Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds
Abstract
1. Introduction
2. VLP Involvement in Intracellular Delivery
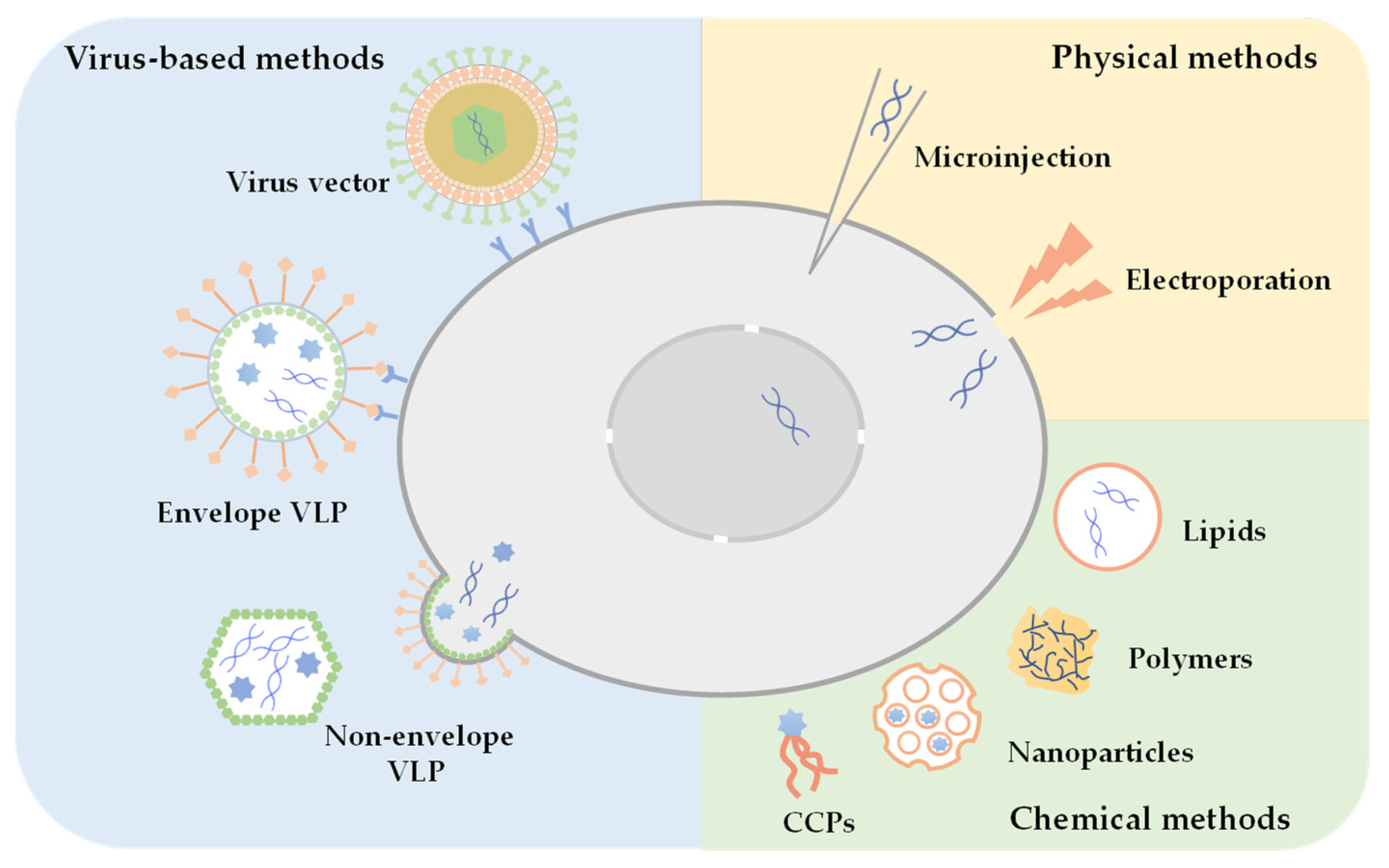
2.1. Strategies for Intracellular Delivery
2.2. VLP-Mediated Intracellular Delivery
2.2.1. VLPs for Protein Delivery
2.2.2. VLPs for Nucleic Acid Delivery
2.2.3. VLPs for Compound Delivery
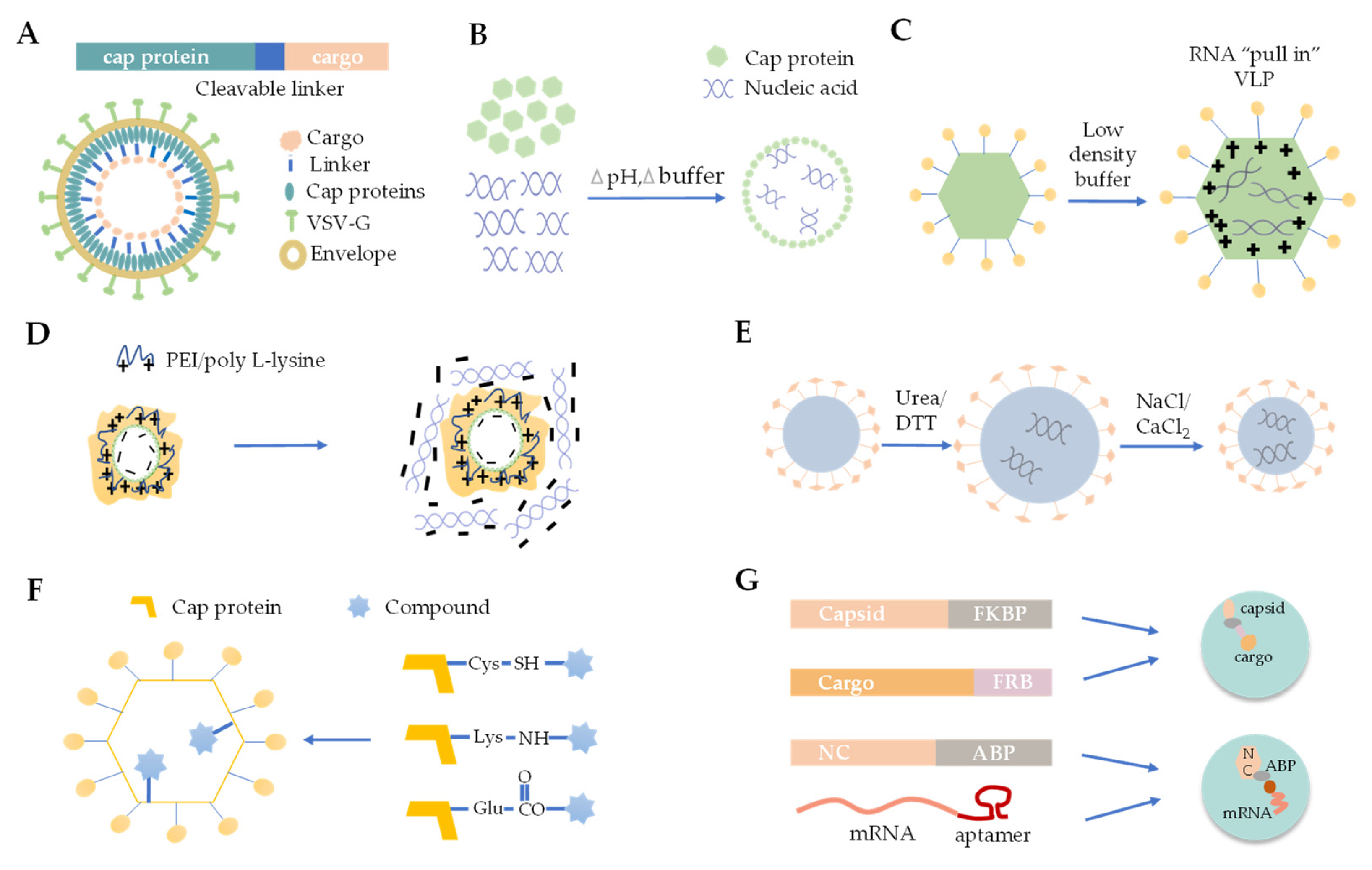
3. The Strategies for Cargo Loading into VLPs
3.1. Foreign Protein Fusion with VLPs
3.2. De Novo Packaging with Nucleic Acids
3.3. Osmotic Shock
3.4. Polymer Mediated Adsorption
3.5. Disassembly and Reassembly
3.6. Chemical Linking
3.7. Physical Interaction between VLP and Cargo
4. The Manufacture and Application of VLPs
4.1. Production, Stability and Immunogenicity of VLP for Cargo Delivery
4.2. VLP-Mediated Cargo Delivery in Biological and Biomedical Research
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Shirbaghaee, Z.; Bolhassani, A. Different Applications of Virus-like Particles in Biology and Medicine: Vaccination and Delivery Systems: Different Applications of Virus-Like Particles. Biopolymers 2016, 105, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wei, Y.-Q.; Guo, H.-C.; Sun, S.-Q. The Application of Virus-like Particles as Vaccines and Biological Vehicles. Appl. Microbiol. Biotechnol. 2015, 99, 10415–10432. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, J.; Tullman-Ercek, D. Production and Applications of Engineered Viral Capsids. Appl. Microbiol. Biotechnol. 2014, 98, 5847–5858. [Google Scholar] [CrossRef]
- Ramqvist, T.; Andreasson, K.; Dalianis, T. Vaccination, Immune and Gene Therapy Based on Virus-like Particles against Viral Infections and Cancer. Expert Opin. Biol. Ther. 2007, 7, 997–1007. [Google Scholar] [CrossRef]
- Pushko, P.; Pumpens, P.; Grens, E. Development of Virus-Like Particle Technology from Small Highly Symmetric to Large Complex Virus-Like Particle Structures. Intervirology 2013, 56, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Suffian, I.F.B.M.; Al-Jamal, K.T. Bioengineering of Virus-like Particles as Dynamic Nanocarriers for in Vivo Delivery and Targeting to Solid Tumours. Adv. Drug Deliv. Rev. 2022, 180, 114030. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, D.; Booth, G.; Gao, W.; Lu, Y. Virus-Like Particle Engineering: From Rational Design to Versatile Applications. Biotechnol. J. 2018, 13, 1700324. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and Methods, Perspectives for Drug Delivery, Gene Therapy and Research: Electroporation: Aspects and Perspectives. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Dean, D.A. Cell-Specific Targeting Strategies for Electroporation-Mediated Gene Delivery in Cells and Animals. J. Membr. Biol. 2013, 246, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Cool, S.K.; Geers, B.; Roels, S.; Stremersch, S.; Vanderperren, K.; Saunders, J.H.; De Smedt, S.C.; Demeester, J.; Sanders, N.N. Coupling of Drug Containing Liposomes to Microbubbles Improves Ultrasound Triggered Drug Delivery in Mice. J. Control. Release 2013, 172, 885–893. [Google Scholar] [CrossRef]
- Yamoah, M.; Moshref, M.; Sharma, J.; Chen, W.C.; Ledford, H.; Lee, J.H.; Chavez, K.; Wang, W.; López, J.; Lieu, D.; et al. Highly Efficient Transfection of Human Induced Pluripotent Stem Cells Using Magnetic Nanoparticles. Int. J. Nanomed. 2018, 13, 6073–6078. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Lin, D.; Nguyen, A.H.; Abdelrasoul, G.N.; Chen, J.; Mar, A.; Qian, F.; Fang, Q.; Kovalchuk, I.; Wang, Y.; et al. Transfection of Difficult-to-Transfect Rat Primary Cortical Neurons with Magnetic Nanoparticles. J. Biomed. Nanotechnol. 2018, 14, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, J.; Zhou, Q.; Zhang, L.; Wang, S.; Zhang, Z.; Yao, C. Advanced Physical Techniques for Gene Delivery Based on Membrane Perforation. Drug Deliv. 2018, 25, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, L.-C. Microinjection as a Tool of Mechanical Delivery. Curr. Opin. Biotechnol. 2008, 19, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.; Wood, M.J. Biological Gene Delivery Vehicles: Beyond Viral Vectors. Mol. Ther. 2009, 17, 767–777. [Google Scholar] [CrossRef]
- Moraes, F.C.; Pichon, C.; Letourneur, D.; Chaubet, F. MiRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics 2021, 13, 1901. [Google Scholar] [CrossRef]
