Virological, Serological and Clinical Analysis of Chikungunya Virus Infection in Thai Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement Approval
2.2. Study Population
2.3. Determination of CHIKV Viral Load
2.4. Virus Isolation and Propagation
2.5. Neutralizing Assay
2.6. Sequencing and Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
3.1. Demographic Data
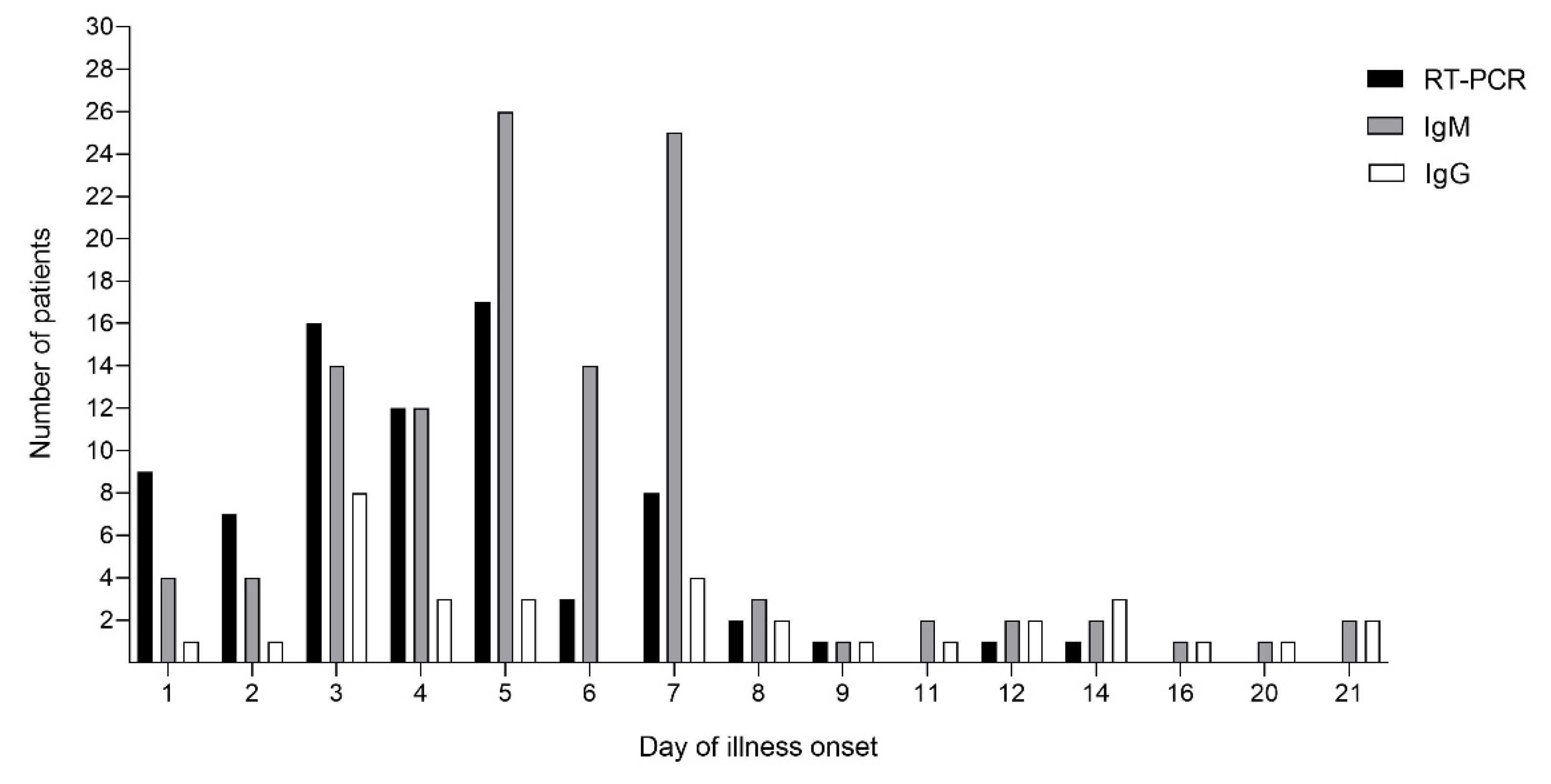
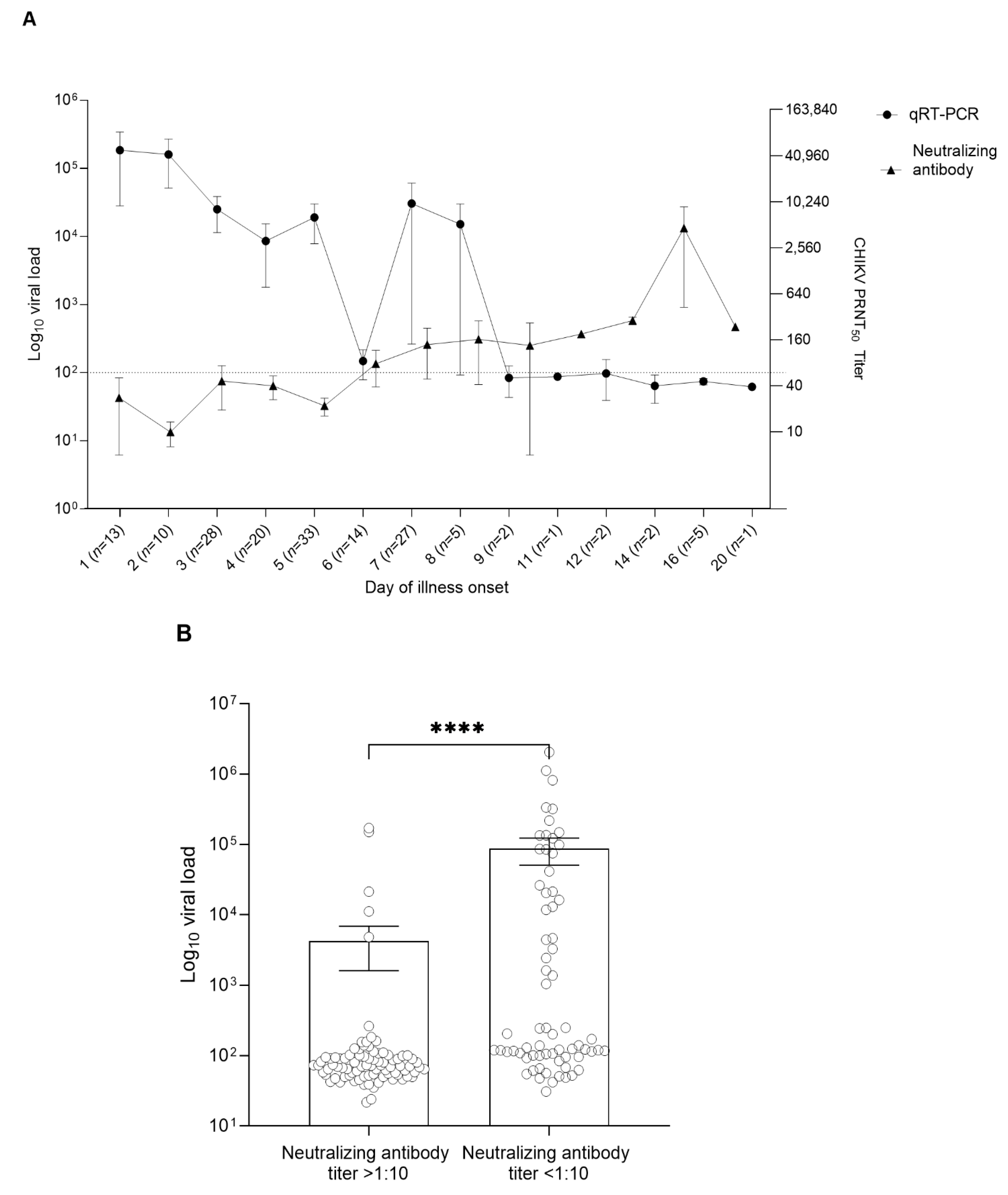
3.2. CHIKV Viral Load and CHIKV Antibody Profile in the Patient Serum Samples
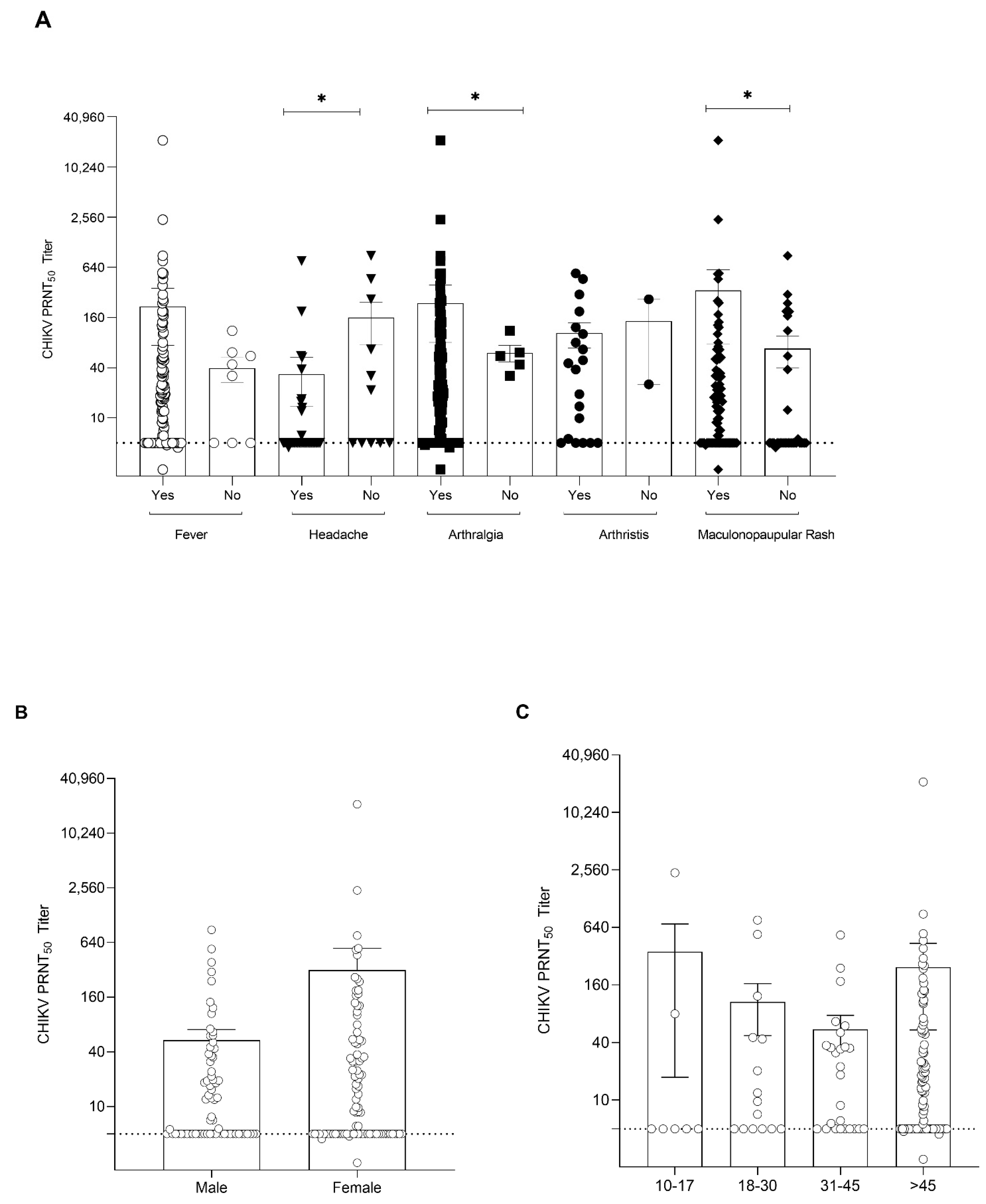
3.3. Clinical Features, Hematological Profile, and Viral Load Analysis
3.4. Antibody Responses against CHIKV Virus in Infected Patients
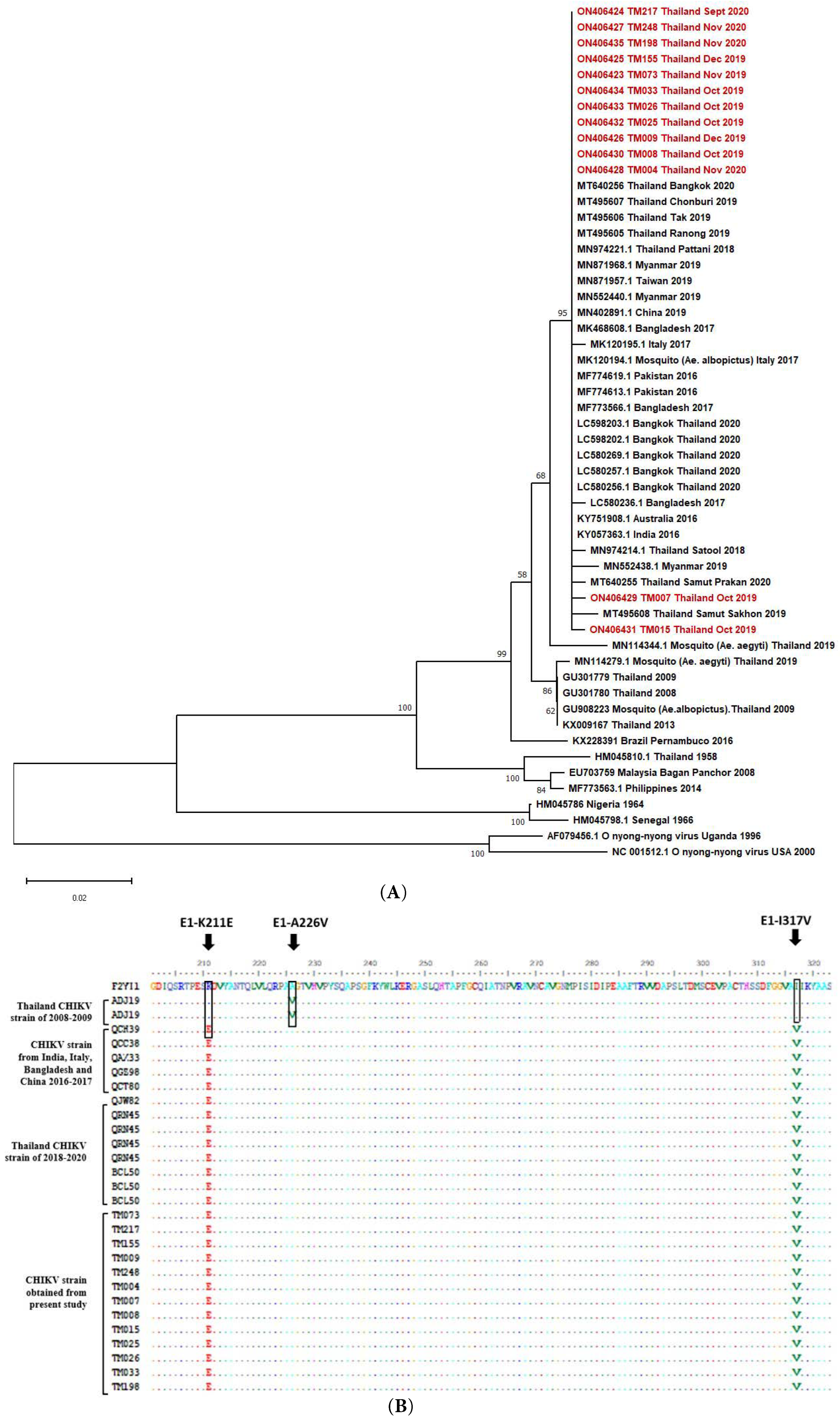
3.5. CHIKV Sequence Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, C.R.; Plante, K.S.; Heise, M.T. Chikungunya Virus: Current Perspectives on a Reemerging Virus. Microbiol. Spectr. 2016, 4, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S. Cost and burden of dengue and chikungunya from the Americas to Asia. Dengue Bull. 2010, 34, 1–5. [Google Scholar]
- Cardona-Ospina, J.A.; Villamil Gomez, W.; Jiménez-Canizales, C.; Castañeda Hernandez, D.; Rodriguez-Morales, A. Estimating the burden of disease and the economic cost attributable to chikungunya, Colombia, 2014. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef]
- Bureau of Epidemiology MoPH National Disease Surveillance: Chikungunya. Available online: https://ddc.moph.go.th/en/ (accessed on 1 January 2022).
