious Laryngotracheitis (ILT) with Recombinant Herpesvirus of Turkey (RHVT-LT) and Chicken Embryo Origin (CEO) Vaccines Applied Alone or in Combination. Avian Pathol. 2019, 48, 573–581. [Google Scholar] [CrossRef]
- Vagnozzi, A.; Zavala, G.; Riblet, S.M.; Mundt, A.; García, M. Protection Induced by Commercially Available Live-Attenuated and Recombinant Viral Vector Vaccines against Infectious Laryngotracheitis Virus in Broiler Chickens. Avian Pathol. 2012, 41, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR Signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, R.; Zhu, J.; Allan, B.; Mutwiri, G.K.; Babiuk, L.A.; Potter, A.; Griebel, P. Chicken TLR21 Acts as a Functional Homologue to Mammalian TLR9 in the Recognition of CpG Oligodeoxynucleotides. Mol. Immunol. 2009, 46, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a Vaccine Adjuvant. Expert. Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef]
- Dalpke, A.H.; Heeg, K. CpG-DNA as Immune Response Modifier. Int. J. Med. Microbiol. 2004, 294, 345–354. [Google Scholar] [CrossRef]
- Gupta, S.; Deb, R.; Dey, S.; Chellappa, M.M. Toll-like receptor-based adjuvants: Enhancing the immune response to vaccines against infectious diseases of chicken. Expert. Rev. Vaccines 2014, 13, 909–925. [Google Scholar] [CrossRef]
- Abdul-Cader, M.S.; Ahmed-Hassan, H.; Amarasinghe, A.; Nagy, E.; Sharif, S.; Abdul-Careem, M.F. Toll-like Receptor (TLR)21 Signaling-Mediated Antiviral Response against Avian Influenza Virus Infection Correlates with Macrophage Recruitment and Nitric Oxide Production. J. Gen. Virol. 2017, 98, 1209–1223. [Google Scholar] [CrossRef]
- Abdul-Cader, M.S.; De Silva Senapathi, U.; Nagy, E.; Sharif, S.; Abdul-Careem, M.F. Antiviral Response Elicited against Avian Influenza Virus Infection Following Activation of Toll-like Receptor (TLR)7 Signaling Pathway Is Attributable to Interleukin (IL)-1β Production. BMC Res. Notes 2018, 11, 859. [Google Scholar] [CrossRef]
- Gunawardana, T.; Ahmed, K.A.; Goonewardene, K.; Popowich, S.; Kurukulasuriya, S.; Karunarathna, R.; Gupta, A.; Lockerbie, B.; Foldvari, M.; Tikoo, S.K.; et al. Synthetic CpG-ODN Rapidly Enriches Immune Compartments in Neonatal Chicks to Induce Protective Immunity against Bacterial Infections. Sci. Rep. 2019, 9, 341. [Google Scholar] [CrossRef]
- Bavananthasivam, J.; Read, L.; Astill, J.; Yitbarek, A.; Alkie, T.N.; Abdul-Careem, M.F.; Wootton, S.K.; Behboudi, S.; Sharif, S. The Effects of in ovo Administration of Encapsulated Toll-like Receptor 21 Ligand as an Adjuvant with Marek’s Disease Vaccine. Sci. Rep. 2018, 8, 16370. [Google Scholar] [CrossRef]