- Schott, J.W.; Galla, M.; Godinho, T.; Baum, C.; Schambach, A. Viral and Non-Viral Approaches for Transient Delivery of MRNA and Proteins. Curr. Gene Ther. 2011, 11, 382–398. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as Novel Bio-Carriers for Gene and Drug Delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-Viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Yu, M.; Gu, Z.; Ottewell, T.; Yu, C. Silica-Based Nanoparticles for Therapeutic Protein Delivery. J. Mater. Chem. B 2017, 5, 3241–3252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Keiser, M.S.; Davidson, B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018, 8, e58. [Google Scholar] [CrossRef] [PubMed]
- Lukashev, A.N.; Zamyatnin, A.A. Viral Vectors for Gene Therapy: Current State and Clinical Perspectives. Biochemistry 2016, 81, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Jha, D.; Mishra, R.; Gottschalk, S.; Wiesmüller, K.-H.; Ugurbil, K.; Maier, M.E.; Engelmann, J. CyLoP-1: A Novel Cysteine-Rich Cell-Penetrating Peptide for Cytosolic Delivery of Cargoes. Bioconjug. Chem. 2011, 22, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular Vesicles: A Bright Star of Nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Tan, E.; Wang, Y.; Fan, Q.; Yu, J.; Cheng, Y. Tailoring Guanidyl-Rich Polymers for Efficient Cytosolic Protein Delivery. J. Control. Release 2020, 320, 412–420. [Google Scholar] [CrossRef]
- Ren, L.; Lv, J.; Wang, H.; Cheng, Y. A Coordinative Dendrimer Achieves Excellent Efficiency in Cytosolic Protein and Peptide Delivery. Angew. Chem. Int. Ed. 2020, 59, 4711–4719. [Google Scholar] [CrossRef]
- Wang, M.; Zuris, J.A.; Meng, F.; Rees, H.; Sun, S.; Deng, P.; Han, Y.; Gao, X.; Pouli, D.; Wu, Q.; et al. Efficient Delivery of Genome-Editing Proteins Using Bioreducible Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 2868–2873. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-responsive Liposomes for Drug Delivery. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Voelkel, C.; Galla, M.; Maetzig, T.; Warlich, E.; Kuehle, J.; Zychlinski, D.; Bode, J.; Cantz, T.; Schambach, A.; Baum, C. Protein Transduction from Retroviral Gag Precursors. Proc. Natl. Acad. Sci. USA 2010, 107, 7805–7810. [Google Scholar] [CrossRef]
- Kaczmarczyk, S.J.; Sitaraman, K.; Young, H.A.; Hughes, S.H.; Chatterjee, D.K. Protein Delivery Using Engineered Virus-like Particles. Proc. Natl. Acad. Sci. USA 2011, 108, 16998–17003. [Google Scholar] [CrossRef]
- Wu, D.-T.; Roth, M.J. MLV Based Viral-like-Particles for Delivery of Toxic Proteins and Nuclear Transcription Factors. Biomaterials 2014, 35, 8416–8426. [Google Scholar] [CrossRef] [PubMed]
- Mangeot, P.E.; Risson, V.; Fusil, F.; Marnef, A.; Laurent, E.; Blin, J.; Mournetas, V.; Massouridès, E.; Sohier, T.J.M.; Corbin, A.; et al. Genome Editing in Primary Cells and in Vivo Using Viral-Derived Nanoblades Loaded with Cas9-SgRNA Ribonucleoproteins. Nat. Commun. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered Virus-like Particles for Efficient in Vivo Delivery of Therapeutic Proteins. Cell 2022, 185, 250–265.e16. [Google Scholar] [CrossRef] [PubMed]
- Thuenemann, E.C.; Le, D.H.T.; Lomonossoff, G.P.; Steinmetz, N.F. Bluetongue Virus Particles as Nanoreactors for Enzyme Delivery and Cancer Therapy. Mol. Pharm. 2021, 18, 1150–1156. [Google Scholar] [CrossRef]
- Panthi, S.; Schmitt, P.T.; Lorenz, F.J.; Stanfield, B.A.; Schmitt, A.P. Paramyxovirus-Like Particles as Protein Delivery Vehicles. J. Virol. 2021, 95, e01030-21. [Google Scholar] [CrossRef]
- Peretti, S.; Schiavoni, I.; Pugliese, K.; Federico, M. Cell Death Induced by the Herpes Simplex Virus-1 Thymidine Kinase Delivered by Human Immunodeficiency Virus-1-Based Virus-like Particles. Mol. Ther. 2005, 12, 1185–1196. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, L.; Wang, T.; Li, Y.; Wu, B.; Du, S.; Zhu, Q.; Zhu, C.; Wang, Y.; Zhang, R.; et al. A System Based on Novel Parainfluenza Virus PIV5-L for Efficient Gene Delivery of B-Lymphoma Cells. J. Virol. 2022, 96, e00257-22. [Google Scholar] [CrossRef]
- Fehér, A.; Boross, P.; Sperka, T.; Miklóssy, G.; Kádas, J.; Bagossi, P.; Oroszlan, S.; Weber, I.T.; Tözsér, J. Characterization of the Murine Leukemia Virus Protease and Its Comparison with the Human Immunodeficiency Virus Type 1 Protease. J. Gen. Virol. 2006, 87, 1321–1330. [Google Scholar] [CrossRef]
- Weber, I.T.; Wang, Y.-F.; Harrison, R.W. HIV Protease: Historical Perspective and Current Research. Viruses 2021, 13, 839. [Google Scholar] [CrossRef]
- Dalba, C.; Bellier, B.; Kasahara, N.; Klatzmann, D. Replication-Competent Vectors and Empty Virus-like Particles: New Retroviral Vector Designs for Cancer Gene Therapy or Vaccines. Mol. Ther. 2007, 15, 457–466. [Google Scholar] [CrossRef]
- Zochowska, M.; Paca, A.; Schoehn, G.; Andrieu, J.-P.; Chroboczek, J.; Dublet, B.; Szolajska, E. Adenovirus Dodecahedron, as a Drug Delivery Vector. PLoS ONE 2009, 4, e5569. [Google Scholar] [CrossRef] [PubMed]
