Abstract
Despite the fact that the variola virus is considered eradicated, the search for new small molecules with activity against orthopoxviruses remains an important task, especially in the context of recent outbreaks of monkeypox. As a result of this work, a number of amides of benzoic acids containing an adamantane fragment were obtained. Most of the compounds demonstrated activity against vaccinia virus, with a selectivity index SI = 18,214 for the leader compound 18a. The obtained derivatives also demonstrated activity against murine pox (250 ≤ SI ≤ 6071) and cowpox (125 ≤ SI ≤ 3036). A correlation was obtained between the IC50 meanings and the binding energy to the assumed biological target, the p37 viral protein with R2 = 0.60.
1. Introduction
One of the first infectious diseases defeated by mass vaccination was smallpox, whose causative agent is the variola virus (VARV). Smallpox vaccination has been discontinued since 1980. It is considered that at present more than 50% of the human population does not have immunity against VARV. The World Health Organization considers it necessary to continue to work at finding new low molecules active against VARV [1]. This relates to several reasons, including the possibility of spreading VARV from various types of smallpox burials and the reproduction of VARV or a similar virus for terrorist purposes. [2]. In addition, other orthopoxviruses similar to VARV circulate in nature, such as monkeypox and cowpox viruses, whose mutations can lead to an increase in their pathogenicity for humans. An outbreak of monkeypox in several countries in 2022 brought new public health challenges in addition to the ongoing pandemic of coronavirus disease 2019 (COVID-19). The outbreak has spread to 104 countries on six continents, with the highest incidence in North America and Europe. Monkeypox virus disease has spread rapidly, raising particular concern about human-to-human transmission and community spread in non-endemic regions [3]. Before this, one of the last outbreaks of monkeypox virus diseases in humans was noted in Africa in 2016 [4,5].
Currently, ST-246 (Tecovirimat, TPOXX®) [6] (Figure 1) is approved in the USA for the treatment of smallpox and monkeypox. ST-246 was developed by SIGA Technologies Inc. (New York, NY, USA). In Russia, NIOCH-14 is officially registered as the antipox drug, whose action mechanism is the same as that of Tekovirimat. It should be noted that NIOH-14 is a prodrug of ST-246 [7].
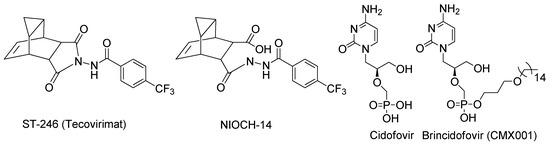
Figure 1.
Some compounds currently approved for the treatment of VARV or are in clinical trials.
Another smallpox drug, CMX001 (Brincidofovir), was approved by the US FDA in June 2021 for the treatment of smallpox in humans [8,9]. CMX001 is a lipophilic nucleotide analogue of Cidofovir (Cidofovir, CDV, Vistide®), which is an antiviral drug used for the treatment of cytomegalovirus retinitis, active in lethal models of orthopoxvirus infection in mice and monkeys [10] (Figure 1). Low oral bioavailability and significant nephrotoxicity were demonstrated for the Cidofovir. Moreover, it was shown to be ineffective when used after the onset of smallpox lesions in VARV-infected monkeys. In the case of CMX001, it was found that it does not provide a sufficient level of protection against a lethal infection of mice with the ectromelia virus in experiments with small rodents [11].
In 2010, the World Health Organization (WHO) published the report “Scientific Review of Research on Variola Virus 1999–2010” [10] and comments of the WHO Advisory Group of Independent Experts on Smallpox Programme Review (AGIES) concerning this issue [12]. AGIES experts noted that VARV was experimentally shown to develop resistance to CMX001 and Tecovirimat and further investigations are necessary to find new agents with different mechanisms of action.
Compounds containing an adamantane fragment demonstrated a wide range of biological activity, including antiviral activity [13]. In particular, semicarbazone 1 containing an isatin fragment, was found to be a vaccinia virus inhibitor (Figure 2), leading to a 42% reduction in vaccinia virus reproduction after a three-hour exposure in cell culture at 2 μg/mL concentration [14]. Some secondary and tertiary amines containing an adamantane fragment, such as compounds 2, 3, and tromantadine 4, demonstrated pronounced vaccinia virus inhibitory effects [15]. It was shown in [16] that diphenyl derivative amide 5 inhibits vaccinia virus reproduction in cell culture in the range from 72% to 100% at a concentration of 2 µg/mL. However, the activity of these compounds against other types of orthopoxviruses was not investigated. Interestingly, there are data concerning the activity of Tecovirimat derivative 6 on orthopoxviruses obtained by replacing the aromatic fragment with adamantyl [17]. For 6, it was shown that concentrations below 0.05 μM are effective against vaccinia and cowpox viruses.
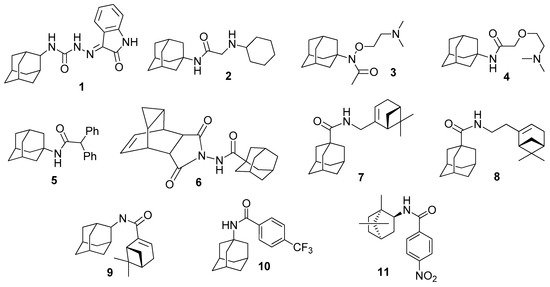
Figure 2.
A number of adamantane or bicyclic monoterpene derivatives (1–11), active against orthopoxviruses.
The activity of 1- and 2-aminoadamantane amides containing monoterpene acid fragments against vaccinia, cowpox, and mousepox viruses was shown in [18]. The highest activity simultaneous with low toxicity was demonstrated for amides 7–9 (Figure 2). The highest selectivity indices (SI), i.e., the ratio of TC50 (test compound concentration at which 50% of cells in the non-infected monolayer are destroyed) to IC50 (concentration of the compound at which 50% of cells in the infected monolayer are not destroyed and remain viable) were shown for 7, being from 406 (for cowpox virus) to 1123 (for vaccinia virus). At the same time, the selectivity index for the vaccinia virus in the reference drug cidofovir was 12. It should be noted that the exhibited activity critically depended on the type of terpene substituent [18].
On the other hand, introduction of aromatic fragments instead of monoterpene fragments into the compounds seems to be a promising approach, in particular due to the relatively greater availability of the starting compounds. It is important that the structure of Tecovirimat, which demonstrates a high activity against poxviruses, contains a fragment of 4-trifluoro-substituted benzoic acid. In [19], the synthesis and investigation of the activity against orthopoxviruses for a wide range of aromatic amides containing adamantane fragments were described with the selectivity index of 2325 in the case of the 10.
