Trends and Challenges in the Surveillance and Control of Avian Metapneumovirus
Abstract
:1. Introduction
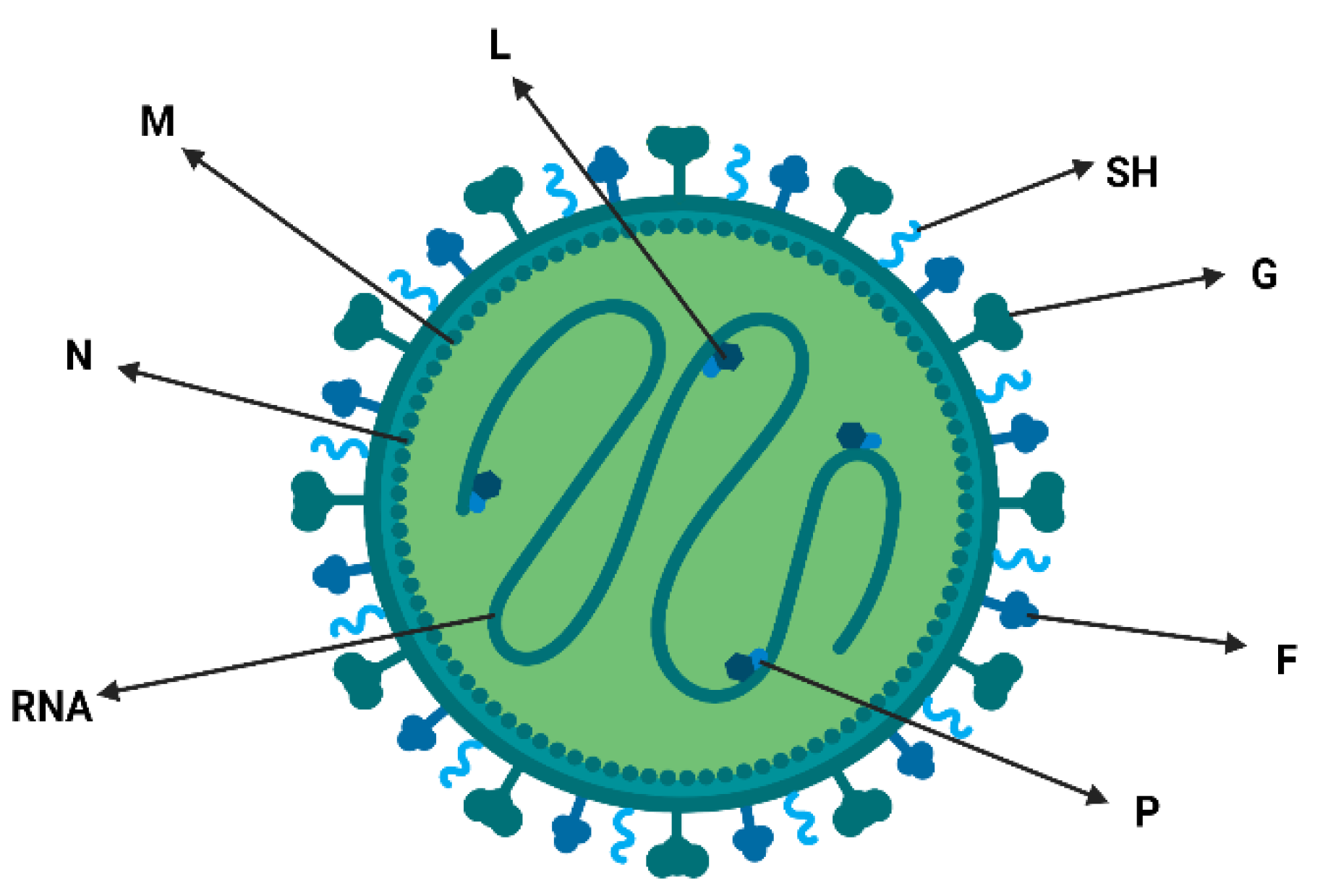
2. Characteristics of Viral Particles, Classification and Nomenclature
3. Discovery and Distribution of Avian Metapneumovirus (aMPV)
4. Replication, Viral Persistence and Clinical Signs
5. Transmission and Economic Losses
6. Co-Infection of aMPV and Bacteria
7. Vaccination against aMPV and Viral Selective Pressure
8. Methodological Trends for the Discovery of New Viral Strains in Poultry
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goossens, E.; Dehau, T.; Ducatelle, R.; Van Immerseel, F. Omics technologies in poultry health and productivity—Part 2: Future applications in the poultry industry. Avian Pathol. 2022, 51, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Dehau, T.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. Omics technologies in poultry health and productivity—Part 1: Current use in poultry research. Avian Pathol. 2022, 51, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, G.; Mescolini, G.; Silveira, F.; Lupini, C.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M.; Legnardi, M.; Gobbo, F.; Terregino, C.; et al. First detection of avian metapneumovirus subtype C Eurasian lineage in a Eurasian wigeon (Mareca penelope) wintering in Northeastern Italy: An additional hint on the role of migrating birds in the viral epidemiology. Avian Pathol. 2022, 51, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kaboudi, K.; Lachheb, J. Avian metapneumovirus infection in turkeys: A review on turkey rhinotracheitis. J. Appl. Poult. Res. 2021, 30, 100211. [Google Scholar] [CrossRef]
- Zaffuto, K.M.; Estevez, C.N.; Afonso, C.L. Primary chicken tracheal cell culture system for the study of infection with avian respiratory viruses. Avian Pathol. 2008, 37, 25–31. [Google Scholar]
- Croville, G.; Foret, C.; Heuillard, P.; Senet, A.; Delpont, M.; Mouahid, M.; Ducatez, M.F.; Kichou, F.; Guerin, J.-L. Disclosing respiratory co-infections: A broad-range panel assay for avian respiratory pathogens on a nanofluidic PCR platform. Avian Pathol. 2018, 47, 253–260. [Google Scholar] [CrossRef]
- Cook, J.K. Avian rhinotracheitis. Rev. Sci. Tech. OIE 2000, 19, 602–613. [Google Scholar]
- Cook, J.K.; Cavanagh, D. Detection and differentiation of avian pneumoviruses. Avian Pathol. 2002, 31, 117–132. [Google Scholar]
- Sugiyama, M.; Koimaru, H.; Shiba, M.; Ono, E.; Nagata, T.; Ito, T. Drop of egg production in chickens by experimental infection with an avian metapneumovirus strain PLE8T1 derived from swollen head syndrome and the application to evaluate vaccine. J. Vet. Med. Sci. 2006, 68, 783–787. [Google Scholar]
- Alexander, D.J.; Jones, R.C. Paramyxoviridae. In Poultry Diseases; Pattison, M., McMullin, P., Bradbury, J.M., Alexander, D., Eds.; Saunders Elsevier Publ.: London, UK, 2008; pp. 294–316. [Google Scholar]
- Cecchinato, M.; Catelli, E.; Lupini, C.; Ricchizzi, E.; Clubbe, J.; Battilai, M.; Naylor, C. Avian metapneumovirus (AMPV) attachment protein involvement in probable virus evolution concurrent with mass live vaccine introduction. Veter.-Microbiol. 2010, 146, 24–34. [Google Scholar] [CrossRef]