- Zochowska, M.; Piguet, A.-C.; Jemielity, J.; Kowalska, J.; Szolajska, E.; Dufour, J.-F.; Chroboczek, J. Virus-like Particle-Mediated Intracellular Delivery of MRNA Cap Analog with in Vivo Activity against Hepatocellular Carcinoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Jekhmane, S.; de Haas, R.; Filho, O.P.D.S.; van Asbeck, A.H.; Favretto, M.E.; Hernandez-Garcia, A.; Brock, R.; de Vries, R. Virus-Like Particles of MRNA with Artificial Minimal Coat Proteins: Particle Formation, Stability, and Transfection Efficiency. Nucleic Acid Ther. 2017, 27, 159–167. [Google Scholar] [CrossRef]
- Hernandez-Garcia, A.; Kraft, D.J.; Janssen, A.F.J.; Bomans, P.H.H.; Sommerdijk, N.A.J.M.; Thies-Weesie, D.M.E.; Favretto, M.E.; Brock, R.; de Wolf, F.A.; Werten, M.W.T.; et al. Design and Self-Assembly of Simple Coat Proteins for Artificial Viruses. Nat. Nanotechnol. 2014, 9, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian Retrovirus-like Protein PEG10 Packages Its Own MRNA and Can Be Pseudotyped for MRNA Delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Shao, M.W.; Paul, A.; Abbasi, S.; Chahal, P.; Mena, J.A.; Montes, J.; Kamen, A. A Novel Polyethyleneimine-Coated Adeno-Associated Virus-like Particle Formulation for Efficient SiRNA Delivery in Breast Cancer Therapy: Preparation and in Vitro Analysis. Int. J. Nanomed. 2012, 7, 1575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suffian, I.F.M.; Wang, J.T.-W.; Faruqu, F.N.; Benitez, J.; Nishimura, Y.; Ogino, C.; Kondo, A.; Al-Jamal, K.T. Engineering Human Epidermal Growth Receptor 2-Targeting Hepatitis B Virus Core Nanoparticles for SiRNA Delivery in Vitro and in Vivo. ACS Appl. Nano Mater. 2018, 1, 3269–3282. [Google Scholar] [CrossRef]
- Yadav, M.; Atala, A.; Lu, B. Developing All-in-One Virus-like Particles for Cas9 MRNA/Single Guide RNA Co-Delivery and Aptamer-Containing Lentiviral Vectors for Improved Gene Expression. Int. J. Biol. Macromol. 2022, 209, 1260–1270. [Google Scholar] [CrossRef]
- Singsuksawat, E.; Onnome, S.; Posiri, P.; Suphatrakul, A.; Srisuk, N.; Nantachokchawapan, R.; Praneechit, H.; Sae-kow, C.; Chidpratum, P.; Sa-ngiamsuntorn, K.; et al. Potent Programmable Antiviral against Dengue Virus in Primary Human Cells by Cas13b RNP with Short Spacer and Delivery by VLP. Mol. Ther. Methods Clin. Dev. 2021, 21, 729–740. [Google Scholar] [CrossRef]
- Yan, D.; Teng, Z.; Sun, S.; Jiang, S.; Dong, H.; Gao, Y.; Wei, Y.; Qin, W.; Liu, X.; Yin, H.; et al. Foot-and-Mouth Disease Virus-like Particles as Integrin-Based Drug Delivery System Achieve Targeting Anti-Tumor Efficacy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1061–1070. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, W.; Chen, Y.; Zhang, L.; Zhang, J.; Zhang, Z. Self-Assembled Virus-Like Particles from Rotavirus Structural Protein VP6 for Targeted Drug Delivery. Bioconjug. Chem. 2011, 22, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yui, M.; Deo, V.K.; Park, E.Y. Development of Rous Sarcoma Virus-like Particles Displaying HCC49 ScFv for Specific Targeted Drug Delivery to Human Colon Carcinoma Cells. Pharm. Res. 2015, 32, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Mu, Y.; Jiang, L.; Yu, H.; Yang, S.; Zhang, Y.; Wang, J.; Zhang, H.; Sun, H.; Xiao, C.; et al. Multifunctional TK-VLPs Nanocarrier for Tumor-Targeted Delivery. Int. J. Pharm. 2016, 502, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L.; et al. Cell-Specific Delivery of Diverse Cargos by Bacteriophage MS2 Virus-like Particles. ACS Nano 2011, 5, 5729–5745. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Zhao, R. Establishment of MicroRNA Delivery System by PP7 Bacteriophage-like Particles Carrying Cell-Penetrating Peptide. J. Biosci. Bioeng. 2017, 124, 242–249. [Google Scholar] [CrossRef]
- Wang, G.; Jia, T.; Xu, X.; Chang, L.; Zhang, R.; Fu, Y.; Li, Y.; Yang, X.; Zhang, K.; Lin, G.; et al. Novel MiR-122 Delivery System Based on MS2 Virus like Particle Surface Displaying Cell-Penetrating Peptide TAT for Hepatocellular Carcinoma. Oncotarget 2016, 7, 59402–59416. [Google Scholar] [CrossRef]
- Qazi, S.; Miettinen, H.M.; Wilkinson, R.A.; McCoy, K.; Douglas, T.; Wiedenheft, B. Programmed Self-Assembly of an Active P22-Cas9 Nanocarrier System. Mol. Pharm. 2016, 13, 1191–1196. [Google Scholar] [CrossRef]
- Crooke, S.N.; Schimer, J.; Raji, I.; Wu, B.; Oyelere, A.K.; Finn, M.G. Lung Tissue Delivery of Virus-Like Particles Mediated by Macrolide Antibiotics. Mol. Pharm. 2019, 16, 2947–2955. [Google Scholar] [CrossRef]
- Cho, C.-W.; Cho, Y.-S.; Kang, B.-T.; Hwang, J.-S.; Park, S.-N.; Yoon, D.-Y. Improvement of Gene Transfer to Cervical Cancer Cell Lines Using Non-Viral Agents. Cancer Lett. 2001, 162, 75–85. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Fang, P.-Y.; Ramos, L.M.G.; Holguin, S.Y.; Hsiao, C.; Bowman, J.C.; Yang, H.-W.; Williams, L.D. Functional RNAs: Combined Assembly and Packaging in VLPs. Nucleic Acids Res. 2017, 45, 3519–3527. [Google Scholar] [CrossRef] [PubMed]
