Abstract
Hepatitis B “e” antigen (HBeAg) negative chronic hepatitis B (CHB), 40 years since discovery in the Mediterranean area, has become the most prevalent form of HBV-induced liver disease worldwide and a major health care burden caused by HBV infection. A great deal of knowledge accumulated over the last decades provides consistent evidence on the bimodal dynamics of the expression of structural and non-structural forms of the viral core proteins which associate with different virologic and clinic–pathologic outcomes of HBV infection. In absence of serum HBeAg, the presence and persistence of HBV replication causes and maintains virus-related liver injury. Thus, in clinical practice it is mandatory to screen HBV carriers with HBeAg-negative infection for the early diagnosis of HBeAg-negative CHB since antiviral therapy can cure HBV-induced liver disease when started at early stages.
1. Discovery
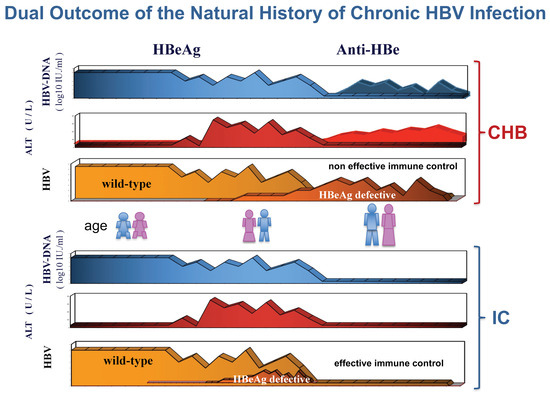
In the mid-1970s, intrahepatic hepatitis B core antigen (HBcAg) was the hallmark of active hepatitis B virus (HBV) replication in carriers of hepatitis B surface antigen (HBsAg) [1]. The HBcAg-positive immunohistochemical staining of the nuclei of HBV infected hepatocytes was indirect evidence of 22 nm viral nucleocapsid particles (core), seen in the electron microscope and associated with HBV-induced inflammatory liver disease [2]. Since its discovery in the serum, hepatitis B “e” antigen (HBeAg) was significantly associated with intrahepatic detection of HBcAg [3,4]. However, a significant proportion of HBeAg-negative/anti-HBe-positive carriers without intrahepatic HBcAg had evidence of unexplained chronic hepatitis, as only a proportion of patients showed hepatitis delta virus (HDV) coinfection [5]. The development of molecular diagnostic assays for detection of serum HBV-DNA, firstly presented at the 1980 AASLD meeting in Chicago, allowed a better understanding of their pathology [6]. Using this technique, our group found that serum HBV-DNA was present in both HBeAg-positive and HBeAg-negative patients with intrahepatic HBcAg [6]. Similar findings were confirmed worldwide after the diffusion of molecular biology techniques for detection of serum HBV-DNA and HBeAg-negative/anti-HBe-positive CHB was shown to prevail in the Mediterranean patients [7,8,9,10]. Serum HBV-DNA levels were lower in HBeAg-negative than HBeAg-positive patients [11,12,13,14]. In addition, a study from our group underlined a peculiar feature of the intrahepatic staining of HBcAg in HBeAg-negative/anti-HBe-positive patients, namely, the concomitant nuclear and cytoplasmic, rather than exclusively nuclear, localization [14]. According to the specific features of HBV infection in anti-HBe-positive patients, their disease was proposed as a distinct clinical entity from HBeAg-positive CHB [15]. Subsequently the study of its virologic characteristics led to the identification of HBeAg defective HBV mutants and their pathogenetic role in HBeAg-negative/anti-HBe-positive CHB [16,17,18,19]. The first study of the dynamic ratios between wild-type HBeAg-positive and HBeAg-negative variant populations within the circulating viral quasispecies of HBeAg-positive carriers revealed that precore G1896A HBeAg-minus HBV variants emerged at the time of hepatitis B exacerbations and were followed by the appearance of circulating antibodies against HBeAg (anti-HBe) [20]. Furthermore, CHB associated with HBeAg-minus HBV infection was characterized by transaminases (ALT) flares intervened with periods of complete ALT normalization: ALT flares were preceded by major increases of viral load that remained very low during the remission phases [20,21]. Altogether, these clinic–pathologic evidences suggested that genetic heterogeneity of the HBV precore region might significantly influence the course and outcome of chronic hepatitis B. The hypothesis was that wild-type HBV favors the induction of chronic infection secreting HBeAg, whereas HBeAg-defective variants, surging after the establishment of chronic infection, selectively prevail, displacing wild-type virus once HBeAg is recognized as immune target, since the lack of HBeAg expression provides a selective advantage to the virus during chronic inflammatory infection (Figure 1). In the last 30 years, a great deal of studies in animals and humans confirmed and further extended this seminal hypothesis.