It is also necessary to mention the article [20], which describes a synthesis of a number of derivatives, combining fragments of benzoic acids and camphor or fenchone scaffolds (adamantane bioisosteres). In particular, for amide of an endo-bornylamine derivative 11, a selectivity index of 31,500 was shown.
Molecular modeling of interactions between the adamantane amide derivatives and the proposed molecular target—the p37 protein encoded by the F13L gene [21]—was performed in [19]. The p37 protein plays an important role in the viral envelope formation and the virus release from the host cell [22,23,24]. For the protein, a wide spectrum of lipase activity was shown as well [25]. The p37 was previously shown to be the molecular target of Tecovirimat [26], however, its mechanism of action and the binding site with p37 were not elucidated. Nevertheless, in [19] the binding site was chosen based on the molecular modeling results and the assumption that p37 exhibits phospholipase activity. In particular, based on the presence of the HKD domain (H—histidine; K—lysine; D—asparagine) in p37, this protein was compared with phospholipases and the binding site was used to describe the antiviral activity mechanism of a number of compounds containing an adamantane fragment. On the other hand, the adamantane fragment is similar geometrically and by lipophilicity to the cage fragment of Tecovirimat, which can determine the adamantane derivatives activity against orthopoxviruses.
Thus, the data indicates the importance of further investigations aimed at the search for new, safe, and effective agents active against orthopoxviruses. The goal of this work was to synthesize both previously described and new amides of aromatic carboxylic acids containing adamantane fragments and to investigate their biological activity against a wide range of orthopoxviruses. Special attention was paid to the crystal structures of the synthesized substances and discussion of the proposed action mechanism of the compounds.
2. Materials and Methods
2.1. Chemistry
2.1.1. General Information
All chemicals were purchased from commercial vendors and used without further purification, unless indicated otherwise. 1H- and 13C-NMR spectra were registered on a Bruker Avance—III 400 (400.13 MHz (1H) and 100.71 MHz (13C) in CDCl3). Chemical shifts obtained are given in ppm, relative to residual chloroform (δH 7.24, δC 76.90 ppm), and J are given in Hz. Numeration of atoms in the compounds (see Supplementary Materials, Figure S1) is given for assigning the signals in the NMR spectra and does not coincide with the nomenclature of compounds. The elemental composition of compounds was determined from high-resolution mass spectra (HR-MS) recorded on a DFS Thermo Scientific spectrometer in full scan mode (0–500 m/z, 70 eV electron impact ionization, direct sample injection). The conversion of reagents and the purity of the target compounds were determined using gas chromatography methods: 7820A gas chromatograph (Agilent Tech., Santa Clara, CA, USA), flame-ionization detector, HP-5 capillary column (0.25 mm × 3 m × 0.25 μm), helium carrier gas (flow rate 2 mL/min, flow division 99:1), temperature range from 120 °C to 280 °C, heating of 20 °C/min. The purity of the target compounds for biological testing was confirmed to be more than 95%.
Suitable for XRD crystals of 12a, 12b, 15a, 18a, 16b, 17b were obtained by slow evaporation of Et2O solution; 14a, 16a, 12b, 13b, 18b were obtained from hot EtOH solution and 17a was obtained from hot toluene solution.
XRD of 12a–18a, 12b, 13b, 15–18b was performed on Oxford Gemini R Ultra (Rigaku Oxford Diffraction) with MoKα (λ = 0.71073 Å), graphite monochromator, and CCD detector. The reflex intensities were measured by ω-scanning of frames (1°). Absorption correction was applied using CrysAlisPro v.1.171.41.93a [27] by the multi-scan method. The structures were solved and refined using SHELXS [28] and SHELXL [29] with the help of Olex2 version 1.5 [30] software. All non-hydrogen atoms were refined in anisotropic approximation. Hydrogen atom positions were refined freely, anisotropic displacement parameter of hydrogen atoms was Uiso(H) = 1.2·Ueq(atom). Nitro group of 17b structure was disordered, for O2B of a nitro group ISOR was performed, O3B-N2, O3A-N2, N2-O2B, N2-O2A distances were restrained by SADI, as well as for N2-C16-O3B-O2B fragment FLAT was performed with standard deviation. XRD data have been deposited with the Cambridge Structural Database (CCDC) (see Supporting Information, Tables S1–S3) and are available from the authors or at the address: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 1 December 2022).
2.1.2. General Method of Synthesis of Amides 12a,b–18a,b
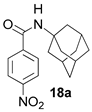
To solutions of 1 eq corresponding benzoic acids chlorides and 2 eq of triethylamine in dry toluene, 1 eq of 1- or 2-aminoadamantanes hydrochlorides was added at 0 °C. The resulted mixtures were stirred for 1 h at 0 °C and 12 h at room temperature. After solvent evaporation, the mixtures were suspended in EtOAc and treated with 5% HCl, 5% NaOH, and saturated NaCl water solutions consequentially with following drying over sodium sulfate. The resulted amides were isolated by crystallization from Et2O. NMR data of 13a,b–16a,b, 19a agree with the literary ones: 12a [31], 12b [32], 13a [33], 13b [34], 14a [35], 14b [36], 15a [37], 15b [36], 18a [38].
2.1.3. N-(adamantan-1-yl)-2-nitrobenzamide 16a
Yield 70%. 1H-NMR (CDCl3): 1.69 (s, 6H, 2H-4, 2H-6, 2H-10); 2.10 (s, 9H, 2H-2, 2H-8, 2H-9, H-3, H-5, H-7); 5.44 (br. s, 1H, H-11); 7.42–7.52 (m, 1H, H-17); 7.50–7.55 (m, 1H, H-18); 7.57–7.66 (m, 1H, H-16); 7.95–8.05 (m, 1H, H-15). 13C-NMR (CDCl3): 52.77 (C-1), 41.33 (C-2, C-9, C-8) 29.24 (C-3, C-5, C-7), 36.07 (C-4, C-10, C-6), 163.92 (C-12), 137.43 (C-13), 147.84 (C-14), 121.30 (C-15), 129.56 (C-16), 132.96 (C-17), 125.47 (C-18). HR MS: 300.1464 (M+, C17H20O3N2+; calculated 300.1468).