- Rossini, G.; Landini, M.P.; Sambri, V. Evolution and Epidemiology of Chikungunya Virus. Methods Mol. Biol. 2016, 1426, 3–10. [Google Scholar]
- Tuite, A.R.; Watts, A.G.; Khan, K.; Bogoch, I.I. Countries at risk of importation of chikungunya virus cases from Southern Thailand: A modeling study. Infect. Dis. Model. 2019, 4, 251–256. [Google Scholar] [CrossRef]
- Mohan, A.; Kiran, D.H.N.; Manohar, I.; Kumar, D. Epidemiology, clinical manifestations, and diagnosis of chikungunya fever: Lessons learned from the re-emerging epidemic. Indian J. Dermatol. 2010, 55, 54–63. [Google Scholar] [CrossRef]
- Thiberville, S.-D.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; de Lamballerie, X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antivir. Res. 2013, 99, 345–370. [Google Scholar] [CrossRef]
- Alvarado, L.I.; Lorenzi, O.D.; Torres-Velásquez, B.C.; Sharp, T.M.; Vargas, L.; Muñoz-Jordán, J.L.; Hunsperger, E.A.; Pérez-Padilla, J.; Rivera, A.; González-Zeno, G.E.; et al. Distinguishing patients with laboratory-confirmed chikungunya from dengue and other acute febrile illnesses, Puerto Rico, 2012–2015. PLoS Negl. Trop. Dis. 2019, 13, e0007562. [Google Scholar] [CrossRef]
- Pathak, H.; Mohan, M.C.; Ravindran, V. Chikungunya arthritis. Clin. Med. 2019, 19, 381–385. [Google Scholar] [CrossRef]
- Hoarau, J.-J.; Jaffar Bandjee, M.-C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Kabra, S.K.; Lodha, R.; Ratageri, V.H.; Ray, P. Virus load and clinical features during the acute phase of Chikungunya infection in children. PLoS ONE 2019, 14, e0211036. [Google Scholar]
- Natrajan, M.S.; Rojas, A.; Waggoner, J.J. Beyond Fever and Pain: Diagnostic Methods for Chikungunya Virus. J. Clin. Microbiol. 2019, 57, e00350-19. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Gérardin, P.; Taylor, A.; Mostafavi, H.; Malvy, D.; Mahalingam, S. Chikungunya Arthritis: Implications of Acute and Chronic Inflammation Mechanisms on Disease Management. Arthritis Rheumatol. 2018, 70, 484–495. [Google Scholar] [CrossRef]
- Jain, J.; Nayak, K.; Tanwar, N.; Gaind, R.; Gupta, B.; Shastri, J.S.; Bhatnagar, R.K.; Kaja, M.K.; Chandele, A.; Sunil, S. Clinical, Serological, and Virological Analysis of 572 Chikungunya Patients From 2010 to 2013 in India. Clin. Infect. Dis. 2017, 65, 133–140. [Google Scholar] [CrossRef]
- Nayak, K.; Jain, V.; Kaur, M.; Khan, N.; Gottimukkala, K.; Aggarwal, C.; Sagar, R.; Gupta, S.; Rai, R.C.; Dixit, K.; et al. Antibody response patterns in chikungunya febrile phase predict protection versus progression to chronic arthritis. JCI Insight. 2020, 5, e130509. [Google Scholar] [CrossRef]
- Wang, S.M.; Ali, U.H.; Sekaran, S.D.; Thayan, R. Detection and Quantification of Chikungunya Virus by Real-Time RT-PCR Assay. In Chikungunya Virus: Methods and Protocols; Chu, J.J.H., Ang, S.K., Eds.; Springer: New York, NY, USA, 2016; pp. 105–117. [Google Scholar]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Edwards, C.J.; Welch, S.R.; Chamberlain, J.; Hewson, R.; Tolley, H.; Cane, P.A.; Lloyd, G. Molecular diagnosis and analysis of Chikungunya virus. J. Clin. Virol. 2007, 39, 271–275. [Google Scholar] [CrossRef]