Figure 1.
At the meeting on the Pathogenetic Implications of HBV Heterogeneity of 5–7 April 1991 in Sestriere, Italy, our group proposed, in a cartoon, depicted as poster, a hypothetical theory to explain the clinic–pathologic implications of the differential expression of HBeAg provided by wild-type HBeAg-positive virus and HBeAg-defective variant. The 4 pictures on the left side of the cartoon outline the induction of persistent HBV infection without liver injury favored by wild-type HBV population. The 4 pictures on the right side represent, in sequence from top to bottom, the induction of anti-HBV inflammatory response that leads to the selection of HBeAg-negative variants behaving as immune escape mutants.
2. Pathogenesis
HBV is not directly cytopathic, and chronic infection results from virus persistence because of a defective host’s immune response and inability to clear viral infection. A concerted innate and adaptive immune response is thought to be responsible for the control of viral infection and the clearance of intrahepatic necro-inflammation in primary HBV infection [22,23,24]. Humoral antibody response contributes to clearance of circulating virus particles, preventing viral spread, while cellular immune response silences the transcriptional activity of cccDNA by both non-cytolytic pathways and direct infected cell elimination. Anti-HBV T-cell response is vigorous, polyclonal, and multi-specific in acutely infected patients who successfully clear the infection, whereas a weak immune response leads to chronic infection and persistent liver cell injury, eventually leading to cirrhosis and hepatocellular carcinoma [22,23,24]. The stealth virus behavior that favors chronic infection as well as inflammation that causes acute and chronic hepatitis B are both influenced by the expression of proteins encoded by the precore/core region of the HBV genome [25].
The HBV nucleocapsid protein, core antigen (HBcAg), is a multifunctional protein that plays key roles in both viral lifecycle and the relationship between the virus and host’s immune system [26,27]. Within the nucleus of infected cells, HBcAg participates in the epigenetic regulation of the viral genome, interacting with supercoiled HBV-DNA, cccDNA, and host proteins and regulating the transcription of viral genes. Within the cytoplasm, HBcAg self-assembles into icosahedral viral nucleocapsids containing the HBV genome. HBcAg is strongly immunogenic, inducing antigen-specific T-cell responses which are critical for the immune control of HBV infection; however the role played by HBV nucleocapsid protein, particularly the secretory form, HBeAg, can change over time, favoring either the HBV immune evasion or eventually leading to the immune control of HBV infection.
HBeAg is a non-structural protein translated from precore mRNA that is processed in the ER, secreted in the extracellular space, and circulated in the blood. HBeAg is not required for viral replication or infection; nevertheless, it plays a key role in the viral–host interplay and the establishment of chronic HBV infection [28,29]. The importance of this non-structural protein is demonstrated by the fact that it is conserved in all ortho-hepadnaviruses [30]. Since its discovery, HBeAg has been regarded primarily as an HBV accessory protein and used in clinical practice as ancillary marker of active viral replication [31]. Subsequent studies in both animal models and humans provided consistent evidence that HBeAg contributes to the establishment of viral persistence in the absence of inflammatory liver disease in vertical, mother-to-child transmission because of the pivotal role played by serum HBeAg, which crosses the human placenta [28,32,33]. The multifaceted immunomodulatory functions of HBeAg include the downregulation of TLR2, NF-κB activation, and IL-18-mediated signal of IFNγ expression, promoting viral replication. The analysis of T-cell responses to HBe/HBcAg in cord blood of HBeAg-positive and HBeAg-negative newborns reported no HBe/HBcAg-specific responses in T-cells derived from HBeAg-positive cord blood [34,35]. Accordingly, HBeAg expressed as a transgene in utero or 3 days after birth was shown to elicit T-cell tolerance to HBeAg and HBcAg [35,36]. Furthermore, maternal-derived HBeAg was shown to alter macrophage function in non-transgenic offspring, where viral persistence requires both maternal-derived HBeAg and the presence of HBeAg in the periphery [36].