- Abed, M.; Verschueren, E.; Budayeva, H.; Liu, P.; Kirkpatrick, D.S.; Reja, R.; Kummerfeld, S.K.; Webster, J.D.; Gierke, S.; Reichelt, M.; et al. The Gag Protein PEG10 Binds to RNA and Regulates Trophoblast Stem Cell Lineage Specification. PLoS ONE 2019, 14, e0214110. [Google Scholar]
- Pastuzyn, E.D.; Day, C.E.; Kearns, R.B.; Kyrke-Smith, M.; Taibi, A.V.; McCormick, J.; Yoder, N.; Belnap, D.M.; Erlendsson, S.; Morado, D.R.; et al. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein That Mediates Intercellular RNA Transfer. Cell 2018, 172, 275–288.e18. [Google Scholar] [CrossRef] [PubMed]
- Tokatlian, T.; Segura, T. SiRNA Applications in Nanomedicine: SiRNA Applications in Nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 305–315. [Google Scholar] [CrossRef]
- Laufer, S.D.; Detzer, A.; Sczakiel, G.; Restle, T. Selected Strategies for the Delivery of SiRNA In Vitro and In Vivo. In RNA Technologies and Their Applications; Erdmann, V.A., Barciszewski, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 29–58. [Google Scholar]
- Choi, K.; Kim, K.; Kwon, I.C.; Kim, I.-S.; Ahn, H.J. Systemic Delivery of SiRNA by Chimeric Capsid Protein: Tumor Targeting and RNAi Activity in Vivo. Mol. Pharm. 2013, 10, 18–25. [Google Scholar] [CrossRef]
- Choi, K.; Choi, S.-H.; Jeon, H.; Kim, I.-S.; Ahn, H.J. Chimeric Capsid Protein as a Nanocarrier for SiRNA Delivery: Stability and Cellular Uptake of Encapsulated SiRNA. ACS Nano 2011, 5, 8690–8699. [Google Scholar] [CrossRef]
- Kong, J.; Liu, X.; Jia, J.; Wu, J.; Wu, N.; Chen, J.; Fang, F. Pokemon SiRNA Delivery Mediated by RGD-Modified HBV Core Protein Suppressed the Growth of Hepatocellular Carcinoma. Hum. Gene Ther. Methods 2015, 26, 175–180. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Jia, T.; Zhang, K.; Li, J.; Wang, L. Development of a MicroRNA Delivery System Based on Bacteriophage MS2 Virus-like Particles: Development of a MicroRNA Delivery System. FEBS J. 2012, 279, 1198–1208. [Google Scholar] [CrossRef]
- Knopp, Y.; Geis, F.K.; Heckl, D.; Horn, S.; Neumann, T.; Kuehle, J.; Meyer, J.; Fehse, B.; Baum, C.; Morgan, M.; et al. Transient Retrovirus-Based CRISPR/Cas9 All-in-One Particles for Efficient, Targeted Gene Knockout. Mol. Ther. Nucleic Acids 2018, 13, 256–274. [Google Scholar] [CrossRef]
- Lu, B.; Javidi-Parsijani, P.; Makani, V.; Mehraein-Ghomi, F.; Sarhan, W.M.; Sun, D.; Yoo, K.W.; Atala, Z.P.; Lyu, P.; Atala, A. Delivering SaCas9 MRNA by Lentivirus-like Bionanoparticles for Transient Expression and Efficient Genome Editing. Nucleic Acids Res. 2019, 47, e44. [Google Scholar] [CrossRef]
- Yin, D.; Ling, S.; Wang, D.; Dai, Y.; Jiang, H.; Zhou, X.; Paludan, S.R.; Hong, J.; Cai, Y. Targeting Herpes Simplex Virus with CRISPR–Cas9 Cures Herpetic Stromal Keratitis in Mice. Nat. Biotechnol. 2021, 39, 567–577. [Google Scholar] [CrossRef]
- Prel, A.; Caval, V.; Gayon, R.; Ravassard, P.; Duthoit, C.; Payen, E.; Maouche-Chretien, L.; Creneguy, A.; Nguyen, T.H.; Martin, N.; et al. Highly Efficient in Vitro and in Vivo Delivery of Functional RNAs Using New Versatile MS2-Chimeric Retrovirus-like Particles. Mol. Ther. Methods Clin. Dev. 2015, 2, 15039. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.-S. Highly Efficient RNA-Guided Genome Editing in Human Cells via Delivery of Purified Cas9 Ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Choi, J.G.; Dang, Y.; Abraham, S.; Ma, H.; Zhang, J.; Guo, H.; Cai, Y.; Mikkelsen, J.G.; Wu, H.; Shankar, P.; et al. Lentivirus Pre-Packed with Cas9 Protein for Safer Gene Editing. Gene Ther. 2016, 23, 627–633. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like Particles: Next-Generation Nanoparticles for Targeted Therapeutic Delivery. Bioeng. Transl. Med. 2017, 2, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral Nanoparticles for Drug Delivery, Imaging, Immunotherapy, and Theranostic Applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Shoja, Z.; Jalilvand, S.; Latifi, T.; Roohvand, F. Rotavirus VP6: Involvement in Immunogenicity, Adjuvant Activity, and Use as a Vector for Heterologous Peptides, Drug Delivery, and Production of Nano-Biomaterials. Arch. Virol. 2022, 167, 1013–1023. [Google Scholar] [CrossRef]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins Avβ3 and Avβ5 Promote Adenovirus Internalization but Not Virus Attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Fender, P.; Schoehn, G.; Perron-Sierra, F.; Tucker, G.C.; Lortat-Jacob, H. Adenovirus Dodecahedron Cell Attachment and Entry Are Mediated by Heparan Sulfate and Integrins and Vary along the Cell Cycle. Virology 2008, 371, 155–164. [Google Scholar] [CrossRef]
