Clinical Severity of SARS-CoV-2 Variants during COVID-19 Vaccination: A Systematic Review and Meta-Analysis
Abstract
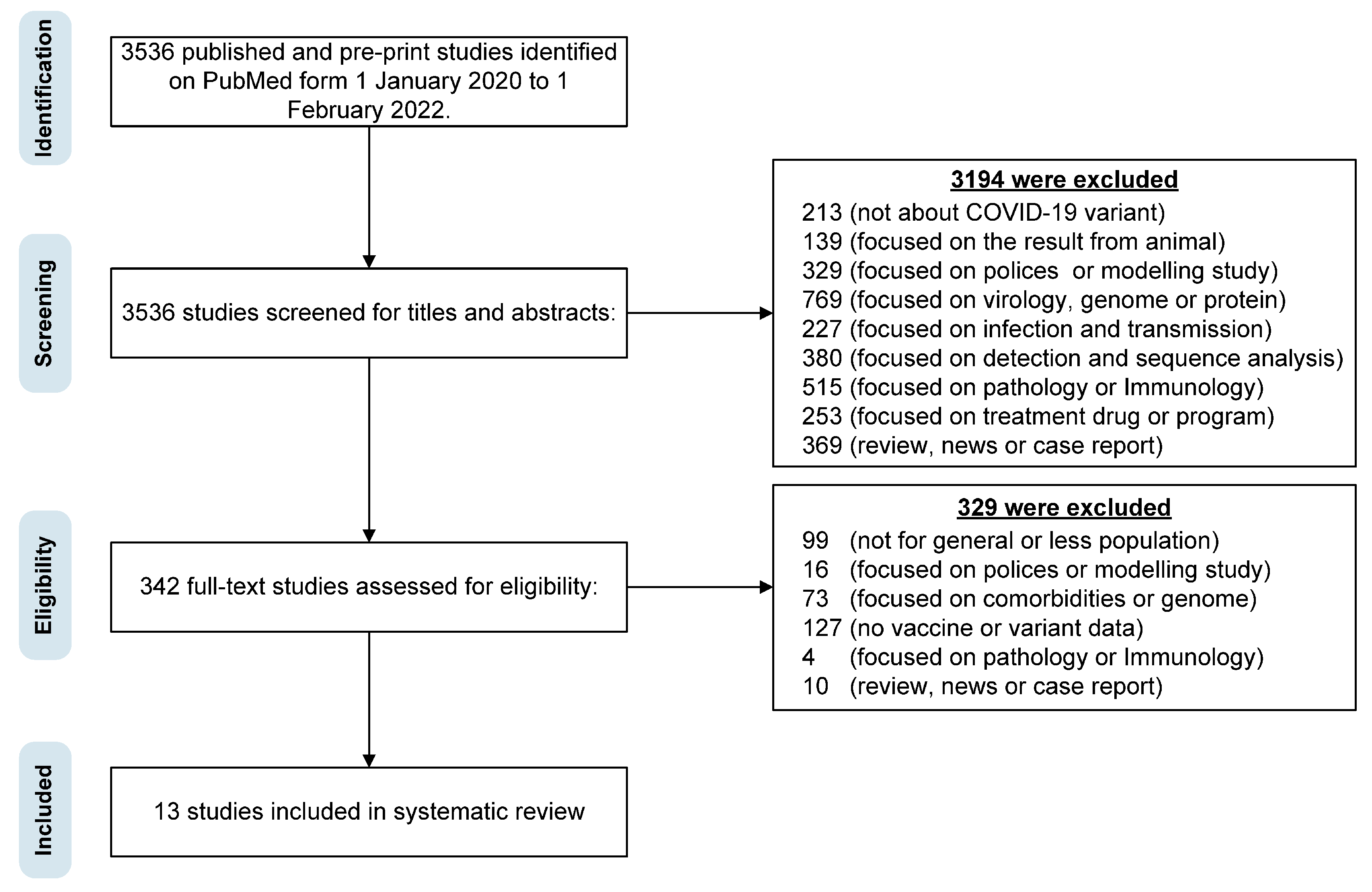
:1. Introduction
2. Methods
2.1. Measures of Clinical Severity of COVID-19 Disease
2.2. Search Strategy and Selection Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Archived: WHO Timeline—COVID-19. Available online: https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 (accessed on 24 June 2022).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World Data 2020. [Google Scholar]
- Khalid, F.; Tahir, R.; Ellahi, M.; Amir, N.; Rizvi, S.F.A.; Hasnain, A. Emerging Trends of Edible Vaccine Therapy for Combating Human Diseases Especially COVID-19: Pros, Cons, and Future Challenges. Phytother. Res. 2022, 36, 2746–2766. [Google Scholar] [CrossRef] [PubMed]
- Houhamdi, L.; Gautret, P.; Hoang, V.T.; Fournier, P.-E.; Colson, P.; Raoult, D. Characteristics of the First 1119 SARS-CoV-2 Omicron Variant Cases, in Marseille, France, November-December 2021. J. Med. Virol. 2022, 94, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian Health System and the COVID-19 Challenge. Lancet Public Health 2020, 5, e253. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Jorge, D.C.P.; Veiga, R.V.; Rodrigues, M.S.; Torquato, M.F.; da Silva, N.B.; Fiaccone, R.L.; Cardim, L.L.; Pereira, F.A.C.; de Castro, C.P.; et al. Mathematical Modeling of COVID-19 in 14.8 Million Individuals in Bahia, Brazil. Nat. Commun. 2021, 12, 333. [Google Scholar] [CrossRef]
- Tsang, T.K.; Wang, C.; Yang, B.; Cauchemez, S.; Cowling, B.J. Using Secondary Cases to Characterize the Severity of an Emerging or Re-Emerging Infection. Nat. Commun. 2021, 12, 6372. [Google Scholar] [CrossRef]
- Lyu, P.; Liu, X.; Zhang, R.; Shi, L.; Gao, J. The Performance of Chest CT in Evaluating the Clinical Severity of COVID-19 Pneumonia: Identifying Critical Cases Based on CT Characteristics. Investig. Radiol. 2020, 55, 412–421. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating Clinical Severity of COVID-19 from the Transmission Dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of COVID-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef]
- Butt, A.A.; Dargham, S.R.; Chemaitelly, H.; Al Khal, A.; Tang, P.; Hasan, M.R.; Coyle, P.V.; Thomas, A.G.; Borham, A.M.; Concepcion, E.G.; et al. Severity of Illness in Persons Infected With the SARS-CoV-2 Delta Variant vs Beta Variant in Qatar. JAMA Intern. Med. 2022, 182, 197–205. [Google Scholar] [CrossRef]
