Evaluation of Heat Inactivation of Human Norovirus in Freshwater Clams Using Human Intestinal Enteroids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of Temperature Kinetics in Freshwater Clams Subjected to Heat Treatment
2.2. Preparation of HuNoV-Containing Stool Filtrate
2.3. Artificial Inoculation of HuNoV into Freshwater Clams Followed by Heat Treatment and Sample Processing
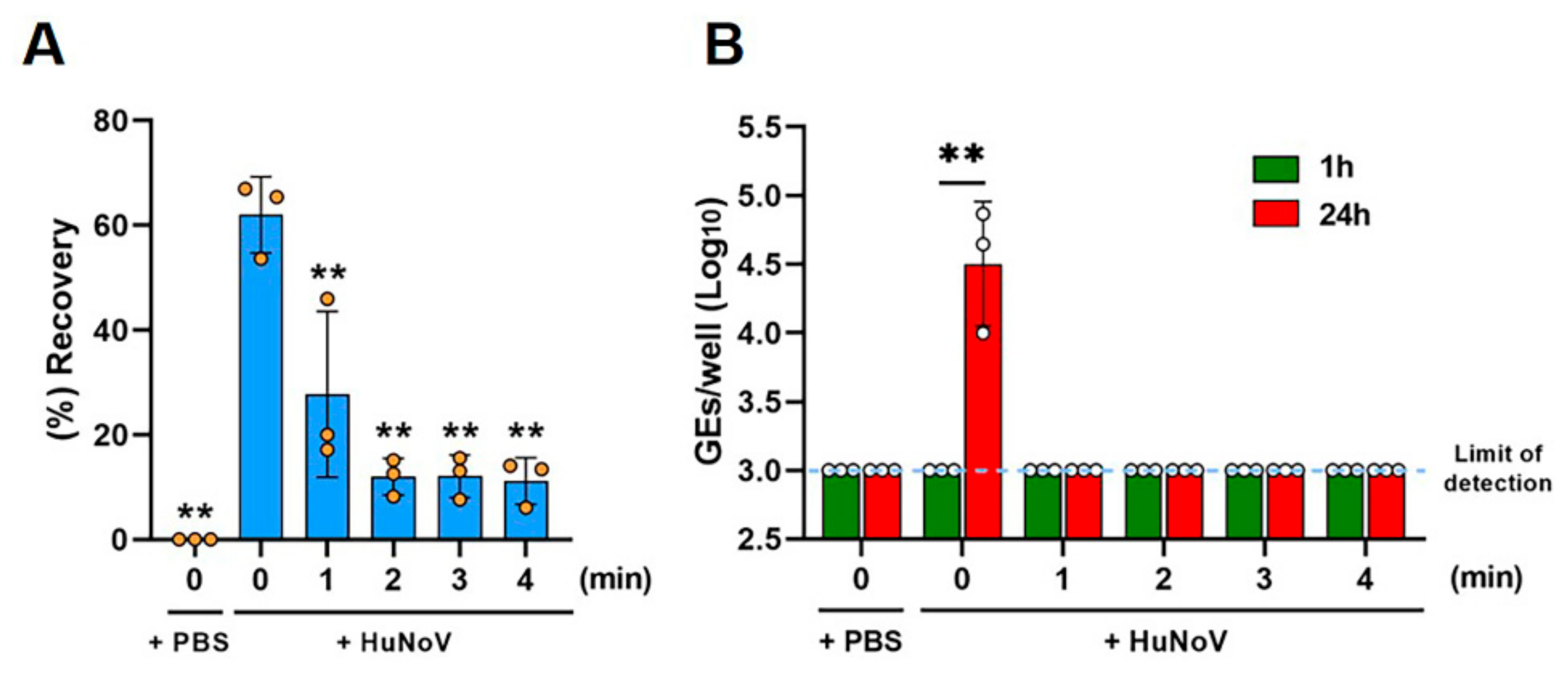
2.4. Evaluation of Infectivity of HuNoV in Clam Extracts Using HIEs
2.5. Evaluation of Viral Recovery Efficiency after Homogenization
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Yoon, Y. Etiological Agents Implicated in Foodborne Illness World Wide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- WHO. Food Safety. 2020. Available online: https://www.who.int/NEWS-ROOM/FACT-SHEETS/DETAIL/FOOD-SAFETY (accessed on 11 November 2021).
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng.pdf?sequence=1 (accessed on 11 November 2021).