- Giovanardi, D.; Lupini, C.; Pesente, P.; Rossi, G.; Ortali, G.; Catelli, E. Longitudinal field studies of Avian Metapneumovirus and Turkey Hemorrhagic Enteritis Virus in turkeys suffering from colibacillosis associated mortality. Vet. Res. Commun. 2014, 38, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.L.; Amarasinghe, G.K.; Bányai, K.; Bào, Y.; Basler, C.F.; Bavari, S.; Bejerman, N.; Blasdell, K.R.; Briand, F.-X.; Briese, T.; et al. Taxonomy of the order Mononegavirales: Update 2016. Arch. Virol. 2016, 161, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M. Comparative investigation on different turkey rhinotracheitis (TRT) virus isolates from different countries. Dtsch. Tierarztl. Wochenschr. 1992, 99, 486–488. [Google Scholar] [PubMed]
- Jing, L.; Cook, J.K.A.; David, T.; Brown, K.; Shaw, K.; Cavanagh, D. Detection of turkey rhinotracheitis virus in turkeys using the polymerase chain reaction. Avian Pathol. 1993, 22, 771–783. [Google Scholar] [CrossRef]
- Cook, J.K.A.; Kinloch, S.; Ellis, M.M. In vitro and in vivo studies in chickens and turkeys on strains of turkey rhinotracheitis virus isolated from the two species. Avian Pathol. 1993, 22, 157–170. [Google Scholar] [CrossRef]
- Collins, M.S.; Gough, R.E.; Alexander, D.J. Antigenic differentiation of Avian pneumovirus isolates using polyclonal antisera and mouse monoclonal antibodies. Avian Pathol. 1993, 22, 469–479. [Google Scholar]
- Juhasz, K.; Easton, A.J. Extensive sequence variation in the attachment (G) protein gene of avian pneumovirus: Evidence for two distinct subgroups. J. Gen. Virol. 1994, 75, 2873–2880. [Google Scholar] [CrossRef]
- Milwright, R.D.P.; Toquin, D.; Bayon-Auboyer, M.H.; Jestin, V.; Morin, H. Isolation of a pneumovirus from a Muscovy duck. Vet. Rec. 1999, 145, 680. [Google Scholar]
- Seal, B.S.; Sellers, H.S.; Meinersmann, R.J. Fusion protein predicted amino acid sequence of the first US Avian pneumovirus isolate and lack of heterogeneity among other US isolates. Virus Res. 2000, 66, 139–147. [Google Scholar]
- Easton, A.J.; Domachowske, J.B.; Rosenberg, H.F. Animal Pneumoviruses: Molecular genetics and pathogenesis. Clin. Microbiol. Rev. 2004, 17, 390–412. [Google Scholar] [CrossRef]
- Franzo, G.; Legnardi, M.; Mescolini, G.; Tucciarone, C.M.; Lupini, C.; Quaglia, G.; Catelli, E.; Cecchinato, M. Avian Metapneumovirus subtype B around Europe: A phylodynamic reconstruction. Vet. Res. 2020, 51, 88. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, E.; Bougeard, S.; Allée, C.; Eterradossi, N.; Courtillon, C.; Brown, P.A. Establishment and application of a quadruple real-time RT-PCR for detecting avian metapneumovirus. PLoS ONE 2022, 17, e0270708. [Google Scholar] [CrossRef]