- Veneti, L.; Valcarcel Salamanca, B.; Seppälä, E.; Starrfelt, J.; Storm, M.L.; Bragstad, K.; Hungnes, O.; Bøås, H.; Kvåle, R.; Vold, L.; et al. No Difference in Risk of Hospitalization between Reported Cases of the SARS-CoV-2 Delta Variant and Alpha Variant in Norway. Int. J. Infect. Dis. 2021, 115, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Wu, J.T.; Liu, D.; Leung, G.M. First-Wave COVID-19 Transmissibility and Severity in China Outside Hubei after Control Measures, and Second-Wave Scenario Planning: A Modelling Impact Assessment. Lancet 2020, 395, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Cabezudo-García, P.; Ciano-Petersen, N.L.; Mena-Vázquez, N.; Pons-Pons, G.; Castro-Sánchez, M.V.; Serrano-Castro, P.J. Incidence and Case Fatality Rate of COVID-19 in Patients with Active Epilepsy. Neurology 2020, 95, e1417–e1425. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.-E.; Houhamdi, L.; Colson, P.; Cortaredona, S.; Delorme, L.; Cassagne, C.; Lagier, J.-C.; Chaudet, H.; Tissot-Dupont, H.; Giraud-Gatineau, A.; et al. SARS-CoV-2 Vaccination and Protection Against Clinical Disease: A Retrospective Study, Bouches-Du-Rhône District, Southern France, 2021. Front. Microbiol. 2022, 12, 4204. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and Effectiveness of mRNA BNT162b2 Vaccine against SARS-CoV-2 Infections and COVID-19 Cases, Hospitalisations, and Deaths Following a Nationwide Vaccination Campaign in Israel: An Observational Study Using National Surveillance Data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital Admission and Emergency Care Attendance Risk for SARS-CoV-2 Delta (B.1.617.2) Compared with Alpha (B.1.1.7) Variants of Concern: A Cohort Study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- Seppälä, E.; Veneti, L.; Starrfelt, J.; Danielsen, A.S.; Bragstad, K.; Hungnes, O.; Taxt, A.M.; Watle, S.V.; Meijerink, H. Vaccine Effectiveness against Infection with the Delta (B.1.617.2) Variant, Norway, April to August 2021. Euro Surveill. 2021, 26, 2100793. [Google Scholar] [CrossRef]
- Fisman, D.N.; Tuite, A.R. Evaluation of the Relative Virulence of Novel SARS-CoV-2 Variants: A Retrospective Cohort Study in Ontario, Canada. CMAJ 2021, 193, E1619–E1625. [Google Scholar] [CrossRef]
- Gupta, N.; Kaur, H.; Yadav, P.D.; Mukhopadhyay, L.; Sahay, R.R.; Kumar, A.; Nyayanit, D.A.; Shete, A.M.; Patil, S.; Majumdar, T.; et al. Clinical Characterization and Genomic Analysis of Samples from COVID-19 Breakthrough Infections during the Second Wave among the Various States of India. Viruses 2021, 13, 1782. [Google Scholar] [CrossRef]
- Sheikh, A.; Robertson, C.; Taylor, B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. N. Engl. J. Med. 2021, 385, 2195–2197. [Google Scholar] [CrossRef]