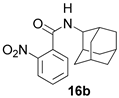
2.1.4. N-(adamantan-2-yl)-2-nitrobenzamide 16b
Yield 57%. 1H-NMR (CDCl3): 1.61–1.71 (m, 2H, H-4, H-9); 1.71–1.79 (m, 4H, H′-4, 2H-6, H′-9); 1.76–1.93 (m, 6H, H-5, H-7, 2H-8, 2H-10); 2.04–2.13 (m, 2H, H-1, H-3); 4.17–4.32 (m, 1H, H-2); 6.02–6.19 (br. d, 1H, J(11, 2) = 6.8 Hz, H-11); 7.47–7.52 (m, 1H, H-18); 7.51–7.58 (m, 1H, H-17); 7.61–7.68 (m, 1H, J(16, 17) = 8.04 Hz, H-16); 7.99–8.07 (m, 1H, H-15). 13C-NMR (CDCl3): 31.39 (C-1, C-3), 53.96 (C-2), 31.69 (C-4, C-9), 26.87 and 26.93 (C-5, C-7), 37.24 (C-6), 36.88 (C-8, C-10), 165.54 (C-12), 133.25 (C-13), 146.18 (C-14), 124.34 (C-15), 130.10 (C-16), 133.55 (C-17), 128.63 (C-18). HR MS: 300.1464 (M+, C17H20O3N2+; calculated 300.1468).
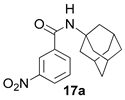
2.1.5. N-(adamantan-1-yl)-3-nitrobenzamide 17a
Yield 37%. 1H-NMR (CDCl3): 1.68–1.73 (m, 6H, 2H-4, 2H-6, 2H-10); 2.07–2.17 (m, 9H, 2H-2, 2H-8, 2H-9, H-3, H-5, H-7); 5.94–5.83 (br. s, 1H, H-11); 7.60 (t, 1H, H-17); 8.05–8.11 (dm, 1H, J(18, 17) = 7.84 Hz, H-18); 8.26–8.33 (dm, 1H, J(16, 17) = 7.84 Hz, H-16); 8.46–8.51 (m, 1H, H-14). 13C-NMR (CDCl3): 52.77 (C-1), 41.33 (C-2, C-9, C-8) 29.24 (C-3, C-5, C-7), 36.07 (C-4, C-10, C-6), 163.92 (C-12), 137.43 (C-13), 121.31 (C-14), 147.84 (C-15), 125.47 (C-16), 129.56 (C-17), 132.96 (C-18). HR MS: 300.1470 (M+, C17H20O3N2+; calculated 300.1468).
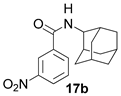
2.1.6. N-(adamantan-2-yl)-3-nitrobenzamide 17b
Yield 34%. 1H-NMR (CDCl3): 1.68–1.74 (m, 2H, H-4, H-9); 1.75–1.86 (m, 4H, H′-4, 2H-6, H′-9); 1.87–1.96 (m, 6H, H-5, H-7, 2H-8, 2H-10); 2.00–2.10 (m, 2H, H-1, H-3); 4.2–4.29 (m, 1H, H-2); 6.38–6.55 (br. d 1H, J (11, 8) = 6.15 Hz, H-11); 7.63 (t, 1H, H-17); 8.08–8.16 (dm, 1H, J (18, 17) = 7.73 Hz, H-18); 8.29–8.37 (dm, 1H, J (16, 17) = 8.04 Hz, H-16); 8.50–8.59 (m, 1H, H-14). 13C-NMR (CDCl3): 31.68 (C-1, C-3), 54.08 (C-2), 31.9 (C-4, C-9), 26.87 and 26.98 (C-5, C-7), 37.24 (C-6), 36.9 (C-8, C-10), 164.17 (C-12), 136.35 (C-13), 121.54 (C-14), 148.01 (C-15), 125.76 (C-16), 129.72 (C-17), 133 (C-18). HR MS: 300.1473 (M+, C17H20O3N2+; calculated 300.1468).
2.1.7. N-(adamantan-2-yl)-4-nitrobenzamide 18b
Yield 79%. 1H-NMR (CDCl3): 1.66–1.74 (m, 2H, H-4, H-9); 1.75–1.85 (m, 4H, H′-4, 2H-6, H′-9); 1.86–1.95 (m, 6H, H-5, H-7, 2H-8, 2H-10); 2.01–2.08 (m, 2H, H-1, H-3); 4.2–4.27 (m, 1H, H-2); 6.38–6.52 (br. d, 1H, J (11, 8) = 6.41 Hz, H-11); 7.86–7.95 (dm, 2H, J (14,15) = J (18, 17) = 8.75 Hz, H-14, H-18); 8.2–8.31 (dm, 2H, J (15,14) = J (17, 18) = 8.7 Hz, H-15, H-17). 13C-NMR (CDCl3): 31.71 (C-1, C-3), 54.07 (C-2), 31.94 (C-4, C-9), 26.87 and 27.01 (C-5, C-7), 37.24 (C-6), 36.9 (C-8, C-10), 164.57 (C-12), 140.77 (C-13), 127.94 (C-14, C-18), 123.73 (C-15, C-17), 149.32 (C-16). HR MS: 300.1471 (M+, C17H20O3N2+; calculated 300.1468).
2.2. Biology
Cytotoxicity and antiviral activity of synthesized amides against vaccinia, cowpox, and mousepox (ectromelia) viruses were evaluated using an adapted colorimetric method in Vero cell culture [39]. The vaccinia virus (strain Copenhagen), cowpox virus (strain Grishak), mousepox virus—ectromelia (strain K-1), obtained from the State collection of pathogens of viral infections and rickettsioses of the SRC VB Vector (Koltsovo, Novosibirsk region, Russia) were used in this work. Viruses were produced in Vero cell culture in DMEM medium (BioloT, Russia). The concentration of viruses in the culture fluid was determined by the method of plaques by titration of the samples in Vero cell culture, calculated and expressed in decimal logarithms of plaque-forming units per ml (log10 PFU per ml). The concentration of the virus in the samples used in this work was from 5.6 to 6.1 log10 PFU per mL. The used series of viruses with the indicated titer was stored at −70 °C. The antiviral efficacy of compounds was evaluated according to the adapted and modified method [39]. The commercially available drug Cidofovir (Cidofovir, Vistide) manufactured by GileadSciencesInc. was used as a reference (Foster City, CA, USA). To evaluate the antiviral activity, firstly, a 50 μL dilution of samples was added to the wells of 96-well plates with a monolayer of cells containing 100 μL of DMEM medium with 2% fetal serum, and then 50 μL of a 1000 PFU per well virus dose was added. The cytotoxicity of compounds was determined based on cell destruction under the derivative influence in the wells, into which no virus was introduced. Cell monolayers in the plate wells were used as controls, into which the virus without compounds was added (virus control) and cell monolayers in the wells, into which neither the virus nor the compound was added (cell culture control). After the incubation of cell monolayers infected with orthopoxvirus and treated with the tested compounds for 4 days, a vital dye “neutral red” was added to the culture medium for 1.5 h. Next, the monolayer was washed twice with saline solution, the lysis buffer was added and after 30 min the optical density (OD), which is an indicator of the number of cells in the monolayer not destroyed in the presence of the virus, was measured on an Emax plate reader (Molecular Devices, San Jose, CA, USA) at 490 nm. The OD values were used to calculate a 50% cytotoxic concentration (CC50 μM) and a 50% virus inhibiting concentration (IC50 μM) using the SoftMax Pro-4.0 computer program. Based on these indicators, the selectivity index (SI) was calculated: SI = TC50/IC50.