- Vivès, R.R.; Lortat-Jacob, H.; Chroboczek, J.; Fender, P. Heparan Sulfate Proteoglycan Mediates the Selective Attachment and Internalization of Serotype 3 Human Adenovirus Dodecahedron. Virology 2004, 321, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Garay, E.; Cervera, L.; Kratje, R.; Prieto, C.; Gòdia, F. Chimeric VLPs Based on HIV-1 Gag and a Fusion Rabies Glycoprotein Induce Specific Antibodies against Rabies and Foot-and-Mouth Disease Virus. Vaccines 2021, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Rangel, G.; Bárcena, J.; Moreno, N.; Mata, C.P.; Castón, J.R.; Alejo, A.; Blanco, E. Chimeric RHDV Virus-Like Particles Displaying Foot-and-Mouth Disease Virus Epitopes Elicit Neutralizing Antibodies and Confer Partial Protection in Pigs. Vaccines 2021, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, S.; Zou, Y.; Yu, W.; Jiang, Y.; Zhan, Y.; Wang, N.; Dong, Y.; Yang, Y. Structure-Based Design of Porcine Circovirus Type 2 Chimeric VLPs (CVLPs) Displays Foreign Peptides on the Capsid Surface. Front. Cell. Infect. Microbiol. 2018, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.R.; Tsuchida, C.A.; Nguyen, D.N.; Shy, B.R.; McGarrigle, E.R.; Espinoza, C.R.S.; Carr, D.; Blaeschke, F.; Marson, A.; Doudna, J.A. Targeted Delivery of CRISPR-Cas9 and Transgenes Enables Complex Immune Cell Engineering. Cell Rep. 2021, 35, 109207. [Google Scholar] [CrossRef]
- Lu, Z.; Yao, X.; Lyu, P.; Yadav, M.; Yoo, K.; Atala, A.; Lu, B. Lentiviral Capsid-Mediated Streptococcus pyogenes Cas9 Ribonucleoprotein Delivery for Efficient and Safe Multiplex Genome Editing. CRISPR J. 2021, 4, 914–928. [Google Scholar] [CrossRef]
- Baron, Y.; Sens, J.; Lange, L.; Nassauer, L.; Klatt, D.; Hoffmann, D.; Kleppa, M.-J.; Barbosa, P.V.; Keisker, M.; Steinberg, V.; et al. Improved Alpharetrovirus-Based Gag.MS2 Particles for Efficient and Transient Delivery of CRISPR-Cas9 into Target Cells. Mol. Ther. Nucleic Acids 2022, 27, 810–823. [Google Scholar] [CrossRef]
- Mianné, J.; Nasri, A.; Van, C.N.; Bourguignon, C.; Fieldès, M.; Ahmed, E.; Duthoit, C.; Martin, N.; Parrinello, H.; Louis, A.; et al. CRISPR/Cas9-Mediated Gene Knockout and Interallelic Gene Conversion in Human Induced Pluripotent Stem Cells Using Non-Integrative Bacteriophage-Chimeric Retrovirus-like Particles. BMC Biol. 2022, 20, 8. [Google Scholar] [CrossRef]
- Hwang, B.J.; Jang, Y.; Kwon, S.B.; Yu, J.E.; Lim, J.; Roh, Y.H.; Seong, B.L. RNA-Assisted Self-Assembly of Monomeric Antigens into Virus-like Particles as a Recombinant Vaccine Platform. Biomaterials 2021, 269, 120650. [Google Scholar] [CrossRef]
- Touzé, A.; Bousarghin, L.; Ster, C.; Combita, A.-L.; Roingeard, P.; Coursaget, P. Gene Transfer Using Human Polyomavirus BK Virus-like Particles Expressed in Insect Cells. J. Gen. Virol. 2001, 82, 3005–3009. [Google Scholar] [CrossRef][Green Version]
- Chou, M.-I.; Hsieh, Y.-F.; Wang, M.; Chang, J.T.; Chang, D.; Zouali, M.; Tsay, G.J. In Vitro and in Vivo Targeted Delivery of IL-10 Interfering RNA by JC Virus-like Particles. J. Biomed. Sci. 2010, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Porterfield, J.Z.; Dhason, M.S.; Loeb, D.D.; Nassal, M.; Stray, S.J.; Zlotnick, A. Full-Length Hepatitis B Virus Core Protein Packages Viral and Heterologous RNA with Similarly High Levels of Cooperativity. J. Virol. 2010, 84, 7174–7184. [Google Scholar] [CrossRef] [PubMed]
- Dhason, M.S.; Wang, J.C.-Y.; Hagan, M.F.; Zlotnick, A. Differential Assembly of Hepatitis B Virus Core Protein on Single- and Double-Stranded Nucleic Acid Suggest the DsDNA-Filled Core Is Spring-Loaded. Virology 2012, 430, 20–29. [Google Scholar] [CrossRef]
- Strods, A.; Ose, V.; Bogans, J.; Cielens, I.; Kalnins, G.; Radovica, I.; Kazaks, A.; Pumpens, P.; Renhofa, R. Preparation by Alkaline Treatment and Detailed Characterisation of Empty Hepatitis B Virus Core Particles for Vaccine and Gene Therapy Applications. Sci. Rep. 2015, 5, 11639. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Mukhopadhyay, R.; Mukhija, R.; Krishnan, A.; Garg, L.C.; Panda, A.K. Optimization of Inclusion Body Solubilization and Renaturation of Recombinant Human Growth Hormone from Escherichia Coli. Protein Expr. Purif. 2000, 18, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Gyoo Park, S.; Yoo, J.; Jung, G. Calcium Ions Affect the Hepatitis B Virus Core Assembly. Virology 2005, 332, 454–463. [Google Scholar] [CrossRef]
- Dell’Orco, D.; Xue, W.-F.; Thulin, E.; Linse, S. Electrostatic Contributions to the Kinetics and Thermodynamics of Protein Assembly. Biophys. J. 2005, 88, 1991–2002. [Google Scholar] [CrossRef]