Epidemiologic evidence pointed out that in the absence of prophylaxis, perinatal transmission of HBV is frequent when the mothers are HBeAg-positive (70–90% within 3 months), but significantly less frequent in HBeAg-negative mothers with lower viral loads (<10%) [37]. These evidence agree with previous clinical observations that acute fulminant hepatitis B occurs in newborns to HBeAg-negative, but not in those born to HBeAg-positive, mothers. Furthermore, HBV viral strains with consistent prevalence of mutations in precore and core promoter regions of the viral genome (HBeAg defective variants) were isolated from fulminant hepatitis B cases at any age [38,39,40,41]. Accordingly, primary infections with HBeAg-defective variants in both adults and neonates rarely become chronic, and their presence is associated with an increased risk of severe acute hepatitis [42,43,44]. The evidence that acute and even fulminant hepatitis B and viral clearance can occur in neonates infected with HBeAg-defective variants argues against the hypothesis that an immature immune system and liver microenvironment of newborns are responsible for chronic HBV infection without liver inflammation in children born from HBeAg-positive mothers. In actuality, HBV exposure in utero was demonstrated to induce complex changes in the newborn’s immune system, including an advanced immune maturation state or “trained immunity” with pronounced Th1 profile, but associated with the absence of HBV-specific T-cell responses [45]. By contrast, in young patients, it was shown that the immune profile of T-cell was not of tolerance, but characterized by an HBV-specific immune profile less compromised than that observed in older patients [46]. Thus, the old concept of immune tolerance versus immune activation/elimination HBV infection phases was more properly changed in that of not-inflammatory vs. inflammatory chronic HBV infection [47]. Nevertheless, age can only partially explain some of the epidemiologic and clinic–pathological reports [48], and there is compelling evidence in vitro and in vivo in both animal models and humans that the inflammatory switch of HBV infection parallels the dynamics of HBeAg expression prompted by significant variations of the wild-type/HBV-defective HBV ratio within the infecting viral quasispecies. The impact of HBeAg expression on virus persistence was confirmed in the animal model where the infection of neonatal woodchucks with wild-type woodchuck hepatitis virus (WHV) expressing WHeAg elicited chronic infection, whereas infection with a WHeAg-negative virus caused acute self-limited hepatitis in the newborns [49].
The relevance of HBeAg secretion in the natural history of HBV infection is also supported by the evidence that its production is modulated at both transcriptional and translational levels. Interestingly, all HBV genotypes that may differentially support the G1896A switch, creating a translational stop codon on the leader protein of HBeAg, may produce variants able to modulate HBeAg production, such as basic core promoter mutants or other mutants in the ATG of precore sequence, which are more frequently observed in genotype A, B, and C [50,51]. The G1896A mutation prevails in HBV genotype D because it confers a higher base-pairing stability of the stem loop of the encapsidation signal of the pregenomic RNA of this genotype. Such an advantage does not occur in genotypes A and H and to a lesser extent in genotypes B, C, and E [52,53,54], that, however, carry other mutations responsible for the lack of HBeAg production.