- Gomis, S.; Babiuk, L.; Allan, B.; Willson, P.; Waters, E.; Ambrose, N.; Hecker, R.; Potter, A. Protection of Neonatal Chicks against a Lethal Challenge of Escherichia Coli Using DNA Containing Cytosine-Phosphodiester-Guanine Motifs. Avian Dis. 2004, 48, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Gaghan, C.; Browning, M.; Cortes, A.L.; Gimeno, I.M.; Kulkarni, R.R. Effect of CpG-Oligonucleotide in Enhancing Recombinant Herpes Virus of Turkey-Laryngotracheitis Vaccine-Induced Immune Responses in One-Day-Old Broiler Chickens. Vaccines 2023, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- López De, B.; Abad, J.; Cortes, A.L.; Correa, M.; Gimeno, I.M. Evaluation of Factors That Influence Dose Variability of Marek’s Disease Vaccines. Avian Dis. 2019, 63, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.G.; McElhaney, J.E.; Verschoor, C.P. Reliable Reference Genes for the Quantification of MRNA in Human T-Cells and PBMCs Stimulated with Live Influenza Virus. BMC Immunol. 2020, 2, 4. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acid. Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Bavananthasivam, J.; Kulkarni, R.R.; Read, L.; Sharif, S. Reduction of Marek’s Disease Virus Infection by Toll-Like Receptor Ligands in Chicken Embryo Fibroblast Cells. Viral Immunol. 2018, 31, 389–396. [Google Scholar] [CrossRef]
- Senapathi, U.D.S.; Abdul-Cader, M.S.; Amarasinghe, A.; van Marle, G.; Czub, M.; Gomis, S.; Abdul-Careem, M.F. The in ovo Delivery of CpG Oligonucleotides Protects against Infectious Bronchitis with the Recruitment of Immune Cells into the Respiratory Tract of Chickens. Viruses 2018, 10, 635. [Google Scholar] [CrossRef]
- Johnson, D.I.; Vagnozzi, A.; Dorea, F.; Riblet, S.M.; Mundt, A.; Zavala, G.; García, M. Protection against Infectious Laryngotracheitis by in ovo Vaccination with Commercially Available Viral Vector Recombinant Vaccines. Avian Dis. 2010, 54, 1251–1259. [Google Scholar] [CrossRef]
- Korsa, M.G.; Browning, G.F.; Coppo, M.J.C.; Legione, A.R.; Gilkerson, J.R.; Noormohammadi, A.H.; Vaz, P.K.; Lee, S.W.; Devlin, J.M.; Hartley, C.A. Protection Induced in Broiler Chickens Following Drinking-Water Delivery of Live Infectious Laryngotracheitis Vaccines against Subsequent Challenge with Recombinant Field Virus. PLoS ONE 2015, 10, e0137719. [Google Scholar] [CrossRef]
- Palomino-Tapia, V.A.; Zavala, G.; Cheng, S.; García, M. Long-Term Protection against a Virulent Field Isolate of Infectious Laryngotracheitis Virus Induced by Inactivated, Recombinant, and Modified Live Virus Vaccines in Commercial Layers. Avian Pathol. 2019, 48, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.P.; van der Meer, F.; Checkley, S.; Jospeh, T.; King, R.; Ravi, M.; Peters, D.; Fonseca, K.; Gagnon, C.A.; Provost, C.; et al. Analysis of Whole-Genome Sequences of Infectious laryngotracheitis Virus Isolates from Poultry Flocks in Canada: Evidence of Recombination. Viruses 2020, 12, 1302. [Google Scholar] [CrossRef] [PubMed]
- Coppo, M.J.C.; Hartley, C.A.; Devlin, J.M. Immune Responses to Infectious Laryngotracheitis Virus. Dev. Comp. Immunol. 2013, 41, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, G.; Hurley, D.J.; Gogal, R.M.; Sharif, S.; Read, L.R.; Williams, S.M.; Jerry, C.F.; Maekawa, D.A.; García, M. Immune Responses in the Eye-Associated Lymphoid Tissues of Chickens after Ocular Inoculation with Vaccine and Virulent Strains of the Respiratory Infectious Laryngotracheitis Virus (ILTV). Viruses 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, D.; Whang, P.; Riblet, S.M.; Hurley, D.J.; Guy, J.S.; García, M. Assessing the Infiltration of Immune Cells in the Upper Trachea Mucosa after Infectious Laryngotracheitis Virus (ILTV) Vaccination and Challenge. Avian Pathol. 