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses-The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- Sow, H.; Desbiens, M.; Morales-Rayas, R.; Ngazoa, S.E.; Jean, J. Heat inactivation of hepatitis A virus and a norovirus surrogate in soft-shell clams (Mya arenaria). Foodborne Pathog. Dis. 2011, 8, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, H.; D’Souza, D.H.; Davidson, P.M. Thermal Inactivation of Foodborne Enteric Viruses and Their Viral Surrogates in Foods. J. Food Prot. 2015, 78, 1597–1617. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Watanabe, M.; Zhu, S.; Graves, C.L.; Keyes, L.R.; Grau, K.R.; Gonzalez-Hernandez, M.B.; Iovine, N.M.; Wobus, C.E.; Vinjé, J.; et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014, 346, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Ettayebi, K.; Tenge, V.R.; Cortes-Penfield, N.W.; Crawford, S.E.; Neill, F.H.; Zeng, X.L.; Yu, X.; Ayyar, B.V.; Burrin, D.; Ramani, S.; et al. New Insights and Enhanced Human Norovirus Cultivation in Human Intestinal Enteroids. mSphere 2021, 6, e01136-20. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hisaie, K.; Kurokawa, S.; Suzuki, A.; Sakon, N.; Uchida, Y.; Yuki, Y.; Kiyono, H. Human Norovirus Propagation in Human Induced Pluripotent Stem Cell-Derived Intestinal Epithelial Cells. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 686.e685–688.e685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dycke, J.; Ny, A.; Conceição-Neto, N.; Maes, J.; Hosmillo, M.; Cuvry, A.; Goodfellow, I.; Nogueira, T.C.; Verbeken, E.; Matthijnssens, J.; et al. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019, 15, e1008009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, V.; Morantz, E.K.; Browne, H.; Ettayebi, K.; Zeng, X.L.; Atmar, R.L.; Estes, M.K.; Vinjé, J. Human Norovirus Replication in Human Intestinal Enteroids as Model to Evaluate Virus Inactivation. Emerg. Infect. Dis. 2018, 24, 1453–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, S.; Matsumoto, N.; Hisaie, K.; Uematsu, S. Alcohol abrogates human norovirus infectivity in a pH-dependent manner. Sci. Rep. 2020, 10, 15878. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Murakami, K.; Hirano, J.; Fujii, Y.; Yamaoka, Y.; Ohashi, H.; Watashi, K.; Estes, M.K.; Muramatsu, M. Dasabuvir Inhibits Human Norovirus Infection in Human Intestinal Enteroids. mSphere 2021, 6, e0062321. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Tenge, V.R.; Karandikar, U.C.; Lin, S.C.; Ramani, S.; Ettayebi, K.; Crawford, S.E.; Zeng, X.L.; Neill, F.H.; Ayyar, B.V.; et al. Bile acids and ceramide overcome the entry restriction for GII.3 human norovirus replication in human intestinal enteroids. Proc. Natl. Acad. Sci. USA 2020, 117, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cromeans, T.; Park, G.W.; Costantini, V.; Lee, D.; Wang, Q.; Farkas, T.; Lee, A.; Vinjé, J. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl. Environ. Microbiol. 2014, 80, 5743–5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, C.; Pérez-Rodríguez, F.J.; Sabrià, A.; Beguiristain, N.; Pintó, R.M.; Guix, S.; Bosch, A. Inactivation of Hepatitis A Virus and Human Norovirus in Clams Subjected to Heat Treatment. Front. Microbiol. 2021, 11, 578328. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Qu, L.; Ettayebi, K.; Crawford, S.E.; Blutt, S.E.; Robertson, M.J.; Zeng, X.L.; Tenge, V.R.; Ayyar, B.V.; Karandikar, U.C.; et al. Human norovirus exhibits strain-specific sensitivity to host interferon pathways in human intestinal enteroids. Proc. Natl. Acad. Sci. USA 2020, 117, 23782–23793. [Google Scholar] [CrossRef] [PubMed]
- Guix, S.; Pintó, R.M.; Bosch, A. Final Consumer Options to Control and Prevent Foodborne Norovirus Infections. Viruses 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, T.; Yamaoka, Y.; Ito, A.; Kamaishi, T.; Sugiyama, R.; Estes, M.K.; Muramatsu, M.; Murakami, K. Evaluation of Heat Inactivation of Human Norovirus in Freshwater Clams Using Human Intestinal Enteroids. Viruses 2022, 14, 1014. https://doi.org/10.3390/v14051014
Hayashi T, Yamaoka Y, Ito A, Kamaishi T, Sugiyama R, Estes MK, Muramatsu M, Murakami K. Evaluation of Heat Inactivation of Human Norovirus in Freshwater Clams Using Human Intestinal Enteroids. Viruses. 2022; 14(5):1014. https://doi.org/10.3390/v14051014
Chicago/Turabian StyleHayashi, Tsuyoshi, Yoko Yamaoka, Atsushi Ito, Takashi Kamaishi, Ryuichi Sugiyama, Mary K. Estes, Masamichi Muramatsu, and Kosuke Murakami. 2022. "Evaluation of Heat Inactivation of Human Norovirus in Freshwater Clams Using Human Intestinal Enteroids" Viruses 14, no. 5: 1014. https://doi.org/10.3390/v14051014
APA StyleHayashi, T., Yamaoka, Y., Ito, A., Kamaishi, T., Sugiyama, R., Estes, M. K., Muramatsu, M., & Murakami, K. (2022). Evaluation of Heat Inactivation of Human Norovirus in Freshwater Clams Using Human Intestinal Enteroids. Viruses, 14(5), 1014. https://doi.org/10.3390/v14051014