2.3. Molecular Modeling
All theoretical calculations were carried out using software Schrodinger Small Molecule Drug Discovery Suite 2022-1 [40]. The geometric parameters of the ligands were optimized in the OPLS4 [41] force field considering all possible conformations. To perform the AIM analysis, amides 16a,b were additionally optimized at the DFT level using M062 functional [42] with GD3 dispersion correction [43] and 6-311 + G(d,p) basis set [44]. The AIM analysis and estimation of hydrogen bonds strength was carried out using software Multiwfn version 2.1.2 [45].
For molecular modeling, we used geometric parameters of the p37 protein obtained as a result of folding [20]. We considered the cavity in the protein as a proposed binding site, as described in [19,20]. In particular, Phe52, Leu118, Cys120, Ser135, Asn312, Lys314, Asn329, and Asp331 amino acids were considered to form the binding site. For molecular docking we used the reference ligand–protein model (ST-246 [26,46]—p37), obtained as a result of molecular modeling described in [20]. Molecular docking was performed using the forced ligand positioning protocol (IFD) [47,48,49] with the following conditions: flexible protein and ligand; grid matrix size of 20 Å; amino acids (within a radius of 5 Å from the ligand) restrained and optimized, taking into account the influence of the ligand; the maximum number of positions was limited to 20; docking solutions were ranked by evaluating the following calculated parameters: docking score (based on Glide score minus penalties); parameter of model energy value (Emodel), including Glide score value, energy unrelated interactions, and the parameters of energy spent on formation of the laying of the compound in the binding site and binding energy of ligand and protein (IFD score). The absence or minimum number of unfavorable clash interactions was taken into account during the analysis of protein–ligand interactions. Binding energies (ΔGMM-GBSA) for ligand–protein complexes were estimated using variable-dielectric generalized Born model for best docking positions, with water taken as a solvent. The influence of amino acid residues located at a distance of 5 Å on the ligand was considered.
3. Results and Discussion
3.1. Organic Synthesis
Firstly, the synthesis of a number of aromatic amides containing adamantane fragments was performed. Substances 12a,b–18a,b were obtained by interaction of substituted aromatic carboxylic acid chlorides with 1- and 2-aminoadamantane hydrochlorides in toluene in the presence of two excesses of triethylamine (Scheme 1). After reaction mixture treatment and crystallization of the products from diethyl ether no additional purification was needed.
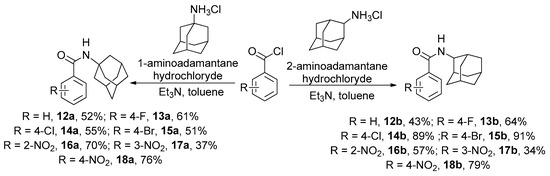
Scheme 1.
Synthesis of amides 12a,b–18a,b.
It should be mentioned that the synthesis of some of the obtained amides was previously described in [31,32,33,34,35,36,37,38].
To reveal the influence of the linker type between the adamantane fragment and the aromatic one, bromantane was synthesized by reductive amination of adamantan-2-one with 4-bromoaniline under the conditions of the Leuckart–Wallach reaction according to the procedure [50] with a yield of 68% (Scheme 2).
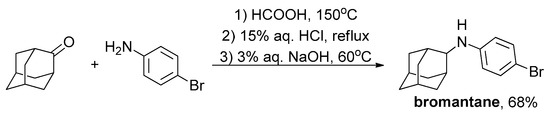
Scheme 2.
Bromantane synthesis.
3.2. X-ray Analysis of Single Crystals
The structures of all compounds except 12a [51] were determined for the first time. Compounds 12a–15a were crystallized in orthorhombic space group Pbca and they were isostructural. Their molecular structure consists of an adamantan-1-yl group, attached to N benzamides with different substituents in the 4th position: hydrogen, fluorine, chlorine, and bromine. Cell packing shows zigzag chains along the c-axis direction resulting from weak N-H⋯O hydrogen bonds (Figure 3A). The molecules are staggered and the angle of deviation between the planes that pass through the aromatic rings is 47.6°, 48.5°, 56.4°, and 58.7° for 12a, 13a, 14a, and 15a, respectively (Figure 3B).
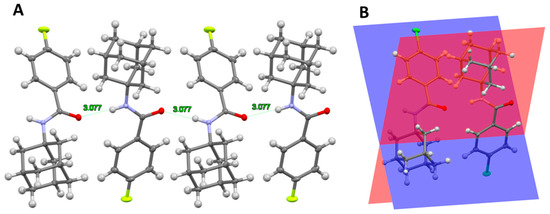
Figure 3.
(A) A partial packing plot of 13a showing zigzag chains along the c-axis direction resulting from weak N-H⋯O contacts; (B) a partial packing plot of 14a showing the angle of deviation between the planes that pass through the aromatic rings.
Compounds 13a, 16a, 16b, 17b, 18a, and 18b were crystallized in the monoclinic space group P21/c, 17a was crystallized in the non-centrosymmetric space group Pc, and 12b was crystallized in the triclinic space group P-1. The asymmetric unit cell of 12b and 17a contains 4 and 2 molecules, and the others have one independent molecule. All structures except 17a have N-H⋯O contacts which can be classified as weak hydrogen bonds. For these compounds, the N⋯O distance is from 2.971 to 3.151 Å, while in 17a these contacts have lengths of 3.254 Å and 3.262 Å. 4-F and 2-NO2 groups of 13b, 16a, and 16b compounds are not involved in the formation of hydrogen bonds or short contact. The 3-NO2 group of 17a and 17b has weak O-π interactions with the aromatic ring neighboring molecule. Compounds 13a, 14a, and 15a have halogen⋯π interaction, 17a and 17b have π-π interaction. Compounds 18a and 18b form a short contact between 4-NO2-group and H-atoms neighboring molecules of two different types: in 18a a non-planar cycle of four molecules and in 18b a planar network of multiple molecules are formed (Figure 4a,b). The crystal structures of all compounds have a lot of different types of intermolecular interactions and most of these structures (except 12a–15a) have different spatial organization.