- Lemaitre, E.; Bougeard, S.; Allée, C.; Eterradossi, N.; Courtillon, C.; Brown, P.A. Avian metapneumovirus: A five-plex digital droplet RT-PCR method for identification of subgroups A, B, C, and D. Front. Veter. Sci. 2022, 9, 1058294. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Suderman, M.; Koziuk, J.; Ojkic, D.; Berhane, Y. Development of A recombinant nucleocapsid based indirect ELISA for the detection of antibodies to avian metapneumovirus subtypes, A, B, and C. Veter.-Immunol. Immunopathol. 2020, 231, 110151. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, M.; Lupini, C.; Silveira, F.; Listorti, V.; Mescolini, G.; Morandini, E.; Franzo, G.; Catelli, E. Molecular characterization of avian metapneumovirus from Guinea fowls (Numida meleagridis). Pak. Vet. J. 2018, 38, 419–423. [Google Scholar] [CrossRef]
- Catelli, E.; De Marco, M.A.; Delogu, M.; Terregino, C.; Guberti, V. Serological evidence of Avian pneumovirus infection in reared and free-living pheasants. Vet. Rec. 2001, 149, 56–58. [Google Scholar] [CrossRef]
- Buys, S.B. A preliminary report on the isolation of a virus causing sinusitis in turkeys in South Africa and attempts to attenuate the virus. Turkeys 1980, 28, 36. [Google Scholar]
- Morley, A.J.; Thomson, D.K. Swollen-head syndrome in broiler chickens. Avian Dis. 1984, 28, 238–243. [Google Scholar] [CrossRef]
- Anon, K.R. Turkey rhinotracheitis of unknown aetiology in England and Wales. Vet. Rec. 1985, 117, 653–654. [Google Scholar]
- Giraud, P.; Bennejean, G.; Guittet, M.; Toquin, D. Turkey rhinotracheitis in France: Preliminary investigations on a ciliostatic virus. Vet. Rec. 1986, 119, 606–607. [Google Scholar]
- Hafez, H.M.; Woernle, H. Turkey rhinotracheitis, serological results in Baden-Wurttemnberg. Tierarztl. Umsch. 1989, 44, 476–480. [Google Scholar]
- Lantos, C. Actual problems of poultry hygenes. Bfiteny. Es Feldogozas 1990, 37, 54–58. [Google Scholar]
- Fabris, G.; D’aprile, P.N. Rinotracheite infettiva del tacchino: Osservazioni sul campo ed indagini di laboratorio. Zootec. Int. 1990, 6, 36–40. [Google Scholar]
- Catelli, E.; Cecchinato, M.; Delogu, M.; De Matteo, P.; Ortali, G.; Franciosi, C.; De Marco, M.A.; Naylor, C.J. Avian pneumovirus infection in turkey and broiler farms in Italy: A virological, molecular and serological field survey. Ital. J. Anim. Sci. 2004, 3, 287–292. [Google Scholar] [CrossRef]
- Senne, D.; Edson, J.C.; Pederson, B.P. Avian pneumovirus update. In Proceedings of the American Veterinary Medical Association, 134th Annual Congress, Reno, NV, USA, 19–24 July 1997; p. 190. [Google Scholar]
- Dani, M.A.; Arns, C.W.; Durigon, E.L. Molecular characterization of Brazilian Avian pneumovirus isolates using reverse transcription-polymerase chain reaction, restriction endonuclease analysis and sequencing of a G gene fragment. Avian Pathol. 2016, 28, 473–476. [Google Scholar] [CrossRef]
- Picault, J.P.; Giraud, P.; Drouin, P.; Gulttet, M.; Bennejean, G.; Lamande, J.; Toquin, D.; Gueguen, C. Isolation of a TRTV-like virus from chickens with swollen head syndrome. Vet. Rec. 1987, 121, 135. [Google Scholar] [CrossRef]
- Umar, S.; Teillaud, A.; Aslam, H.B.; Guerin, J.L.; Ducatez, M.F. Molecular epidemiology of respiratory viruses in commercial chicken flocks in Pakistan from 2014 through to 2016. BMC Vet. Res. 2019, 15, 351. [Google Scholar] [CrossRef]