- Cho, B.; Jeon, B.-Y.; Kim, J.; Noh, J.; Kim, J.; Park, M.; Park, S. Expression and Evaluation of Chikungunya Virus E1 and E2 Envelope Proteins for Serodiagnosis of Chikungunya Virus Infection. Yonsei Med. J. 2008, 49, 828–835. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Gupta, S.; Dudha, N.; Appaiahgari, M.; Bharati, K.; Gupta, D.; Gupta, Y.; Kumar, K.; Gabrani, R.; Sharma, S.; Gupta, A.; et al. Molecular cloning and characterization of chikungunya virus genes from Indian isolate of 2006 outbreak. J. Pharm. Res. 2012, 20125, 3860–3863. [Google Scholar]
- Panning, M.; Grywna, K.; van Esbroeck, M.; Emmerich, P.; Drosten, C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg. Infect. Dis. 2008, 14, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Wongkrajang, P.; Chinswangwatanakul, W.; Mokkhamakkun, C.; Chuangsuwanich, N.; Wesarachkitti, B.; Thaowto, B.; Laiwejpithaya, S.; Komkhum, O. Establishment of new complete blood count reference values for healthy Thai adults. Int. J. Lab. Hematol. 2018, 40, 478–483. [Google Scholar] [CrossRef]
- McCarthy, M.K.; Davenport, B.J.J.; Morrison, T.E. Chronic Chikungunya Virus Disease. In Chikungunya Virus; Heise, M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 55–80. [Google Scholar]
- Chelluboina, S.; Robin, S.; Aswathyraj, S.; Arunkumar, G. Persistence of antibody response in chikungunya. VirusDisease 2019, 30, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Coêlho, M.; Gouveia, P.; Bezerra, L.; Marques, C.; Duarte, A.; Valente, L.M.; Magalhães, V. Long-Term Persistence of Serum-Specific Anti-Chikungunya IgM Antibody-A Case Series of Brazilian Patients. Rev. Soc. Bras. Med. Trop. 2021, 54, e0855. [Google Scholar] [CrossRef]
- Suhrbier, A. Rheumatic manifestations of chikungunya: Emerging concepts and interventions. Nat. Rev. Rheumatol. 2019, 15, 597–611. [Google Scholar] [CrossRef]
- Paixao, E.S.; Rodrigues, L.C.; Costa, M.; Itaparica, M.; Barreto, F.; Gerardin, P.; Teixeira, M.G. Chikungunya chronic disease: A systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 301–316. [Google Scholar] [CrossRef]
- Parola, P.; de Lamballerie, X.; Jourdan, J.; Rovery, C.; Vaillant, V.; Minodier, P.; Brouqui, P.; Flahault, A.; Raoult, D.; Charrel, R.N. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg. Infect. Dis. 2006, 12, 1493–1499. [Google Scholar] [CrossRef]
- Huits, R.; De Kort, J.; Van Den Berg, R.; Chong, L.; Tsoumanis, A.; Eggermont, K.; Bartholomeeusen, K.; Ariën, K.K.; Jacobs, J.; Van Esbroeck, M.; et al. Chikungunya virus infection in Aruba: Diagnosis, clinical features and predictors of post-chikungunya chronic polyarthralgia. PLoS ONE. 2018, 13, e0196630. [Google Scholar] [CrossRef]
- Lim, C.K.; Nishibori, T.; Watanabe, K.; Ito, M.; Kotaki, A.; Tanaka, K.; Kurane, I.; Takasaki, T. Chikungunya virus isolated from a returnee to Japan from Sri Lanka: Isolation of two sub-strains with different characteristics. Am. J. Trop. Med. Hyg. 2009, 81, 865–868. [Google Scholar] [CrossRef]
- Chua, C.-L.; Sam, I.C.; Chiam, C.-W.; Chan, Y.-F. The neutralizing role of IgM during early Chikungunya virus infection. PLoS ONE. 2017, 12, e0171989. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.E.; Seaton, B.L.; Matud, J.L.; Batterman, H.J. Chikungunya virus RNA and antibody testing at a National Reference Laboratory since the emergence of Chikungunya virus in the Americas. Clin. Vaccine Immunol. CVI. 2015, 22, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Taslem Mourosi, J.; Khan, M.F.; Ullah, M.O.; Vanakker, O.M.; Hosen, M.J. Chikungunya outbreak in Bangladesh (2017): Clinical and hematological findings. PLoS Negl. Trop. Dis. 2020, 14, e0007466. [Google Scholar] [CrossRef] [PubMed]