- Naleway, A.L.; Groom, H.C.; Crawford, P.M.; Bianca Salas, S.; Henninger, M.L.; Donald, J.L.; Smith, N.; Thompson, M.G.; Blanton, L.H.; Bozio, C.H.; et al. Incidence of SARS-CoV-2 Infection, Emergency Department Visits, and Hospitalizations Because of COVID-19 Among Persons Aged ≥ 12 Years, by COVID-19 Vaccination Status—Oregon and Washington, July 4–September 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Hagan, L.M.; McCormick, D.W.; Lee, C.; Sleweon, S.; Nicolae, L.; Dixon, T.; Banta, R.; Ogle, I.; Young, C.; Dusseau, C.; et al. Outbreak of SARS-CoV-2 B.1.617.2 (Delta) Variant Infections Among Incarcerated Persons in a Federal Prison—Texas, July-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Espenhain, L.; Funk, T.; Overvad, M.; Edslev, S.M.; Fonager, J.; Ingham, A.C.; Rasmussen, M.; Madsen, S.L.; Espersen, C.H.; Sieber, R.N.; et al. Epidemiological Characterisation of the First 785 SARS-CoV-2 Omicron Variant Cases in Denmark, December 2021. Euro Surveill. 2021, 26, 2101146. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. 2022. Available online: https://github.com/owid/covid-19-Data (accessed on 9 April 2022).
- Grint, D.J.; Wing, K.; Houlihan, C.; Gibbs, H.P.; Evans, S.J.W.; Williamson, E.; McDonald, H.I.; Bhaskaran, K.; Evans, D.; Walker, A.J.; et al. Severity of Severe Acute Respiratory System Coronavirus 2 (SARS-CoV-2) Alpha Variant (B.1.1.7) in England. Clin. Infect. Dis. 2021, 75, e1120–e1127. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Twohig, K.A.; Harris, R.J.; Seaman, S.R.; Flannagan, J.; Allen, H.; Charlett, A.; De Angelis, D.; Dabrera, G.; Presanis, A.M. Risk of Hospital Admission for Patients with SARS-CoV-2 Variant B.1.1.7: Cohort Analysis. BMJ 2021, 373, n1412. [Google Scholar] [CrossRef] [PubMed]
- Veneti, L.; Seppälä, E.; Storm, M.L.; Salamanca, B.V.; Buanes, E.A.; Aasand, N.; Naseer, U.; Bragstad, K.; Hungnes, O.; Bøås, H.; et al. Increased Risk of Hospitalisation and Intensive Care Admission Associated with Reported Cases of SARS-CoV-2 Variants B.1.1.7 and B.1.351 in Norway, December 2020–May 2021. PLoS ONE 2021, 16, e0258513. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; de Moura Villela, E.F.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac Vaccine in Older Adults during a Gamma Variant Associated Epidemic of COVID-19 in Brazil: Test Negative Case-Control Study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef] [PubMed]
- Hitchings, M.D.T.; Ranzani, O.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; de Moura Villela, E.F.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of ChAdOx1 Vaccine in Older Adults during SARS-CoV-2 Gamma Variant Circulation in São Paulo. Nat. Commun. 2021, 12, 6220. [Google Scholar] [CrossRef]
- Hussain, M.; Zaman, S.; Bari, A. Guidelines for the Treatment of Severe and Critical Cases of COVID-19. BioMedica 2020, 36, 297–298. [Google Scholar] [CrossRef]