- Seal, B.S. Avian pneumoviruses and emergence of a new type in the United States of America. Anim. Health Res. Rev. 2000, 1, 67–72. [Google Scholar] [CrossRef]
- Shin, H.J.; Cameron, K.T.; Jacobs, J.A.; Turpin, E.A.; Halvorson, D.A.; Goyal, S.M.; Nagaraja, K.V.; Kumar, M.C.; Lauer, D.C.; Seal, B.S.; et al. Molecular epidemiology of subgroup C avian pneumoviruses isolated in the United States and comparison with subgroup a and B viruses. J. Clin. Microbiol. 2002, 40, 1687–1693. [Google Scholar] [CrossRef]
- Lee, E.H.; Song, M.-S.; Shin, J.-Y.; Lee, Y.-M.; Kim, C.-J.; Lee, Y.S.; Kim, H.; Choi, Y.K. Genetic characterization of avian metapneumovirus subtype C isolated from pheasants in a live bird market. Virus Res. 2007, 128, 18–25. [Google Scholar] [CrossRef]
- Sun, S.; Chen, F.; Cao, S.; Liu, J.; Lei, W.; Li, G.; Song, Y.; Lu, J.; Liu, C.; Qin, J.; et al. Isolation and characterization of a subtype C avian metapneumovirus circulating in Muscovy ducks in China. Vet. Res. 2014, 45, 74. [Google Scholar] [CrossRef] [PubMed]
- Jardine, C.M.; Parmley, E.J.; Buchanan, T.; Nituch, L.; Ojkic, D. Avian metapneumovirus subtype C in Wild Waterfowl in Ontario, Canada. Transbound. Emerg. Dis. 2018, 65, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Retallack, H.; Clubb, S.; DeRisi, J.L. Genome sequence of a divergent avian metapneumovirus from a Monk Parakeet (Myiopsitta monachus) Microbiol. Resour. Announc. 2019, 8, e00284-19. [Google Scholar]
- Kaboudi, K. Virus-induced immunosuppression in turkeys (Meleagris gallopavo): A review. Open Vet. J. 2019, 9, 349–360. [Google Scholar] [CrossRef]
- Canuti, M.; Kroyer, A.N.; Ojkic, D.; Whitney, H.G.; Robertson, G.J.; Lang, A.S. Discovery and characterization of novel RNA viruses in aquatic North American wild birds. Viruses 2019, 11, 768. [Google Scholar] [CrossRef]
- Cook, J.K.A. Cook Avian pneumovirus infections of turkeys and chickens. Vet. J. 2000, 160, 118–125. [Google Scholar] [CrossRef]
- Rautenschlein, S. Avian metapneumovirus. In Diseases of Poultry, 14th ed; Swayne, D.E., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2020; pp. 135–143. [Google Scholar]
- Cook, J.K.; Huggins, M.B.; Orbell, S.J.; Mawditt, K.; Cavanagh, D. Infectious bronchitis virus vaccine interferes with the replication of Avian pneumovirus vaccine in domestic fowl. Avian Pathol. 2001, 30, 233–242. [Google Scholar] [CrossRef]
- Jones, R.C.; Rautenschlein, S. Avian metapneumovirus. In Diseases of Poultry; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 125–138. [Google Scholar]
- Jones, R.C.; Williams, R.A.; Baxter-Jones, C.; Savage, C.E.; Wilding, G.P. Experimental infection of laying turkeys with rhinotracheitis virus: Distribution of virus in the tissues and serological response. Avian Pathol. 1988, 17, 841–850. [Google Scholar] [CrossRef]
- Shin, H.J.; Rajashekara, G.; Jirjis, F.F.; Shaw, D.P.; Goyal, S.M.; Halvorson, D.A.; Nagaraja, K.V. Specific detection of Avian pneumovirus (APV) US isolates by RT-PCR. Arch. Virol. 2000, 145, 1239–1246. [Google Scholar] [CrossRef]