- Imad, H.; Phadungsombat, J.; Nakayama, E.; Kludkleeb, S.; Matsee, W.; Ponam, T.; Suzuki, K.; Leaungwutiwong, P.; Piyaphanee, W.; Phum, W.; et al. Chikungunya Manifestations and Viremia in Patients Who Presented to the Fever Clinic at Bangkok Hospital for Tropical Diseases During the 2019 Outbreak in Thailand. Trop. Med. Infect. Dis. 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Rianthavorn, P.; Prianantathavorn, K.; Wuttirattanakowit, N.; Theamboonlers, A.; Poovorawan, Y. An outbreak of chikungunya in southern Thailand from 2008 to 2009 caused by African strains with A226V mutation. Int. J. Infect. Dis. 2010, 14 (Suppl. S3), e161–e165. [Google Scholar] [CrossRef]
- Khongwichit, S.; Chansaenroj, J.; Thongmee, T.; Benjamanukul, S.; Wanlapakorn, N.; Chirathaworn, C.; Poovorawan, Y. Large-scale outbreak of Chikungunya virus infection in Thailand, 2018–2019. PLoS ONE 2021, 16, e0247314. [Google Scholar] [CrossRef]
- Intayot, P.; Phumee, A.; Boonserm, R.; Sor-Suwan, S.; Buathong, R.; Wacharapluesadee, S.; Brownell, N.; Poovorawan, Y.; Siriyasatien, P. Genetic Characterization of Chikungunya Virus in Field-Caught Aedes aegypti Mosquitoes Collected during the Recent Outbreaks in 2019, Thailand. Pathogens. 2019, 8, 121. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef]
- Phadungsombat, J.; Imad, H.; Rahman, M.; Nakayama, E.E.; Kludkleeb, S.; Ponam, T.; Rahim, R.; Hasan, A.; Poltep, K.; Yamanaka, A.; et al. A Novel Sub-Lineage of Chikungunya Virus East/Central/South African Genotype Indian Ocean Lineage Caused Sequential Outbreaks in Bangladesh and Thailand. Viruses 2020, 12, 1319. [Google Scholar] [CrossRef]
- Phadungsombat, J.; Imad, H.; Nakayama, E.; Leaungwutiwong, P.; Ramasoota, P.; Nguitragool, W.; Matsee, W.; Piyaphanee, W.; Shioda, T. Spread of a Novel Indian Ocean Lineage Carrying E1-K211E/E2-V264A of Chikungunya Virus East/Central/South African Genotype across the Indian Subcontinent, Southeast Asia, and Eastern Africa. Microorganisms 2022, 10, 354. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, A.K.; Sukumaran, D.; Parida, M.; Dash, P.K. Two novel epistatic mutations (E1:K211E and E2:V264A) in structural proteins of Chikungunya virus enhance fitness in Aedes aegypti. Virology 2016, 497, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chareonviriyaphap, T.; Akratanakul, P.; Nettanomsak, S.; Huntamai, S. Larval habitats and distribution patterns of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Thailand. Southeast Asian J. Trop. Med. Public Health 2003, 34, 529–535. [Google Scholar] [PubMed]
| Patient Characteristics | Patients, N (%) | |
|---|---|---|
| Gender | Male | 69 (42.6) |
| Female | 93 (57.4) | |
| Age | 10 to 17 | 8 (4.9) |
| 18–30 | 31 (19.1) | |
| 31–45 | 50 (30.9) | |
| 46–60 | 54 (33.3) | |
| 60+ | 19 (11.7) | |
| Race | Thai | 154 (95.1) |
| Myanmar | 4 (2.5) | |
| Laos | 1 (0.6) | |
| Cambodia | 1 (0.6) | |
| Others | 2 (1.2) | |
| underlying diseases | Yes | 61 (37.7) |
| No known underlying disease | 91 (56.2) | |
| No data | 10 (6.2) |
| Parameters | RT-PCR | Total | ||
|---|---|---|---|---|
| >100 Copies/ml | <100 Copies/ml | |||
| CHIKV IgM | Positive | 28 | 85 | 113 |