- Munkvik, M.; Alsnes, I.V.; Vatten, L. The Risk of Severe COVID-19: Hospital and ICU Admission Rates in Norway. bioRxiv 2020. [Google Scholar] [CrossRef]
- Varma, A.; Dergaa, I.; Ashkanani, M.; Musa, S.; Zidan, M. Analysis of Qatar’s Successful Public Health Policy in Dealing with the COVID-19 Pandemic. Int. J. Med. Rev. Case Rep. 2021, 5, 6–11. [Google Scholar] [CrossRef]
- Chemaitelly, H.; AlMukdad, S.; Ayoub, H.H.; Altarawneh, H.N.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; et al. COVID-19 Vaccine Protection among Children and Adolescents in Qatar. N. Engl. J. Med. 2022, 387, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Moline, H.L.; Whitaker, M.; Deng, L.; Rhodes, J.C.; Milucky, J.; Pham, H.; Patel, K.; Anglin, O.; Reingold, A.; Chai, S.J.; et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥ 65 Years—COVID-NET, 13 States, February-April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1088–1093. [Google Scholar] [CrossRef]
- Brodin, P. Immune Determinants of COVID-19 Disease Presentation and Severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The Efficacy and Effectiveness of the COVID-19 Vaccines in Reducing Infection, Severity, Hospitalization, and Mortality: A Systematic Review. Hum. Vaccin. Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.B.; Aggarwal, R.; Jani, I.; Jawad, J.; Kochhar, S.; MacDonald, N.; Madhi, S.A.; Mohsni, E.; Mulholland, K.; Neuzil, K.M.; et al. COVID-19 Vaccine Strategies Must Focus on Severe Disease and Global Equity. Lancet 2022, 399, 406–410. [Google Scholar] [CrossRef]
- Sen-Crowe, B.; Sutherland, M.; McKenney, M.; Elkbuli, A. A Closer Look Into Global Hospital Beds Capacity and Resource Shortages During the COVID-19 Pandemic. J. Surg. Res. 2021, 260, 56–63. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective Effectiveness of Previous SARS-CoV-2 Infection and Hybrid Immunity against the Omicron Variant and Severe Disease: A Systematic Review and Meta-Regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England: A Cohort Study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]




| Study | Location | Variant | Study Period | Population | Vaccine Proportion for Population Pf * (%) | Vaccine Proportion for Elderly PO * (%) | cCHR (%) | cCFR(%) | HFR (%) | HIR (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Fournier et al. [15] | France | Alpha − | 2021.1.18–2021.8.13 18 January 2021–13 August 2021 | 7894 | 6.26 | 78.00 | 7.56 (6.99, 8.17) | 1.66 (1.39, 1.97) | 21.94 (18.69, 25.48) | 22.78 (19.47, 26.35) |
| Haas et al. [16] | Israel | Alpha # | 24 January 2021–3 April 2021 | 116,142 | 5.40 | 91.47 | 5.43 (5.31, 5.57) | 0.73 (0.69, 0.79) | 13.51 (12.70, 14.38) | NR |
| Twohig et al. [17] | UK | Alpha + | 29 March 2021–23 May 2021 | 34,656 | 1.31 | 87.04 | 2.20 (2.05, 2.36) | NR | NR | NR |