- Arns, C.W.; Zuanaze, M.; Berchieri, J.A.; Silva, E.N.; Di Fabio, J.; Sesti, L.; Zuanaze, M.A.F. Doenças das Aves. Metapneumovírus Aviário, 2nd ed.; FACTA: Campinas, Brazil, 2009; pp. 777–783. [Google Scholar]
- Gough, R.E.; Jones, R.C. Diseases of Poultry, 12th ed.; Avian Metapneumovirus; Iowa State Press: Ames, IA, USA, 2008; pp. 101–110. [Google Scholar]
- Samy, A.; Naguib, M. Avian Respiratory Coinfection and Impact on Avian Influenza Pathogenicity in Domestic Poultry: Field and experimental findings. Vet. Sci. 2018, 5, 23. [Google Scholar] [CrossRef]
- Mary, J.; Jackwood, P.; Erica, S. Multicausal Respiratory Diseases In Diseases of Poultry, 14th ed.; Swayne David, E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Naylor, C.J.; Al-Ankari, A.R.; Al-Afaleq, A.I.; Bradbury, J.M.; Jones, R.C. Exacerbation of Mycoplasma gallisepticum infection in Turkeys by rhinotracheitis virus. Avian Pathol. 1992, 21, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Marien, M.; Decostere, A.; Martel, A.; Chiers, K.; Froyman, R.; Nauwynck, H. Synergy between Avian pneumovirus and Ornithobacterium rhinotrachealein turkeys. Avian Pathol. 2005, 34, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Turpin, E.A.; Perkins, L.E.L.; Swayne, D.E. Experimental Infection of Turkeys with Avian Pneumovirus and Either Newcastle Disease Virus or Escherichia coli. Avian Dis. 2002, 46, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Jirjis, F.F.; Noll, S.L.; Halvorson, D.A.; Nagaraja, K.V.; Martin, F.; Shaw, D.P. Effects of Bacterial Coinfection on the Pathogenesis of Avian Pneumovirus Infection in Turkeys. Avian Dis. 2004, 48, 34–49. [Google Scholar] [CrossRef]
- Hafez, M.; Awad, A.; Ger, S. Turkey production and health: Current challenges Authors. J. Vet. Res. 2021, 1, 3–14. [Google Scholar] [CrossRef]
- van Empel, P.C.M.; HafezH, M. Ornithobacterium rhinotracheale: A review. Avian Pathol. 1999, 28, 217–227. [Google Scholar] [CrossRef]
- Paudel, S.; Easwaran, M.; Jang, H.; Jung, H.-K.; Kim, J.-H.; Shin, H.-J. Immunization with avian metapneumovirus harboring chicken Fc induces higher immune responses. Virus Res. 2016, 220, 129–135. [Google Scholar] [CrossRef]
- Cook, J.K.; Ellis, M.M.; Dolby, C.A.; Holmes, H.C.; Finney, P.M.; Huggins, M.B. A live attenuated turkey rhinotracheitis virus vaccine. 1. Stability of the attenuated strain. Avian Pathol. 1989, 18, 511–522. [Google Scholar] [CrossRef]
- Cook, J.K.A.; Marjorie, M.E. Attenuation of turkey rhinotracheitis virus by alternative passage in embryonated chicken eggs and tracheal organ cultures. Avian Pathol. 1990, 19, 181–185. [Google Scholar] [CrossRef]
- Naylor, C.; Shaw, K.; Britton, P.; Cavanagh, D. Appearance of type B Avian pneumovirus in great Britain. Avian Pathol. 1997, 26, 327–338. [Google Scholar] [CrossRef]