In spite of a higher replicative fitness shown in vitro by HBeAg-defective HBV variants, serum HBV-DNA levels are lower in HBeAg-negative than HBeAg-positive CHB [55]. This impairment in virion productivity is not thought to be related to precore and basic core promoter (BCP) mutants, but to result from the virus/host’s immune system interplay [56,57,58,59,60]. The different replication fitness conferred by the different BCP/precore mutations according to infecting viral genotypes may explain the different natural history and epidemiology of HBV infection in geographical areas with different HBV genotype prevalence [61,62]. Accordingly, the longer-lasting HBeAg-positive infection in young females, before HBV vaccine implementation, was more frequent in areas with non-D HBV genotype endemicity, causing higher mother-to-child HBV transmission rate, persistent HBeAg-positive infection in newborns, and higher HBV endemicity in the same geographical areas.
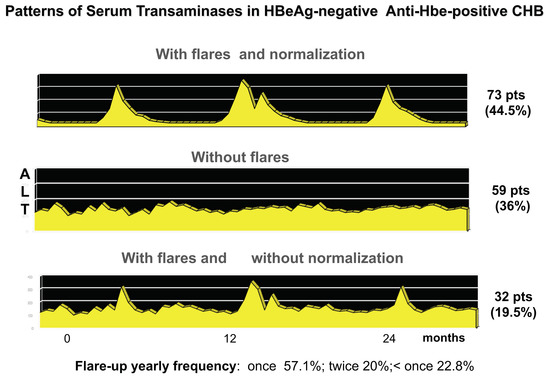
During chronic HBV infection, the reduced production of HBeAg is associated with major changes of intrahepatic HBcAg expression from only nuclear to both nuclear and cytoplasmatic that parallel the activation of anti-HBV immune response [14,19]. Acute fulminant HBeAg-negative hepatitis B was associated with an overwhelming B cell response, specific for HBcAg [63,64], and in HBeAg-positive immune activation or HBV clearance phases, HBe/HBcAgs were primary targets of the T-cell response [65,66,67]. Plasmacytoid dendritic cells pulsed with HBe/HBcAg-peptides stimulate T-cells derived from HBeAg-negative, but not HBeAg-positive, chronic patients [68]. Accordingly, in about one third of cases, the disease pattern of HBeAg-negative CHB is characterized by hepatitis exacerbations intervened by phases of complete normalization of serum transaminases which witness repeated, but ineffective, attempts at immune control of HBV replication [21]. The long-lasting ineffective immune elimination pattern induces the selection of HBeAg defective strains that become prevalent in the later phase of CHB natural history [69]. Thus, secretory HBeAg is tolerogenic, and cytosolic HBcAg is immunogenic and targets for HBe/HBcAg-specific CTLs once HBeAg-specific tolerance subsides (Figure 2).
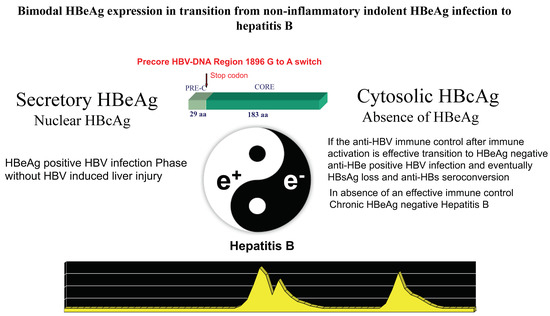
Figure 2.
The dynamic change of the protein expression coded by the precore/core HBV-DNA region from the secretory HBeAg to the cytosolic HBcAg pattern conditions the interplay between HBV replication and the host’s immune response. The G1896A mutation inducing a stop codon that blocks the production of the precore leader sequence necessary for HBeAg secretion provides a major functional switch modulating the dynamics of HBeAg expression associated with HBV-induced liver inflammation during chronic HBV infection.