| Seppälä et al. [18] | Norway | Alpha + | 15 April 2021–15 August 2021 | 13,001 | 1.59 | 92.46 | 2.94 (2.65, 3.24) | 0.19 (0.12, 0.28) | 6.54 (4.28, 9.51) | NR |
| Veneti et al. [12] | Norway | Alpha + | 3 May 2021–15 August 2021 | 12,078 | 1.47 | 94.26 | 1.99 (1.75, 2.25) | 0.12 (0.07, 0.20) | 6.25 (3.54, 10.10) | 16.67 (12.18, 22.00) |
| Butt et al. [11] | Qatar | Beta + | 22 March 2021–7 July 2021 | 451 | 11.97 | NR | 19.96 (16.36, 23.95) | 0.22 (0.01, 1.23) | 1.11 (0.03, 6.04) | 15.56 (8.77, 24.72) |
| Fournier et al. [15] | France | Delta − | 18 January 2021–13 August 2021 | 3730 | 15.12 | 78.00 | 3.54 (2.97, 4.18) | 0.70 (0.46, 1.02) | 19.70 (13.29, 27.51) | 21.21 (14.58, 29.18) |
| Fisman et al. [19] | Canada | Delta + | 7 February 2021–27 June 2021 | 5989 | 1.19 | 60.48 | 5.83 (5.25, 6.45) | 0.67 (0.48, 0.91) | 11.46 (8.32, 15.28) | 25.79 (21.28, 30.71) |
| Gupta et al. [20] | India | Delta − | 1 March 2021–1 June 2021 | 677 | 87.45 | 12.40 | 9.90 (7.75, 12.40) | 0.44 (0.09, 1.29) | 4.48 (0.93, 12.53) | NR |
| Butt et al. [11] | Qatar | Delta + | 22 March 2021–7 July 2021 | 451 | 21.06 | NR | 27.27 (23.21, 31.63) | 0.67 (0.14, 1.93) | 2.44 (0.51, 6.96) | 17.89 (11.56, 25.82) |
| Twohig et al. [17] | UK | Delta + | 29 March 2021–23 May 2021 | 8682 | 3.90 | 87.04 | 2.26 (1.96, 2.59) | NR | NR | NR |
| Sheikh et al. [21] | UK | Delta + | 1 April 2021–16 August 2021 | 72,143 | 11.52 | 95.56 | NR | 0.15 (0.12, 0.18) | NR | NR |
| Sheikh et al. [21] | UK | Delta + | 1 April 2021–16 August 2021 | 71,744 | 11.52 | 95.56 | NR | 0.24 (0.20, 0.28) | NR | NR |
| Seppälä et al. [18] | Norway | Delta + | 15 April 2021–15 August 2021 | 5430 | 10.30 | 94.26 | 1.55 (1.24, 1.91) | 0.09 (0.03, 0.21) | 5.95 (1.96, 13.35) | NR |
| Veneti et al. [12] | Norway | Delta + | 3 May 2021–15 August 2021 | 7977 | 7.89 | 94.26 | 1.34 (1.10, 1.62) | 0.06 (0.02, 0.15) | 4.67 (1.53, 10.57) | 14.95 (8.80, 23.14) |
| Naleway et al. [22] | USA | Delta ^ | 4 July 2021–25 September 2021 | 7155 | 42.05 | 83.85 | 6.26 (5.71, 6.85) | 1.10 (0.88, 1.37) | 17.63 (14.22, 21.49) | 24.33 (20.43, 28.58) |
| Hagan et al. [23] | USA | Delta + | 12 July 2021–14 August 2021 | 172 | 75.00 | 81.80 | 2.33 (0.64, 5.85) | 0.58 (0.01, 3.20) | 25.00 (0.63, 80.59) | NR |
| Espenhain et al. [24] | Denmark | Delta + | 22 November 2021–7 December 2021 | 19,137 | 48.43 | 98.96 | 1.52 (1.35, 1.70) | 0.07 (0.04, 0.12) | 4.80 (2.66, 7.97) | 7.59 (4.82, 11.26) |
| Houhamdi et al. [4] | France | Delta − | 28 November 2021–31 December 2021 | 3075 | 20.49 | 88.34 | 11.93 (10.81, 13.13) | 1.27 (0.90, 1.73) | 10.67 (7.67, 14.24) | 25.61 (21.22, 30.40) |
| Espenhain et al. [24] | Denmark | Omicron + | 22 November 2021–7 December 2021 | 785 | 76.31 | 98.96 | 1.15 (0.53, 2.17) | 0.00 (0.00, 0.47) | 0.00 (0.00, 33.62) | 11.11 (0.28, 48.25) |