- Cook, J.K.; Huggins, M.B.; Woods, M.A.; Orbell, S.J.; Mockett, A.P. Protection provided by a commercially available vaccine against different strains of turkey rhinotracheitis virus. Vet. Rec. 1995, 136, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.; Huggins, M.B.; Orbell, S.J.; Senne, D.A. Preliminary antigenic characterization of an Avian pneumovirus isolated from commercial turkeys in Colorado, USA. Avian Pathol. 1999, 28, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Toquin, D.; Bäyon-Auboyer, M.; Jestin, V.; Eterradossi, N. Résponse Sérologique et Protection Croisée Visa vis de L’infection par Une Souche Non-A Non-B du Virus de La Rhinotrachéite Infectieuse De La Dinde; Comptes Rendus des 3emes Journées de la Recherche Avicole: St Malo, France, 1999; pp. 223–224. [Google Scholar]
- Christopher, B.; Basim, M.; Andreas, H.; Stephane, L.; Kannan, G. Vaccination against Avian metapneumovirus subtype B in commercial broiler chicks: Heterologous protection and selected host transcriptional responses to subtype A or B challenge. Avian Pathol. 2022, 51, 181–196. [Google Scholar] [CrossRef]
- Felippe, P.A.; Silva, L.H.A.; Santos, M.B.; Sakata, S.T.; Arns, C.W. Detection of and phylogenetic studies with avian metapneumovirus recovered from feral pigeons and wild birds in Brazil. Avian Pathol. 2011, 40, 445–452. [Google Scholar] [CrossRef]
- Ganapathy, K.; Bufton, A.; Pearson, A.; Lemiere, S.; Jones, R.C. Vaccination of commercial broiler chicks against avian metapneumovirus infection: A comparison of drinking-water, spray and oculo-oral delivery methods. Vaccine 2010, 28, 3944–3948. [Google Scholar] [CrossRef]
- Tamam, S.M.; Hussein, A.S.; Arafa, A.M.; Madbouly, H.M. Preparation and evaluation of inactivated avian Metapneumovirus vaccine from recently isolated Egyptian strain. J. Appl. Poult. Res. 2015, 24, 168–176. [Google Scholar] [CrossRef]
- Cook, J.K.A.; Orthel, F.; Orbell, S.; Woods, M.A.; Huggins, M.B. An experimental turkey rhinotracheitis (TRT) infection in breeding turkeys and the prevention of its clinical effects using live-attenuated and inactivated TRT vaccines. Avian Pathol. 1996, 25, 231–243. [Google Scholar] [CrossRef]
- Duffy, S.; Shackelton, L.; Holmes, E. Rates of evolutionary change in viruses: Patterns and determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar] [CrossRef]
- Franzo, G.; Massi, P.; Tucciarone, C.M.; Barbieri, I.; Tosi, G.; Fiorentini, L.; Ciccozzi, M.; Lavazza, A.; Cecchinato, M.; Moreno, A. Think globally, act locally: Phylodynamic reconstruction of infectious bronchitis virus (IBV) QX genotype (GI-19 lineage) reveals different population dynamics and spreading patterns when evaluated on different epidemiological scales. PLoS ONE 2017, 12, e0184401. [Google Scholar] [CrossRef]
- Franzo, G.; Cecchinato, M.; Tosi, G.; Fiorentini, L.; Faccin, F.; Tucciarone, C.M.; Trogu, T.; Barbieri, I.; Massi, P.; Moreno, A. GI-16 lineage (624/I or Q1), there and back again: The history of one of the major threats for poultry farming of our era. PLoS ONE 2018, 13, e0203513. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Rautenschlein, S. Investigations on the protective role of passively transferred antibodies against avian metapneumovirus infection in turkeys. Avian Pathol. 