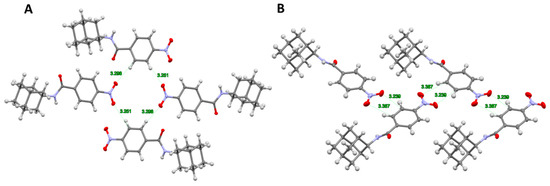
Figure 4.
A partial packing plot of 18a (A) and 18b (B) showing a short contact between 4-NO2-group and H-atoms neighboring molecules.
The X-ray analysis of single crystals is a widely used method to determine crystal structures of subsequent compounds with atomic resolution. These data can be used in future investigations of drug design by modifying the structure to increase the bioavailability of substances and polymorph screening. The performed experiments allow us to obtain atomic coordinates of the studied molecules, thus, to verify structure optimization and docking results.
3.3. Biology
The activity of the obtained compounds against orthopoxviruses was investigated using vaccinia viruses (Copenhagen strain); the values of inhibitory activity (IC50) and cytotoxicity (TC50) were evaluated using an adapted colorimetric method in Vero cell culture [39] (Table 1). Cidofovir (Heritage Consumer Products, LLC, East Brunswick, NJ, USA) was used as a positive control.

Table 1.
TC50, IC50, and SI values for amides 12a,b–18a,b and bromantane.
It should be noted that 15b was previously investigated for activity against orthopoxviruses, in particular, against the vaccinia virus, as well as the cowpox and ectromelia viruses [19] with IC50 of 3.06 µM and TC50 > 299.2 µM. In our experiments, the activity of 15b was confirmed, and even better IC50 and TC50 values were obtained.
Most of the studied compounds showed significant activity against the vaccinia virus with IC50 of up to 0.03 µM for 18a. Isomeric derivatives of adamantane substituted at the 1- and 2-positions showed similar activity, a significant difference was obtained only in the case of 13a,b. However, there was a slight trend towards an increase in the activity of 1-aminoadamantane derivatives. In most cases, except for derivatives of meta-nitrobenzoic acid 17a,b, 1-aminoadamantane amides were shown to be significantly less cytotoxic than isomeric derivatives of 2-aminoadamantane. Thus, these trends led to a significantly higher selectivity index for 1-substituted adamantane derivatives 12a–15a, 18a.
Regarding the substitution position of the aromatic fragment, it can be noted that the compounds containing the aromatic fragment with para-substituents showed the highest activity, in particular, when comparing unsubstituted derivatives 12a,b, as well as nitro derivatives 16a,b–18a,b. At the same time, chlorine and bromine derivatives demonstrated similar activity (IC50 equal to 0.13–0.18 µM), while nitro-derivatives 18a,b were found to be almost five times more active (IC50 equal to 0.03 and 0.04 µM) and significantly less cytotoxic than meta-nitro substituted amides 17a,b, which led to the highest selectivity indices of 18,214 and 12,300, respectively.
It should be noted that the obtained compounds are similar to Tecovirimat in their structure; in particular, they contain a bulky lipophilic scaffold and an aromatic fragment. Interestingly, the synthesis and investigation of activity against poxviruses of Tecovirimat analogues containing 4-fluorine 19, 4-chloro- 20, 4-bromo- 21, and 4-nitro-phenyl 22 fragments (Figure 5) are described in [17,52] with 19–22 being structurally similar to derivatives 13a,b–15a,b, 18a,b.
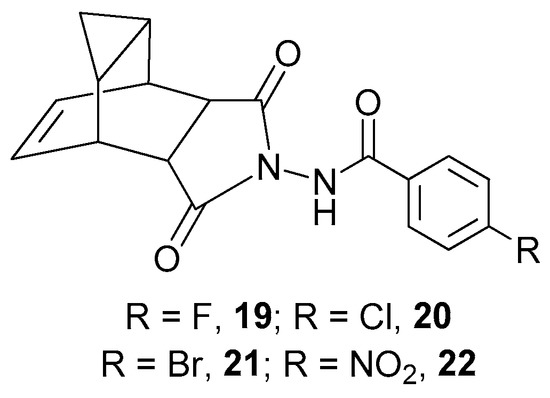
Figure 5.
Tecovirimat derivatives 19–22 with aromatic substituent, contained in 13a,b–15a,b, 18a,b.
The Tecovirimat analogs 19–22 demonstrated activity against vaccinia virus with IC50 < 0.05 μM for 19, and IC50 of 0.02 μM for 20–22. The obtained adamantane derivatives showed generally less activity except the compound 18a with IC50 of 0.03 µM, which is similar to the IC50 of the Tecovirimat analog 22. At the same time, 18a is much easier to synthesize than 22.
It is also worth noting that amides of 4-nitrobenzoic acid 18a,b demonstrated the highest activity, which also agreed with the results obtained in [20], where amide 11 with SI = 31,500 showed the highest activity (Figure 2).
Comparing bromantane with structurally similar 4-bromine substituted amide 15b one may conclude that bromantane was found to be significantly less active (IC50 of 0.22 µM for 15b against 38.8 µM for bromantane) with higher cytotoxicity (TC50 of 129.67 µM for 15b against 86.6 µM for bromantane). It seemed possible that acyl linker led to an increase in activity, but at the same time the linked fragments played the key role in determining the presence of antipox activity.
The most active compounds 13a–15a, 18a,b were investigated for activity against cowpox (CPXV) and ectromelia (ECTV) viruses (Table 2).

Table 2.
Antipox activity of 13a–15a, 18a,b against CPXV and ECTV.
For the activity of compounds 13a–15a, 18a,b, the same patterns were obtained as in the case of vaccinia virus, while lower values of the selectivity index were obtained as a result of higher IC50 in relation to cowpox and ectromelia viruses. At the same time, as well as the reference compound cidofovir, the compounds showed higher activity against the ectromelia virus than against the cowpox virus.