- Le, D.T.; Müller, K.M. In Vitro Assembly of Virus-Like Particles and Their Applications. Life 2021, 11, 334. [Google Scholar] [CrossRef]
- Tang, S.; Xuan, B.; Ye, X.; Huang, Z.; Qian, Z. A Modular Vaccine Development Platform Based on Sortase-Mediated Site-Specific Tagging of Antigens onto Virus-Like Particles. Sci. Rep. 2016, 6, 25741. [Google Scholar] [CrossRef]
- Patel, K.G.; Swartz, J.R. Surface Functionalization of Virus-Like Particles by Direct Conjugation Using Azide—Alkyne Click Chemistry. Bioconjug. Chem. 2011, 22, 376–387. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.-W. Protein-Based Nanoparticles in Cancer Vaccine Development. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Heath, M.D.; Cabral-Miranda, G.; Lipp, C.; Zeltins, A.; Sande, M.; Stein, J.V.; Riether, C.; Roesti, E.; Zha, L.; et al. Vaccination with Nanoparticles Combined with Micro-Adjuvants Protects against Cancer. J. Immunother. Cancer 2019, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Steinmetz, N.F. Development of a Virus-Like Particle-Based Anti-HER2 Breast Cancer Vaccine. Cancers 2021, 13, 2909. [Google Scholar] [CrossRef] [PubMed]
- Buzón, P.; Maity, S.; Roos, W.H. Physical Virology: From Virus Self-assembly to Particle Mechanics. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1613. [Google Scholar] [CrossRef]
- Mateu, M.G. Assembly, Stability and Dynamics of Virus Capsids. Arch. Biochem. Biophys. 2013, 531, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, R.W.; Crépin, T.; Kolakofsky, D. Nucleoproteins and Nucleocapsids of Negative-Strand RNA Viruses. Curr. Opin. Microbiol. 2011, 14, 504–510. [Google Scholar] [CrossRef]
- Ding, Y.; Chuan, Y.P.; He, L.; Middelberg, A.P.J. Modeling the Competition between Aggregation and Self-Assembly during Virus-like Particle Processing. Biotechnol. Bioeng. 2010, 107, 550–560. [Google Scholar] [CrossRef]
- Rolfsson, Ó.; Middleton, S.; Manfield, I.W.; White, S.J.; Fan, B.; Vaughan, R.; Ranson, N.A.; Dykeman, E.; Twarock, R.; Ford, J.; et al. Direct Evidence for Packaging Signal-Mediated Assembly of Bacteriophage MS2. J. Mol. Biol. 2016, 428, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Comas-Garcia, M.; Kroupa, T.; Datta, S.A.; Harvin, D.P.; Hu, W.-S.; Rein, A. Efficient Support of Virus-like Particle Assembly by the HIV-1 Packaging Signal. eLife 2018, 7, e38438. [Google Scholar] [CrossRef] [PubMed]
- Vicente, T.; Roldão, A.; Peixoto, C.; Carrondo, M.J.T.; Alves, P.M. Large-Scale Production and Purification of VLP-Based Vaccines. J. Invertebr. Pathol. 2011, 107, S42–S48. [Google Scholar] [CrossRef]
- Suffian, I.F.B.M.; Wang, J.T.-W.; Hodgins, N.O.; Klippstein, R.; Garcia-Maya, M.; Brown, P.; Nishimura, Y.; Heidari, H.; Bals, S.; Sosabowski, J.K.; et al. Engineering Hepatitis B Virus Core Particles for Targeting HER2 Receptors in Vitro and in Vivo. Biomaterials 2017, 120, 126–138. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like Particles: Fundamentals and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 8579. [Google Scholar] [CrossRef] [PubMed]
- Zeltins, A. Construction and Characterization of Virus-Like Particles: A Review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, K.; Ghahramani Seno, M.M.; Ahmadian, M.R.; Malaekeh-Nikouei, B.; Bassami, M.R.; Dehghani, H.; Afkhami-Goli, A. Optimizing the Synthesis and Purification of MS2 Virus like Particles. Sci. Rep. 2021, 11, 19851. [Google Scholar] [CrossRef] [PubMed]
- Koho, T.; Koivunen, M.R.L.; Oikarinen, S.; Kummola, L.; Mäkinen, S.; Mähönen, A.J.; Sioofy-Khojine, A.; Marjomäki, V.; Kazmertsuk, A.; Junttila, I.; et al. Coxsackievirus B3 VLPs Purified by Ion Exchange Chromatography Elicit Strong Immune Responses in Mice. Antivir. Res. 2014, 104, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.O.; Nicol, C.; Stonehouse, N.J.; Rowlands, D.J. Increasing Type 1 Poliovirus Capsid Stability by Thermal Selection. J. Virol. 2017, 91, e01586-16. [Google Scholar] [CrossRef]
- Sakai, C.; Hosokawa, K.; Watanabe, T.; Suzuki, Y.; Nakano, T.; Ueda, K.; Fujimuro, M. Human Hepatitis B Virus-Derived Virus-like Particle as a Drug and DNA Delivery Carrier. Biochem. Biophys. Res. Commun. 2021, 581, 103–109. [Google Scholar] [CrossRef]
- Schumacher, J.; Bacic, T.; Staritzbichler, R.; Daneschdar, M.; Klamp, T.; Arnold, P.; Jägle, S.; Türeci, Ö.; Markl, J.; Sahin, U. Enhanced Stability of a Chimeric Hepatitis B Core Antigen Virus-like-Particle (HBcAg-VLP) by a C-Terminal Linker-Hexahistidine-Peptide. J. Nanobiotechnol. 2018, 16, 39. [Google Scholar] [CrossRef]
- Thong, Q.X.; Biabanikhankahdani, R.; Ho, K.L.; Alitheen, N.B.; Tan, W.S. Thermally-Responsive Virus-like Particle for Targeted Delivery of Cancer Drug. Sci. Rep. 2019, 9, 3945. [Google Scholar] [CrossRef]