2009, 38, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Yohe, S.; Thyagarajan, B. Review of Clinical Next-Generation Sequencing. Arch. Pathol. Lab. Med. 2017, 141, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Du, S.H.; Li, D.R.; Wang, H.J.; Wang, Q. Application of RT-qPCR in the Study of Forensic Pathology. Fa Yi Xue Za Zhi 2017, 33, 526–531. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Goodwin, S.; Mcpherson, J.D.; Mccombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef]
- Schuster, J.E.; Williams, J.V. Human metapneumovirus. Pediatr. Rev. 2013, 34, 558–565. [Google Scholar] [CrossRef]
- Hodinka, R.L. Respiratory RNA Viruses. Microbiol. Spectr. 2016, 4, 233–271. [Google Scholar] [CrossRef]
- Chacón, J.L.; Mizuma, M.; Vejarano, M.P.; Toquín, D.; Eterradossi, N.; Patnayak, D.P.; Goyal, S.M.; Ferreira, A.J. Avian metapneumovirus subtypes circulating in Brazilian vaccinated and nonvaccinated chicken and turkey farms. Avian Dis. 2011, 55, 82–89. [Google Scholar] [CrossRef]
- Andreopoulou, M.; Franzo, G.; Tucciarone, C.M.; Prentza, Z.; Koutoulis, K.C.; Cecchinato, M.; Chaligianni, I. Molecular epidemiology of infectious bronchitis virus and avian metapneumovirus in Greece. Poult. Sci. 2019, 98, 5374–5384. [Google Scholar] [CrossRef]
- Rizotto, L.S.; Scagion, G.P.; Cardoso, T.C.; Simão, R.M.; Caserta, L.C.; Benassi, J.C.; Keid, L.B.; Oliveira, T.M.F.S.; Soares, R.M.; Arns, C.W.; et al. Complete Genome Sequence of an Avian Metapneumovirus Subtype A Strain Isolated from Chicken (Gallus gallus) in Brazil. Genome Announc. Genome Announc. 2017, 5, e00688-17. [Google Scholar] [CrossRef]
- Aly, S.M.; Sabri, D.M. Next generation sequencing (NGS): A golden tool in forensic toolkit. Arch. Forensic Med. Criminol. 2015, 4, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kariithi, H.M.; Christy, N.; Decanini, E.L.; Lemiere, S.; Volkening, J.D.; Afonso, C.L.; Suarez, D.L. Detection and Genome Sequence Analysis of Avian Metapneumovirus Subtype A Viruses Circulating in Commercial Chicken Flocks in Mexico. Vet. Sci. 2022, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stelzer-Braid, S.; Scotch, M.; Rawlinson, W.D. Detection of respiratory viruses directly from clinical samples using next-generation sequencing: A literature review of recent advances and potential for routine clinical use. Rev. Med. Virol. 2022, 32, e2375. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.G.; Chung, H.C.; Do, H.Q.; Nguyen, T.T.; Cao, T.B.; Truong, H.T.; Mai, T.N.; Le, T.T.; Nguyen, T.H.; Le, T.L.; et al. Serological and Molecular Characterization of Avian Metapneumovirus in Chickens in Northern Vietnam. Vet. Sci. Sep. 2021, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Mayahi, M.; Momtaz, H.; Jafari, R.A.; Zamani, P. Detection and subtyping avian metapneumovirus from turkeys in Iran. Vet. Res. Forum 2017, 8, 105–108. [Google Scholar] [PubMed]
- Tucciarone, C.M.; Franzo, G.; Lupini, C.; Alejo, C.T.; Listorti, V.; Mescolini, G.; Brandão, P.E.; Martini, M.; Catelli, E.; Cecchinato, M. Avian Metapneumovirus circulation in Italian broiler farms. Poult. Sci. 2018, 97, 503–509. [Google Scholar] [CrossRef]