| Negative | 49 | 0 | 49 | |
| Total | 77 | 85 | 162 | |
| Positive | 7 | 26 | 33 | |
| CHIKV IgG | Negative | 70 | 59 | 129 |
| Total | 77 | 85 | 162 | |
| Clinical Features | Presence of Symptom | Log10 Viral Load | p-Value |
|---|---|---|---|
| (%) | Mean ± SD | ||
| Fever | Yes (93.8) | 5.76 ± 2.51 | <0.05 |
| No (4.9) | 4.38 ± 0.38 | ||
| Headache | Yes (24.1) | 6.83 ± 3.46 | 0.028 |
| No (8.0) | 5.06 ± 1.94 | ||
| Arthralgia | Yes (81.5) | 5.90 ± 2.85 | 0.805 |
| No (2.5) | 6.26 ± 3.52 | ||
| Arthritis | Yes (17.3) | 6.32 ± 3.42 | 0.04 |
| No (3.7) | 4.90 ± 0.33 | ||
| Maculopapular rash | Yes (51.2) | 5.33 ± 2.30 | 0.271 |
| No (20.9) | 5.96 ± 2.96 | ||
| Neurological complications | Yes (0.6) | 3.75 ± 0.0 | 0.393 |
| No (3.1) | 7.91 ± 3.97 |
| Clinical Manifestations | All Patients n = 162 | Age Group | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Children 1 n = 8 | Young Adults 2 n = 31 | Middle-Aged Adults 3 n = 50 | Old Adults 4 n = 54 | Seniors 5 n = 19 | |||
| Fever | 152 (93.8) | 8 | 29 | 48 | 48 | 19 | 0.324 |
| Headache | 39 (24.1) | 3 | 7 | 13 | 12 | 4 | 0.464 |
| Arthralgia | 132 (81.5) | 6 | 21 | 41 | 47 | 17 | 0.305 |
| Arthritis | 28 (17.3) | 3 | 7 | 6 | 9 | 3 | 0.664 |
| Maculopapular rash | 83 (51.2) | 5 | 20 | 31 | 23 | 4 | 0.014 |
| Neurological complications | 1 (0.6) | 0 | 0 | 1 | 0 | 0 | 0.301 |
| Parameters | Male n = 69 | Female n = 93 | p-Value |
|---|---|---|---|
| Leukocytes/ | 4.5 (1.50–12.6) | 5.0 (2.4–14.6) | 0.893 |
| Hemoglobin (g/dL) | 14.4 (11.0–17.0) | 13.3 (8.8–15.5) | <0.05 |
| Lymphocytes (%) | 22.5 (3.0–53.0) | 24.7 (4.0–56.0) | 0.290 |
| Neutrophils (%) | 61.9 (3.6–85.0 | 62.0 (32.0–90.0) | 0.896 |
| Eosinophils (%) | 1.0 (0–18.0) | 1.0 (1.0–11.0) | 0.689 |
| Monocytes (%) | 5.0 (1.0–22.0) | 4.2 (1.0–10.0) | 0.084 |
| Basophils (%) | 0 (0–7.0) | 0 (0–2.0) | 0.291 |
| Hematocrit (%) | 43.1 (32.9–52.7) | 39.7 (14.0–46.6) | <0.05 |
| Platelets/ | 174.5 (4.0–507.0) | 216.5 (33.0–495.0) | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tun, Y.M.; Charunwatthana, P.; Duangdee, C.; Satayarak, J.; Suthisawat, S.; Likhit, O.; Lakhotia, D.; Kosoltanapiwat, N.; Sukphopetch, P.; Boonnak, K. Virological, Serological and Clinical Analysis of Chikungunya Virus Infection in Thai Patients. Viruses 2022, 14, 1805. https://doi.org/10.3390/v14081805
Tun YM, Charunwatthana P, Duangdee C, Satayarak J, Suthisawat S, Likhit O, Lakhotia D, Kosoltanapiwat N, Sukphopetch P, Boonnak K. Virological, Serological and Clinical Analysis of Chikungunya Virus Infection in Thai Patients. Viruses. 2022; 14(8):1805. https://doi.org/10.3390/v14081805
Chicago/Turabian StyleTun, Yin May, Prakaykaew Charunwatthana, Chatnapa Duangdee, Jantawan Satayarak, Sarocha Suthisawat, Oranit Likhit, Divya Lakhotia, Nathamon Kosoltanapiwat, Passanesh Sukphopetch, and Kobporn Boonnak. 2022. "Virological, Serological and Clinical Analysis of Chikungunya Virus Infection in Thai Patients" Viruses 14, no. 8: 1805. https://doi.org/10.3390/v14081805
APA StyleTun, Y. M., Charunwatthana, P., Duangdee, C., Satayarak, J., Suthisawat, S., Likhit, O., Lakhotia, D., Kosoltanapiwat, N., Sukphopetch, P., & Boonnak, K. (2022). Virological, Serological and Clinical Analysis of Chikungunya Virus Infection in Thai Patients. Viruses, 14(8), 1805. https://doi.org/10.3390/v14081805