- Shahrivarkevishahi, A.; Luzuriaga, M.A.; Herbert, F.C.; Tumac, A.C.; Brohlin, O.R.; Wijesundara, Y.H.; Adlooru, A.V.; Benjamin, C.; Lee, H.; Parsamian, P.; et al. PhotothermalPhage: A Virus-Based Photothermal Therapeutic Agent. J. Am. Chem. Soc. 2021, 143, 16428–16438. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Bachmann, M.F. Virus-like Particle Vaccinology, from Bench to Bedside. Cell. Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef] [PubMed]
| VLP Origin | Components of the VLP | Cargo Packaging Strategies | Specialties of the Delivery System | VLP Production Systems | Refs. |
|---|---|---|---|---|---|
| Murine leukemia virus (MMLV or FMLV) | Gag, Gag-Pol, VSV-G | Foreign protein fusion to VLP | Enveloped virus; protein delivery; allow for loading large molecular proteins such as Cas9 | HEK-293T producer cells Gesicle 293T producer cells | [29,31,32,33,40] |
| Avian sarcoma leukosis virus (ASLVs) | Gag, VSV-G | Foreign protein fusion to VLP | Enveloped virus; protein delivery | Mammalian cell expression system | [30] |
| Paramyxovirus (PIV5, Nipah) | NP, M, glycoprotein | Physical interaction between cargo and scaffold proteins | Adaption to suspension cell cultures for large-scale production; enveloped virus; few limitations in the size of cargos | Mammalian cell expression system | [35] |
| Human Immunodeficiency Virus-1 (HIV-1) | Capsid p24 protein, Nef7, VSV-G | Foreign protein fusion to VLP | Enveloped virus; more safety compared with traditional treatments of cancer | 293T producer cells | [36] |
| Bluetongue virus | VP3, VP7, VP5 and VP2 | Foreign protein fusion to VLP | Non-enveloped virus; efficiently kill tumor cells | Plant expression system | [34] |
| Adenovirus | Penton-dodecahedron (Pt-Dd) | Conjugation reaction | Non-enveloped virus; chemical linking does not affect the VLP capability to enter cells, even easier internalization; low immune response | Baculovirus-insect cell expression system | [41,42] |
| Artificial proteins | C-S10-K12 protein | Electrostatic adsorption | Non-enveloped artificial VLP; pronounced physical stability; scarcely existing cytotoxicity and hemolysis for target cells | Pichia pastoris expression system | [43,44] |
| Endogenous retrovirus (PEG10) | VSV-G, PEG10 | Physical interaction between mRNA and VLP | VLPs derived from a full human system for mRNA delivery | HEK 293T cells expression system | [45] |
| AAV2 | PEI, Cap (Vp1, Vp2, Vp3) | Electrostatic adsorption | Non-enveloped virus; no pronounced cytotoxicity; engineered for targeting | Baculovirus-insect cell expression system | [46] |
| HBV | Hepatitis B virus core protein (HBc) | Disassembly and reassembly; osmotic shock | Enveloped virus; compatible for siRNA delivery; good biocompatibility; diminish a strong immune response; good stability in serum | E. coli expression system | [47] |
| Lentivirus | 1. Gag (NC, MA, CA) 2. Gag-pol, Gag, VSV-G | Physical interaction between VLP capsid proteins and cargos | Enveloped virus; more highly efficient in genome editing | HEK 293T producer cells | [48,49] |
| Foot-and-mouth disease virus (FMDV) | VP0, VP1 and VP3 | Covalent connection | Non-enveloped virus; targeted delivery to tumor cells avoids side effects in normal tissues | E. coli expression system | [50] |
| Rotavirus | VP6 | Covalent connection | Non-enveloped virus; DOX releases at low pH preventing leak in the bloodstream | E. coli expression system | [51] |
| Rous sarcoma virus (RSV) | Gag | Physical method (electroporation) | Enveloped virus; same amount of DOX loading into VLP is more efficient for killing cells | Silkworm larvae expression system | [52] |
| Porcine parvovirus | VP2 | Covalent connection | Non-enveloped virus; TK peptide is a dual-functional ligand | Baculovirus-Sf9 insect cell expression system | [53] |
| Bacteriophage (MS2, Qβv) | SP94, H5WYG, PEG, Coat protein dimers | Disassembly and reassembly; physical interaction between VLP and cargo | Keep good stability in different conditions; various cargos can be packaged into VLP (RNA, DNA, proteins, compounds) | E. coli expression system | [54] |
| Bacteriophage (PP7, MS2) | Coat protein dimers, TAT peptide | Physical interaction between bacteriophage-like particles and miRNA linked to stem-loop | Heat-resistant at high temperature (≤ 60℃) | E. coli expression system | [55,56] |
| Bacteriophage P22 | Scaffold proteins, Capsid proteins | Foreign protein fusion to VLP | Keep good stability and protect cargo from degradation | E. coli expression system | [57] |