2021, 50, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, D.; Riblet, S.M.; Whang, P.; Hurley, D.J.; Garcia, M. Activation of Cytotoxic Lymphocytes and Presence of Regulatory T Cells in the Trachea of Non-Vaccinated and Vaccinated Chickens as a Recall to an Infectious Laryngotracheitis Virus (ILTV) Challenge. Vaccines 2021, 9, 865. [Google Scholar] [CrossRef]
- Koskela, K.; Arstila, T.P.; Lassila, O. Costimulatory Function of CD28 in Avian Γδ T Cells Is Evolutionarily Conserved. Scand. J. Immunol. 1998, 48, 635–641. [Google Scholar] [CrossRef]
- Baaten, B.J.G.; Li, C.R.; Deiro, M.F.; Lin, M.M.; Linton, P.J.; Bradley, L.M. CD44 Regulates Survival and Memory Development in Th1 Cells. Immunity 2010, 32, 104–115. [Google Scholar] [CrossRef]
- Dai, M.; Xu, C.; Chen, W.; Liao, M. Progress on Chicken T Cell Immunity to Viruses. Cell. Mol. Life Sci. 2019, 76, 2779–2788. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Varghese, B.; Brody, J.D.; Rajapaksa, R.; Kohrt, H.; Czerwinski, D.K.; Levy, S.; Levy, R. A CpG-Loaded Tumor Cell Vaccine Induces Antitumor CD4+ T Cells That Are Effective in Adoptive Therapy for Large and Established Tumors. Blood 2011, 117, 118–127. [Google Scholar] [CrossRef][Green Version]
- Vagnozzi, A.E.; Beltrán, G.; Zavala, G.; Read, L.; Sharif, S.; Garcia, M. Cytokine Gene Transcription in the Trachea, Harderian Gland, and Trigeminal Ganglia of Chickens Inoculated with Virulent Infectious Laryngotracheitis Virus (ILTV) Strain. Avian Pathol. 2018, 47, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, C.; Chu, T.H.; Sheridan, B.S. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front. Immunol. 2018, 9, 2636. [Google Scholar] [CrossRef] [PubMed]
- Crippen, T.L.; Sheffield, C.L.; He, H.; Lowry, V.K.; Kogut, M.H. Differential Nitric Oxide Production by Chicken Immune Cells. Dev. Comp. Immunol. 2003, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhang, F.; Yang, Y.; Shang, S. The Evaluation of Cellular Immunity to Avian Viral Diseases: Methods, Applications, and Challenges. Front. Microbiol. 2021, 12, 794514. [Google Scholar] [CrossRef] [PubMed]
- Elshafiee, E.A.; Isham, I.M.; Najimudeen, S.M.; Perez-Contreras, A.; Barboza-Solis, C.; Ravi, M.; Abdul-Careem, M.F. Host Responses Following Infection with Canadian-Origin Wildtype and Vaccine Revertant Infectious Laryngotracheitis Virus. Vaccines 2022, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Pacini, L.; Savini, C.; Ghittoni, R.; Saidj, D.; Lamartine, J.; Hasan, U.A.; Accardi, R.; Tommasino, M. Downregulation of Toll-Like Receptor 9 Expression by Beta Human Papillomavirus 38 and Implications for Cell Cycle Control. J. Virol. 2015, 89, 11396–11405. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Guo, Y.; Li, Y.; Zhang, Y.; Teng, N.; Zhang, F.; Yang, G. Molecular Characterization and Expression Patterns of a Non-Mammalian Toll-like Receptor Gene (TLR21) in Larvae Ontogeny of Common Carp (Cyprinus carpio L.) and upon Immune Stimulation. BMC Vet. Res. 2018, 14, 153. [Google Scholar] [CrossRef]