3.4. Molecular Docking
The structures of inactive derivatives 16a,b differ from active derivatives 17, 18a,b in the nitro group position. Obviously, the activity of investigated compounds relates to their structural features. The presence of a nitro group in the ortho-position could result in the formation of various intermolecular hydrogen bonds between the oxygen atoms -NO2 and hydrogen of the adamantane fragment. The results of AIM (atoms in molecules) analysis 16a,b allowed a determination of the bond critical points between the atoms of interest. Very weak dispersion interactions (less than −2.5 kcal/mol) were obtained between pairs of atoms 22 and 40 (Figure 6, compound 16a), 22–40 and 22–42 (Figure 6, compound 16b). Weak electrostatic interactions (from −2.5 to −14.0 kcal/mol) were shown between atoms 22 and 7 in both structures. The shown weak dispersion interactions in ortho-substituted structures could shield the adamantane fragment or limit the flexibility of molecules 16a,b.
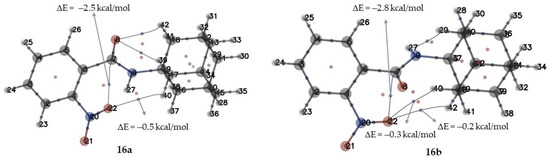
Figure 6.
AIM analysis and estimation of hydrogen bonds strength results: two types of critical points are shown: bond critical points (3; −1) correspond to blue points between nuclei; critical points in the ring (3; +1) correspond to red points; ΔE—value of hydrogen bonds strength.
Molecular docking methods were used to evaluate the affinity of the studied structures to the p37 binding site. The choice of the target was based on the structural similarity of investigated compounds 12a,b–18a,b with derivatives that exhibited antiviral activity against orthopoxviruses due to interaction with p37. In particular, a number of potent antipox agents contained a rigid hydrophobic scaffold (Figure 2 and Scheme 1) [19,20]. We considered the cavity containing Phe52, Leu118, Cys120, Ser135, Asn312, Lys314, Asn329, and Asp331 amino acids as a binding site as it was described in [19,20]. For the molecular docking procedure we used a ligand–protein reference model (Tecovirimat [26,46]—p37), obtained as a result of molecular modeling described in [20].
All the obtained compounds demonstrated activity against orthopoxviruses except 16a, which binds the considering cavity with the formation of a series of intermolecular interactions. For active compounds with 18a being the leader (Figure 7A), realization of the maximum possible number of docking positions was observed (at least 13, Table S3). For inactive 16b, only five docking positions were registered.
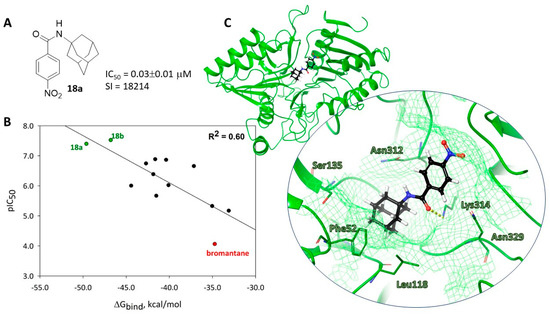
Figure 7.
Molecular docking results: (A)—lead-compound; (B)—correlation between experimental and molecular docking data; (C)—location lead-compound in binding site.
For most compounds, no unfavorable clash interactions were obtained for optimal docking positions except 13a, 16b, 17b, and bromantane. The energy parameters (docking score, Emodel, IFD-score) characterizing the affinities of ligands to the binding site were generally comparable, however, inactive compound 16b was characterized by the maximum binding energy ΔGbind. The results of the biological experiments and the binding energies of ligands and protein correlated with an index of 0.60 (Figure 7B). Since the results of the in vitro experiments are the evaluation of antiviral activity only against vaccinia viruses, and not a study of compound affinity to the specific biological target, the obtained value of the correlation index can be considered quite satisfactory.
The most active compound 18a is located in the binding site with the formation of a hydrogen bridge between the carbonyl oxygen atom of the ligand and the hydrogen atom of the Lys314 NH group. The adamantane fragment of the molecule was surrounded by hydrophobic amino acids such as Phe52, Leu118, Cys12, and Ala134 (Figure 7C).
4. Conclusions
A number of adamantane derivatives were obtained; in particular, benzoic acid amides containing an adamantane fragment substituted at the first and second positions were synthesized. Some of the derivatives were not previously described. The structures of most of the obtained compounds were confirmed using the X-ray diffraction method with obtained crystal structures of 12a–18a, 12b, 13b, 16b–18b. Almost all compounds had different crystal packings and systems of hydrogen bonds; amides 12a–15a were shown to be isostructural. A number of compounds demonstrated the antiviral activity against vaccinia virus with a selectivity index of up to 18,214 for 18a. The most active compounds were tested for activity against cowpox (SI = 3036 for 18a) and ectromelia (SI = 6071 for 18a) viruses. Molecular modeling of interactions for obtained adamantane derivatives with the p37 viral protein considered as a purposed molecular target was performed at the correlation index of the binding energy and IC50 equal to 0.60.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15010029/s1, Table S1: Crystallographic characteristics, details of the experiments and structure refinement for compounds 12a–15a; Table S2: Crystallographic characteristics, details of the experiments and structure refinement for compounds 16a–18a, 12b; Table S3: Crystallographic characteristics, details of the experiments and structure refinement for compounds 13b, 16–18b; Table S4: Molecular docking results; Figure S1. Numeration of atoms in the compounds 16a,b, 17a,b, 18b.
Author Contributions
Chemistry investigation, E.S.M., D.A.R., E.V.S., A.A.V. and M.B.N.; crystallography investigation D.S.K. and S.G.A.; biological investigation, N.I.B., L.N.S., O.A.S., R.V.B., A.P.A. and R.A.M.; molecular modeling E.M.K., S.S.B. and E.S.M.; methodology, O.I.Y., K.P.V. and N.F.S.; project administration O.I.Y.; supervision, E.V.S., O.I.Y., S.S.B. and S.G.A.; writing—original draft preparation E.S.M., E.V.S., S.S.B. and D.S.K.; writing—review and editing, E.V.S. and O.I.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Synthesis of compounds supported by the Russian Ministry of Education and Science No. 1021052605829-1-1.4.1. Biological testing in the orthopoxviruses has been carried out with the State assignment of the State Research Centre of Virology and Biotechnology VECTOR. Crystallographic research and molecular modeling were supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2021-1355 dated 12 October 2021) as part of the implementation of certain activities of the Federal Scientific and Technical Program for the Development of Synchrotron and Neutron Research and Research Infrastructure for 2019–2027.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements. Single-crystal X-ray diffraction data were collected with the equipment of the XRD Facility of the Laboratory of Molecular Design and Ecologically Safe Technologies (MDEST) of the Scientific Educational Center “Institute of Chemical Technology”, Novosibirsk State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The Independent Advisory Group on Public Health Implications of Synthetic Biology Technology Related to Smallpox. Available online: https://apps.who.int/iris/rest/bitstreams/883140/retrieve (accessed on 1 December 2022).