- Yu, M.; Xing, L.; Chang, F.; Bao, Y.; Wang, S.; He, X.; Wang, J.; Wang, S.; Liu, Y.; Farooque, M.; et al. Genomic sequence and pathogenicity of the first avian metapneumovirus subtype B isolated from chicken in China. Vet. Microbiol. 2019, 228, 32–38. [Google Scholar] [CrossRef]
- Goraichuk, I.V.; Kapczynski, D.R.; Seal, B.S.; Suarez, D.L. Complete Genome Sequence of an avian metapneumovirus Subtype B Strain from Hungary. Microbiol. Resour. Announc. Genome Announc. 2020, 9, e00177-20. [Google Scholar] [CrossRef]
- Sugiyama, M.; Ito, H.; Hata, Y.; Ono, E.; Ito, T. Complete nucleotide sequences of avian metapneumovirus subtype B genome. Virus Genes. 2010, 41, 389–395. [Google Scholar] [CrossRef]
| Target | Sample | Methodology | Country | aMPV Subtype | Publication Year | Author |
|---|---|---|---|---|---|---|
| Gene—G | Cloacal/throat double swabs | RT-PCR | China | 2022 | [91] | |
| Gene—G and Protein G | Choanal cleft swab | ELISA | North Vietnam | 2021 | [92] | |
| RT-PCR | aMPV B | 2021 | ||||
| Genes—G, N e M | Choanal cleft swab | RT-PCR | Iran | aMPV B | 2017 | [93] |
| Gene—G | Respiratory tract swabs | RT-PCR | Northern Italy | aMPV B | 2018 | [86] |
| Gene—M | Oropharyngeal and cloacal swabs | RT-PCR | Canada | aMPV C | 2018 | [45] |
| Gene—G | Tracheal swabs | RT-PCR and Sanger sequencing | Greece | aMPV B | 2019 | [94] |
| Gene—G | Throat swabs | - | China | aMPV B | 2019 | [95] |
| Gene—N, M, F, L, M2, SH e G | Tissues swabbed (choana, lung) | Illumina sequencing | Mexico | aMPV A | 2022 | [96] |
| Genome | Uninformed | NGS (Illumina MiSeq) | Hungary | aMPV B | 2020 | [97] |
| Gene—G | Tracheal and cloacal swabs | RT-PCR and Sanger sequencing | Brazil | aMPV A and B | 2011 | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salles, G.B.C.; Pilati, G.V.T.; Muniz, E.C.; de Lima Neto, A.J.; Vogt, J.R.; Dahmer, M.; Savi, B.P.; Padilha, D.A.; Fongaro, G. Trends and Challenges in the Surveillance and Control of Avian Metapneumovirus. Viruses 2023, 15, 1960. https://doi.org/10.3390/v15091960
Salles GBC, Pilati GVT, Muniz EC, de Lima Neto AJ, Vogt JR, Dahmer M, Savi BP, Padilha DA, Fongaro G. Trends and Challenges in the Surveillance and Control of Avian Metapneumovirus. Viruses. 2023; 15(9):1960. https://doi.org/10.3390/v15091960
Chicago/Turabian StyleSalles, Gleidson Biasi Carvalho, Giulia Von Tönnemann Pilati, Eduardo Correa Muniz, Antonio Junior de Lima Neto, Josias Rodrigo Vogt, Mariane Dahmer, Beatriz Pereira Savi, Dayane Azevedo Padilha, and Gislaine Fongaro. 2023. "Trends and Challenges in the Surveillance and Control of Avian Metapneumovirus" Viruses 15, no. 9: 1960. https://doi.org/10.3390/v15091960
APA StyleSalles, G. B. C., Pilati, G. V. T., Muniz, E. C., de Lima Neto, A. J., Vogt, J. R., Dahmer, M., Savi, B. P., Padilha, D. A., & Fongaro, G. (2023). Trends and Challenges in the Surveillance and Control of Avian Metapneumovirus. Viruses, 15(9), 1960. https://doi.org/10.3390/v15091960