| Target Gene | Primer Sequence | Annealing Temp (°C) | Accession Number |
|---|---|---|---|
| HVT-TM-2 | F: 5′-CGGGCCTTACGTTTCACCT-3′ R: 5′-GCGCCGAAAAGCTAGAAAAG-3′ | 60 | NC_002641.1 |
| CKN | F: 5′-GCTACAGCGAGCTCATTTTTTTAGT-3′ R: 5′-TTTACAATGGGTTTAGGTGTCTGAGA-3′ | 55 | CP100558.1 |
| β-actin | F: 5′-CAACACAGTGCTGTCTGGTGGTA-3′ R: 5′-ATCGTACTCCTGCTTGCTGATCC-3′ | 58 | X00182 |
| IFNγ | F:5′-ACACTGACAAGTCAAAGCCGCACA-3′ R:5′-AGTCGTTCATCGGGAGCTTGGC-3′ | 60 | X99774 |
| IFNß | F:5′- CGTGTGCGAGAACAGCATGGAGA-3′ R:5′-TCAGGCATTTCTCCTCGTCGAAGC-3′ | 60 | NM_204628.1 |
| IL-1ß | F:5′-AGCAGATCAAGGAGACGTTC-3′ R:5′-ATCAGCAGGTACTCCTCGAT-3′ | 55 | AJ621614 |
| IL-12 | F:5′-CCAAGACCTGGAGCACACCGAAG-3′ R:5′-CGATCCCTGGCCTGCACAGAGA-3′ | 64 | AY262752.1 |
| TLR21 | F:5′-CCTGCGCAAGTGTCCGCTCA-3′ R:5′-GCCCCAGGTCCAGGAAGCAG-3′ | 60 | NM_001030558.1 |
| iNOS | F: 5′-CCTGGTGATGCTGTGAATTG-3′ R: 5′-CTTCTGTGTCGTTGCATTCAG-3′ | 58 | NM_204665 |
| Marker | Format | |
|---|---|---|
| Panel 1 | KUL-01 | PE |
| IgM | APC | |
| Bu-1 | Pacific Blue | |
| γδTCR | FITC | |
| Live/Dead viable dye | APC-Cy7 | |
| Panel 2 | CD3 | Pacific Blue |
| CD4 | APC | |
| CD28 | PE | |
| CD | FITC | |
| Live/Dead viable dye | APC-Cy7 | |
| Panel 3 | CD3 | Pacific Blue |
| CD8 | APC | |
| CD28 | PE | |
| CD44 | FITC | |
| Live/Dead viable dye | APC-Cy7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaghan, C.; Browning, M.; Fares, A.M.; Abdul-Careem, M.F.; Gimeno, I.M.; Kulkarni, R.R. In Ovo Vaccination with Recombinant Herpes Virus of the Turkey-Laryngotracheitis Vaccine Adjuvanted with CpG-Oligonucleotide Provides Protection against a Viral Challenge in Broiler Chickens. Viruses 2023, 15, 2103. https://doi.org/10.3390/v15102103
Gaghan C, Browning M, Fares AM, Abdul-Careem MF, Gimeno IM, Kulkarni RR. In Ovo Vaccination with Recombinant Herpes Virus of the Turkey-Laryngotracheitis Vaccine Adjuvanted with CpG-Oligonucleotide Provides Protection against a Viral Challenge in Broiler Chickens. Viruses. 2023; 15(10):2103. https://doi.org/10.3390/v15102103
Chicago/Turabian StyleGaghan, Carissa, Matthew Browning, Abdelhamid M. Fares, Mohamed Faizal Abdul-Careem, Isabel M. Gimeno, and Raveendra R. Kulkarni. 2023. "In Ovo Vaccination with Recombinant Herpes Virus of the Turkey-Laryngotracheitis Vaccine Adjuvanted with CpG-Oligonucleotide Provides Protection against a Viral Challenge in Broiler Chickens" Viruses 15, no. 10: 2103. https://doi.org/10.3390/v15102103
APA StyleGaghan, C., Browning, M., Fares, A. M., Abdul-Careem, M. F., Gimeno, I. M., & Kulkarni, R. R. (2023). In Ovo Vaccination with Recombinant Herpes Virus of the Turkey-Laryngotracheitis Vaccine Adjuvanted with CpG-Oligonucleotide Provides Protection against a Viral Challenge in Broiler Chickens. Viruses, 15(10), 2103. https://doi.org/10.3390/v15102103