- Noyce, R.S.; Lederman, S.; Evans, D.H. Construction of an Infectious Horsepox Virus Vaccine from Chemically Synthesized DNA Fragments. PLoS ONE 2018, 13, e0188453. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Ophinni, Y.; Megawati, D.; Frediansyah, A.; Mamada, S.S.; Salampe, M.; Bin Emran, T.; Winardi, W.; Fathima, R.; Sirinam, S.; et al. Monkeypox: A Comprehensive Review. Viruses 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Abubakar, I.; Ihekweazu, C.; Heymann, D.; Ntoumi, F.; Blumberg, L.; Asogun, D.; Mukonka, V.; Lule, S.A.; Bates, M.; et al. Monkeypox—Enhancing Public Health Preparedness for an Emerging Lethal Human Zoonotic Epidemic Threat in the Wake of the Smallpox Post-Eradication Era. Int. J. Infect. Dis. 2019, 78, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, D.B.; Eckburg, P.B. Human Monkeypox: An Emerging Zoonosis. Lancet Infect. Dis. 2004, 4, 15–25. [Google Scholar] [CrossRef]
- FDA Approves the First Drug with an Indication for Treatment of Smallpox. FDA News Release. Available online: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm613496.htm (accessed on 1 December 2022).
- Mazurkov, O.Y.; Kabanov, A.S.; Shishkina, L.N.; Sergeev, A.A.N.A.; Skarnovich, M.O.M.A.; Bormotov, N.I.; Skarnovich, M.O.M.A.; Ovchinnikova, A.S.; Titova, K.A.; Galahova, D.O.; et al. New Effective Chemically Synthesized Anti-Smallpox Compound NIOCH-14. J. Gen. Virol. 2016, 97, 1229–1239. [Google Scholar] [CrossRef]
- Hoy, S.M. Tecovirimat: First Global Approval. Drugs 2018, 78, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Chimerix Receives U.S. Food and Drug Administration Approval for TEMBEXA®(Brincidofovir) for the Treatment of Smallpox. Available online: https://ir.chimerix.com/news-releases/news-release-details/chimerix-receives-us-food-and-drug-administration-approval (accessed on 1 December 2022).
- Scientific Review of Variola Virus Research, 1999–2010. Available online: https://apps.who.int/iris/handle/10665/70508 (accessed on 1 December 2022).
- Parker, S.; Chen, N.G.; Foster, S.; Hartzler, H.; Hembrador, E.; Hruby, D.; Jordan, R.; Lanier, R.; Painter, G.; Painter, W.; et al. Evaluation of Disease and Viral Biomarkers as Triggers for Therapeutic Intervention in Respiratory Mousepox—An Animal Model of Smallpox. Antivir. Res. 2012, 94, 44–53. [Google Scholar] [CrossRef]
- Advisory Group of Independent Experts to Review the Smallpox Research Programme (AGIES). Comments on the Scientific Review of Variola Virus Research, 1999–2010. Available online: https://apps.who.int/iris/handle/10665/70509 (accessed on 1 December 2022).
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef]
- Kreutzberger, A.; Schröders, H.-H.; Stratmann, J. 4-(2-Adamantyl)Thiosemicarbazone. Arch. Pharm. 1984, 317, 767–771. [Google Scholar] [CrossRef]
- May, G.; Peteri, D. Synthesis and Antiviral Effects of Adamantan Derivatives. Arzneimittelforschung 1973, 23, 718–721. [Google Scholar]
- Kreutzberger, A.; Schröders, H.-H. Die Aliphatische Säureamidgruppierung Als Partialstruktur in Virustatika 3. Mitt. Antivirale Wirkstoffe. Arch. Pharm. 1974, 307, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.F.; Bailey, T.R.; Rippin, S.R.; Dai, D. Chemicals, Compositions, and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 2007287735A1, 13 December 2007. [Google Scholar]
- Suslov, E.V.; Mozhaytsev, E.S.; Korchagina, D.V.; Bormotov, N.I.; Yarovaya, O.I.; Volcho, K.P.; Serova, O.A.; Agafonov, A.P.; Maksyutov, R.A.; Shishkina, L.N.; et al. New Chemical Agents Based on Adamantane–Monoterpene Conjugates against Orthopoxvirus Infections. RSC Med. Chem. 2020, 11, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, V.A.; Skomorohov, M.Y.; Leonova, M.V.; Bormotov, N.I.; Serova, O.A.; Shishkina, L.N.; Agafonov, A.P.; Maksyutov, R.A.; Klimochkin, Y.N. Adamantane Derivatives as Potential Inhibitors of P37 Major Envelope Protein and Poxvirus Reproduction. Design, Synthesis and Antiviral Activity. Eur. J. Med. Chem. 2021, 221, 113485. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Kovaleva, K.S.; Kuranov, S.O.; Bormotov, N.I.; Borisevich, S.S.; Zhukovets, A.A.; Yarovaya, O.I.; Serova, O.A.; Nawrozkij, M.B.; Vernigora, A.A.; et al. Design, Synthesis, and Biological Evaluation of (+)-Camphor- and (−)-Fenchone-Based Derivatives as Potent Orthopoxvirus Inhibitors. ChemMedChem 2022, 17, e2021007. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Weisberg, A.; Moss, B. Topology of Epitope-Tagged F13L Protein, a Major Membrane Component of Extracellular Vaccinia Virions. Virology 2003, 308, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Sivan, G.; Weisberg, A.S.; Americo, J.L.; Moss, B. Retrograde Transport from Early Endosomes to the Trans -Golgi Network Enables Membrane Wrapping and Egress of Vaccinia Virus Virions. J. Virol. 2016, 90, 8891–8905. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K.M.; Yang, G.; Jordan, R.; Hruby, D.E. The Vaccinia Virus F13L YPPL Motif Is Required for Efficient Release of Extracellular Enveloped Virus. J. Virol. 2007, 81, 7310–7315. [Google Scholar] [CrossRef]
- Grosenbach, D.W.; Ulaeto, D.O.; Hruby, D.E. Palmitylation of the Vaccinia Virus 37-KDa Major Envelope Antigen. J. Biol. Chem. 1997, 272, 1956–1964. [Google Scholar] [CrossRef]
- Baek, S.-H.; Kwak, J.-Y.; Lee, S.H.; Lee, T.; Ryu, S.H.; Uhlinger, D.J.; Lambeth, J.D. Lipase Activities of P37, the Major Envelope Protein of Vaccinia Virus. J. Biol. Chem. 1997, 272, 32042–32049. [Google Scholar] [CrossRef]
- Yang, G.; Pevear, D.C.; Davies, M.H.; Collett, M.S.; Bailey, T.; Rippen, S.; Barone, L.; Burns, C.; Rhodes, G.; Tohan, S.; et al. An Orally Bioavailable Antipoxvirus Compound (ST-246) Inhibits Extracellular Virus Formation and Protects Mice from Lethal Orthopoxvirus Challenge. J. Virol. 2005, 79, 13139–13149. [Google Scholar] [CrossRef]
- Oxford Diffraction/Agilent Technologies CrysAlisPro 2020. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 1 December 2022).