| Houhamdi et al. [4] | France | Omicron − | 28 November 2021–31 December 2021 | 1119 | 22.97 | 88.34 | 1.88 (1.17, 2.85) | 0.09 (0.00, 0.50) | 4.76 (0.12, 23.82) | 4.76 (0.12, 23.82) |
| Study | Location | Variant | Study Period | Population (Confirmed Cases) | Age Group | cCHR (%) | cCFR (%) | HFR (%) | HIR (%) |
|---|---|---|---|---|---|---|---|---|---|
| Grint et al. [26] | England | Alpha + | 16 November 2020–11 January 2021 | 93,153 | all | 2.90 (2.80, 3.01) | 0.54 (0.49, 0.59) | 13.66 (12.39, 15.01) | 12.74 (11.50, 14.05) |
| 22,795 | 0–24 | 0.31 (0.24, 0.39) | NR | NR | NR | ||||
| 35,820 | 25–44 | 1.50 (1.38, 1.63) | NR | NR | NR | ||||
| 27,334 | 45–64 | 4.37 (4.13, 4.62) | NR | NR | NR | ||||
| 7204 | ≥65 | 12.47 (11.71, 13.25) | NR | NR | NR | ||||
| Nyberg et al. [27] | England | Alpha + | 23 November 2020–31 January 2021 | 592,409 | all | 4.68 (4.62, 4.73) | 0.44 (0.42, 0.46) | 3.29 (3.08, 3.50) | NR |
| 31,935 | 0–24 | 1.20 (1.15, 1.26) | NR | NR | NR | ||||
| 63,084 | 25–44 | 3.35 (3.28, 3.43) | NR | NR | NR | ||||
| 115,296 | 45–64 | 6.99 (6.87, 7.11) | NR | NR | NR | ||||
| 118,229 | ≥65 | 14.49 (14.17, 14.82) | NR | NR | NR | ||||
| Veneti et al. [28] | Norway | Alpha + | 28 December 2020–2 May 2021 | 23,169 | all | 3.82 (3.57, 4.07) | NR | NR | 19.91 (17.32, 22.70) |
| 9915 | 0–24 | 0.36 (0.25, 0.50) | NR | NR | NR | ||||
| 7624 | 25–44 | 2.85 (2.48, 3.24) | NR | NR | NR | ||||
| 1860 | 45–64 | 23.17 (21.27, 25.16) | NR | NR | NR | ||||
| 770 | ≥65 | 25.98 (22.91, 29.22) | NR | NR | NR | ||||
| Haas et al. [16] | Israel | Alpha # | 24 January 2021–3 April 2021 | 116,142 | all | 5.30 (5.17, 5.43) | 0.73 (0.69, 0.79) | 13.87 (13.01, 14.75) | NR |
| 26,818 | 0–24 | 2.40 (2.22, 2.59) | 0.04 (0.02, 0.07) | 1.71 (0.86, 3.04) | NR | ||||
| 59,594 | 25–44 | 2.40 (2.28, 2.53) | 0.04 (0.03, 0.06) | 1.75 (1.13, 2.57) | NR | ||||
| 21,843 | 45–64 | 8.24 (7.87, 8.61) | 6.36 (5.35, 7.51) | 7.73 (6.53, 9.06) | NR | ||||
| 7887 | ≥65 | 28.87 (27.87, 29.88) | 8.60 (7.99, 9.24) | 29.78 (27.90, 31.70) | NR | ||||
| Veneti et al. [12] | Norway | Alpha + | 3 May 2021–15 August 2021 | 12,078 | all | 1.99 (1.75, 2.25) | 0.12 (0.07, 0.20) | 6.25 (3.54, 10.10) | 16.67 (12.18, 21.20) |
| 6292 | 0–24 | 0.17 (0.09, 0.31) | NR | NR | NR | ||||
| 3523 | 25–44 | 1.96 (1.53, 2.47) | NR | NR | NR | ||||
| 2003 | 45–64 | 6.00 (5.00, 7.12) | NR | NR | NR | ||||
| 260 | ≥65 | 15.38 (11.22, 20.35) | NR | NR | NR | ||||
| Veneti et al. [12] | Norway | Beta + | 28 December 2020–2 May 2021 | 548 | all | 4.20 (2.68, 6.23) | NR | NR | 21.74 (7.46, 43.70) |
| 231 | 0–24 | 0 (0, 1.58) | NR | NR | NR | ||||