| Bacteriophage Qβ | Capsid proteins | Covalent connection | Macrophage can be activated by polyvalently displaying macrolides to the surface of Qβ VLPs | E. coli expression system | [58] |
| VLP Origin | Cargo | Applications | Testing | Targeting Strategies | Refs. |
|---|---|---|---|---|---|
| Murine leukemia virus (MLV) | 1. Flp recombinase 2. GFP 3. Nuclear transcription factors 4. Bacterial toxin/anti-toxin system | Gene recombination, cell differentiation, cell death | Murine iPSCs; mouse embryonic fibroblast cell line (SNL cells); HeLa MCAT cell line | Pseudotyped VSV-G envelope; EA6 envelope | [29,31] |
| Avian sarcoma leukosis virus (ASLVs) | 1. Cre recombinase 2. Human caspase-8 3. Active pro-drug enzymes (Fcy and Fur) | Gene recombination, cancer treatment | PC3 cells | VSV-G envelope; ligand/receptor mediated delivery (NA-IFN-γ ligand or HA-TNF ligand) | [30] |
| Friend murine leukemia virus (FMLV) | 1. Cas9-sgRNA ribonucleoproteins 2. Cas9 fusion complexes | Gene editing, gene knock-in, transcriptional activation, transgenic animals | Primary cells (hiPSCs, HSCs, mouse bone marrow); mouse embryos; liver of injected mice | VSV-G envelope; BaEV pseudotyped envelope | [32] |
| Friend murine leukemia virus (FMLV), Moloney murine leukemia virus (MMLV) | ABE8e (base editor) | Gene(base)-editing, genetic disorders treatment | HEK293T cells, primary human and mouse cells (primary human T cells, primary human/mouse fibroblasts), different organs (liver, brain, eye of mouse) in mouse | VSV-G envelope | [33] |
| Paramyxovirus (PIV5, Nipah) | 1. Rluc 2. GFP 3. Superoxide dismutase 4. Cre recombinase | Restore oxidative stress | A549 cells Reporter cells | Tropism of natural virus (such as target sialic acid surface receptors, ephrin-B receptors) | [35] |
| Human Immunodeficiency Virus-1 (HIV-1) | 1. GFP 2. HSV-1 thymidine kinase | Cell suicide therapies | CEM-ss cells and human primary macrophages | VSV-G envelope | [36] |
| Bluetongue virus | HSV-1 thymidine kinase | Anti-tumor treatment | Human glioblastoma derived cells | Natural tropism | [34] |
| Adenovirus | DOX, Bleomycin (BLM) | Anti-hepatocellular carcinoma | Neoplastic cells | Targeting peptides | [41,42] |
| Artificial proteins | mRNA | A therapeutic agent | HeLa and HEK293 cells | Not mentioned | [43] |
| Endogenous retrovirus (PEG10) | Cre mRNA and SpCas9 mRNA/sgRNA | Gene therapy | Reporter N2a cells HEK293FT cells | VSV-G envelope; endogenous MmSYNA envelope | [45] |
| AAV2 | siRNA | Breast cancer treatment | MCF-7 breast cancer cell | Not mentioned | [46] |
| HBc | siPLK1 | Cancer treatment | Cancer cells Mouse tumor model | Ligand/receptor mediated delivery (HER2) | [47] |
| Lentivirus | 1. Cas13 RNP 2. SpCas9 mRNA/sgRNA | Anti-virus infection Gene Knockout | Primary human cells | VSV-G envelope | [48,49,70] |
| Bacteriophage (MS2, Qβ) | siRNA, chemotherapy drugs (DOX, 5-FU, cisplatin), ricin toxin A-chain | Cell apoptosis; cancer treatment | Human hepatocellular carcinoma cell line (HCC) | SP94 | [54] |
| Bacteriophage (PP7, MS2) | MicroRNA (pre-miR-23b, miR-122) | Hepatoma treatment | hepatoma SK-HEP-1 cells, hepatocarcinoma cell lines | Cell-penetrating peptide (TAT peptide) | [55,56] |
| Bacteriophage P22 | Cas9/sgRNA | Gene therapy | dsDNA cleavage assay | Not mentioned | [57] |
| Bacteriophage Qβ | Macrolide antibiotics (azithromycin and clarithromycin) | Antimicrobial infection | RAW 264.7 macrophage cells, lungs tissue in mice | Azithromycin directs the VLPs to the lungs | [58] |
| Foot-and-mouth disease virus (FMDV) | DOX | Tumor treatment | HeLa cells | RGD motif | [50] |
| Rotavirus | DOX | Hepatoma treatment | HepG2 cell | Lactobionic acid | [79] |
| Rous sarcoma virus (RSV) | DOX | Colon carcinoma treatment | LS174T cell | hCC49 antibody scFv | [52] |
| Porcine parvovirus | DOX | Colorectal cancer treatment | Caco-2 cell and HUVEC cell | TK peptide | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Yu, L.; Lin, X.; Liu, X.; Zhang, Y.; Yang, F.; Deng, W. Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds. Viruses 2022, 14, 1905. https://doi.org/10.3390/v14091905
He J, Yu L, Lin X, Liu X, Zhang Y, Yang F, Deng W. Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds. Viruses. 2022; 14(9):1905. https://doi.org/10.3390/v14091905
Chicago/Turabian StyleHe, Junyao, Linying Yu, Xiaodi Lin, Xiaoyan Liu, Yanming Zhang, Fan Yang, and Wen Deng. 2022. "Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds" Viruses 14, no. 9: 1905. https://doi.org/10.3390/v14091905
APA StyleHe, J., Yu, L., Lin, X., Liu, X., Zhang, Y., Yang, F., & Deng, W. (2022). Virus-like Particles as Nanocarriers for Intracellular Delivery of Biomolecules and Compounds. Viruses, 14(9), 1905. https://doi.org/10.3390/v14091905