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Joubert, J.; Foxen, E.; Malan, S. Microwave Optimized Synthesis of N-(Adamantan-1-Yl)-4-[(Adamantan-1-Yl)-Sulfamoyl]Benzamide and Its Derivatives for Anti-Dengue Virus Activity. Molecules 2018, 23, 1678. [Google Scholar] [CrossRef] [PubMed]
- Averina, N.V.; Borisova, G.S.; Zefirova, O.N.; Selyunina, E.V.; Zyk, N.V.; Zefirov, N.S. Synthetic Approaches to Physiologically Active Polycyclic Compounds: III. Ritter Reaction with Ketones of the Adamantane and Oxahomoadamantane Series. Russ. J. Org. Chem. 2004, 40, 497–501. [Google Scholar] [CrossRef]
- Mampuys, P.; Ruijter, E.; Orru, R.V.A.; Maes, B.U.W. Synthesis of Secondary Amides from Thiocarbamates. Org. Lett. 2018, 20, 4235–4239. [Google Scholar] [CrossRef] [PubMed]
- Ebdrup, S.; Andersen, H.S. 11Beta-Hydroxysteroid Dehydrogenase Type 1 Active Compounds. WO2007115935A1, 18 October 2007. [Google Scholar]
- Zhdankin, V.V.; McSherry, M.; Mismash, B.; Bolz, J.T.; Woodward, J.K.; Arbit, R.M.; Erickson, S. 1-Amido-3-(1H)-1,2-Benziodoxoles: Stable Amidoiodanes and Reagents for Direct Amidation of Organic Substrates. Tetrahedron Lett. 1997, 38, 21–24. [Google Scholar] [CrossRef]
- Morozov, I.S.; Artsimovich, N.G.; Klimova, N.V.; Fadeeva, T.A.; Zajtseva, N.M.; Galushina, T.S.; Bykov, N.P.; Pjatin, B.M.; Avdjunina, N.I.; Shcherbakova, O.V.; et al. 2-(Para-Brombenzoyl) or 2-(Para-Chlorobenzoyl)-Aminoadamantanes Increasing Organism Resistance to Environment Inhabitation Extremal Factor Effect and Showing Immunostimulating Activity. SU1646256A1, 10 June 1996. [Google Scholar]
- Bayguzina, A.R.; Lutfullina, A.R.; Khusnutdinov, R.I. Synthesis of N-(Adamantan-1-Yl)Carbamides by Ritter Reaction from Adamantan-1-Ol and Nitriles in the Presence of Cu-Catalysts. Russ. J. Org. Chem. 2018, 54, 1127–1133. [Google Scholar] [CrossRef]
- Hamstra, D.F.J.; Lenstra, D.C.; Koenders, T.J.; Rutjes, F.P.J.T.; Mecinović, J. Poly(Methylhydrosiloxane) as a Green Reducing Agent in Organophosphorus-Catalysed Amide Bond Formation. Org. Biomol. Chem. 2017, 15, 6426–6432. [Google Scholar] [CrossRef]
- Selivanov, B.A.; Tikhonov, A.; Belanov, E.F.; Bormotov, N.I.; Kabanov, A.S.; Mazurkov, O.Y.; Serova, O.A.; Shishkina, L.N.; Agafonov, A.P.; Sergeev, A.N. Synthesis and Antiviral Activity of 1-Aryl-3-{3,5-Dioxo-4- Azatetracyclo[5.3.2.02,6.08,10]-Dodec-11-En-4-Yl}ureas. Khimiko Farmatsevticheskii Zhurnal 2017, 51, 13–17. [Google Scholar] [CrossRef]
- Schrodinger, M. Small Molecule Drug Discovery Suite; Schrödinger LLC: New York, NY, USA, 2020. [Google Scholar]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Grosenbach, D.W.; Honeychurch, K.; Rose, E.A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D.E. Oral Tecovirimat for the Treatment of Smallpox. N. Engl. J. Med. 2018, 379, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an Induced Fit Receptor Structure in Virtual Screening. Chem. Biol. 2006, 67, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New Insights about HERG Blockade Obtained from Protein Modeling, Potential Energy Mapping, and Docking Studies. Bioorg. Med. Chem. 2006, 14, 3160–3173. [Google Scholar] [CrossRef]
- Babushkin, A.S.; Navrotskii, M.B.; Novakov, I.A.; Orlinson, B.S.; Robinovich, M.D.; Sheikin, D.S.; Voloboev, S.N. Potential Synthetic Adaptogens. II. Synthesis and Pharmacological Activity of New Conformationally Labile Bromantane Analogs, N-[(Adamantan-1-YL)Methyl]-4-Bromoanilines. Pharm. Chem. J. 2017, 50, 781–787. [Google Scholar] [CrossRef]
- Mescheryakova, E.S.; Bikmukhametov, K.S.; Bayguzina, A.R.; Lutfullina, A.R.; Tulyabaev, A.R.; Khalilov, L.M. X-Ray Diffraction and Theoretical Study of Molecular and Crystal Structure of New Crystalline Aryl- and Alkyl-Substituted N-(Adamantan-1-Yl)Amides: Similarities and Differences. J. Mol. Struct. 2022, 1261, 132783. [Google Scholar] [CrossRef]
- Bailey, T.R.; Rippin, S.R.; Opsitnick, E.; Burns, C.J.; Pevear, D.C.; Collett, M.S.; Rhodes, G.; Tohan, S.; Huggins, J.W.; Baker, R.O.; et al. N-(3,3a,4,4a,5,5a,6,6a-Octahydro-1,3-Dioxo-4,6-Ethenocycloprop[f]Isoindol-2-(1H)-Yl)Carboxamides: Identification of Novel Orthopoxvirus Egress Inhibitors. J. Med. Chem. 2007, 50, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).