| 185 | 25–44 | 2.70 (0.88, 6.19) | NR | NR | NR | ||||
| 118 | 45–64 | 12.71 (7.29, 20.10) | NR | NR | NR | ||||
| 14 | ≥65 | 21.43 (4.66, 50.80) | NR | NR | NR | ||||
| Sheikh et al. [21] | Scotland | Delta + | 1 April 2021–16 August 2021 | 72,143 | all | NR | 0.15 (0.12, 0.18) | NR | NR |
| 18,833 | 0–24 | NR | 0.03 (0.01, 0.06) | NR | NR | ||||
| 42,965 | 25–44 | NR | 0.05 (0.03, 0.08) | NR | NR | ||||
| 8137 | 45–64 | NR | 0.41 (0.28, 0.57) | NR | NR | ||||
| 2208 | ≥65 | NR | 2.08 (1.53, 2.77) | NR | NR | ||||
| Sheikh et al. [21] | Scotland | Delta + | 1 April 2021–16 August 2021 | 71,700 | all | NR | 0.24 (0.20, 0.27) | NR | NR |
| 13,215 | 0–24 | NR | 0.03 (0.01, 0.08) | NR | NR | ||||
| 34,275 | 25–44 | NR | 0.07 (0.05, 0.10) | NR | NR | ||||
| 18,672 | 45–64 | NR | 0.28 (0.21, 0.37) | NR | NR | ||||
| 5582 | ≥65 | NR | 1.56 (1.25, 1.92) | NR | NR | ||||
| Veneti et al. [12] | Norway | Delta + | 3 May 2021–15 August 2021 | 7977 | all | 1.34 (1.10, 1.62) | 0.06 (0.02, 0.15) | 4.67 (1.53, 10.57) | 14.95 (8.80, 23.14) |
| 3964 | 0–24 | 0.33 (0.17, 0.56) | NR | NR | NR | ||||
| 3036 | 25–44 | 1.45 (1.05–1.94) | NR | NR | NR | ||||
| 1057 | 45–64 | 3.31 (2.32, 4.58) | NR | NR | NR | ||||
| 190 | ≥65 | 7.89 (4.49, 12.69) | NR | NR | NR |
| Dependent Variable (for Delta Variant) | Predictor | R2 (%) | α | p-Value | Intercept | p-Value |
|---|---|---|---|---|---|---|
| cCHR | Pf | 0.00 | 0.0319 | 0.4729 | 3.6411 | 0.0729 |
| cCHR | PO | 15.55 | −0.0744 | 0.1428 | 10.487 | 0.0242 |
| cCFR | Pf | 0.00 | 0.0015 | 0.8055 | 0.4199 | 0.0705 |
| cCFR | PO | 1.36 | −0.0041 | 0.5118 | 0.0080 | 0.1586 |
| HFR | Pf | 0.00 | −0.0390 | 0.6384 | 11.109 | 0.0098 |
| HFR | PO | 0.00 | 0.0181 | 0.8312 | 8.5612 | 0.2436 |
| HIR | Pf | 5.49 | −0.1847 | 0.3411 | 24.202 | 0.0084 |
| HIR | PO | 25.87 | −0.3319 | 0.1753 | 48.086 | 0.0503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Shao, Z.; Ma, L.; Guo, R. Clinical Severity of SARS-CoV-2 Variants during COVID-19 Vaccination: A Systematic Review and Meta-Analysis. Viruses 2023, 15, 1994. https://doi.org/10.3390/v15101994
Yuan Z, Shao Z, Ma L, Guo R. Clinical Severity of SARS-CoV-2 Variants during COVID-19 Vaccination: A Systematic Review and Meta-Analysis. Viruses. 2023; 15(10):1994. https://doi.org/10.3390/v15101994
Chicago/Turabian StyleYuan, Zhilu, Zengyang Shao, Lijia Ma, and Renzhong Guo. 2023. "Clinical Severity of SARS-CoV-2 Variants during COVID-19 Vaccination: A Systematic Review and Meta-Analysis" Viruses 15, no. 10: 1994. https://doi.org/10.3390/v15101994
APA StyleYuan, Z., Shao, Z., Ma, L., & Guo, R. (2023). Clinical Severity of SARS-CoV-2 Variants during COVID-19 Vaccination: A Systematic Review and Meta-Analysis. Viruses, 15(10), 1994. https://doi.org/10.3390/v15101994