4. Diagnosis
In clinical practice, it is mandatory to distinguish HBeAg-negative infection from HBeAg-negative CHB, because the former is characterized by persistently low replicative levels of HBV in the absence of HBV-induced liver disease and shows an overall survival comparable to HBV non-infected individuals [70]. HBeAg-negative CHB is associated with median serum HBV-DNA levels above 20,000 IU/mL, but the fluctuating pattern of viremia makes the diagnostic HBV-DNA testing at a single time point inadequate for a stringent early differential diagnosis of HBeAg-negative CHB from HBeAg-negative infection, particularly because at least one third of patients with HBeAg-negative CHB show, as previously discussed, a viraemia pattern characterized by major fluctuations, with temporary declines of HBV-DNA below the threshold of 2000–20,000 IU/mL. During such phases of low replication, transaminases also normalize, leading to a profile similar to that of HBeAg-negative infection (Figure 4) [21]. Therefore, a prolonged period (at least 1 year) of every 3 months of testing of HBV-DNA is required and recommended by International Guidelines for an accurate differential diagnosis between the two conditions [70].
Alternatively, the single point combined testing of both quantitative HBV-DNA and HBsAg consistently improves the diagnostic performance in the identification of HBeAg-negative infection carrier in the case of HBsAg ≤ 1000 IU/mL and HBV-DNA ≤ 2000 IU/mL [80]. However, this holds true mainly for genotype-D-infected individuals, because of the major influence of HBV genotypes on HBsAg serum levels [78]. Recently it was shown that hepatitis B core-related antigen (HBcrAg) levels may accurately differentiate HBeAg-negative infection from CHB: in a cohort of 1582 European HBeAg-negative carriers, the threshold of 3.14 log U/ml showed an AUROC of 0.968 (% CI 0.958–0.977) with 91 sensitivity, 93% specificity, and 92.4% diagnostic accuracy in the identification of HBeAg-negative CHB, independently of HBV genotype [81]. The question remains about the long-term outcome of HBeAg-negative carriers with viremia > 2000 IU/mL, but persistently ≤ 20,000 IU/mL with normal serum transaminases. An accurate prospective study of 153 HBeAg-negative HBsAg-carriers with HBV-DNA ≤ 20,000 IU/mL with an overall follow-up of 5 years showed that viraemia persistently ≤ 20,000 IU/mL predicts a benign clinical outcome: associated with transition to HBeAg-negative infection in 43% of cases, whereas only 13.1% of them showed progression to HBeAg-negative CHB, usually during the first year of follow-up [82]. Thus, highly stringent criteria, including the combined quantification of serum HBV-DNA, HBsAg, and possibly HBcrAg, should be used to screen HBV carriers with HBeAg-negative infection to diagnose CHB at its early stages when current antiviral therapy can provide a complete cure halting disease progression before advanced liver fibrosis develops [76].
5. Conclusions
HBeAg-negative CHB emergence, persistence, and outcome stem from the dynamics of HBeAg production and expression, which are modulated by naturally occurring precore/BCP mutants whose replicative fitness vary between HBV genotypes. HBeAg-negative variants provide selective advantages over wild-type HBeAg-positive HBV behaving as escape mutants during the spontaneous attempts of antiviral immune clearance. Upon antiviral immune activation, the majority (about 60%) of HBsAg-positive individuals with HBV-induced inflammatory liver disease spontaneously achieve an effective control of viral replication with clearance of intrahepatic inflammation and transition to an indolent HBeAg-negative infection phase; some of them eventually clear circulating HBsAg. Nevertheless, even after HBsAg loss, HBV persists within some hepatocytes in the form of viral mini-chromosome, supercoiled covalently closed HBV-DNA (cccDNA), which eventually triggers the reactivation of viral replication in case of impaired immune competence. The more rapid and effective the immune control of HBV replication after the triggering of immune activation phase, the lower the chance for selection of precore HBV mutants which are associated with both origin and persistence of HBeAg-negative CHB in patients with inadequate immune control of HBV infection. Further studies are needed to understand the fine mechanisms of anti-HBV immune activation that triggers HBV-specific liver inflammation that leads to the selection of HBeAg defective mutants during chronic HBV-induced inflammatory liver disease.
Author Contributions
F.B.: writing—original draft preparation; M.R.B. and F.B.: writing—review and editing; M.R.B. and P.C.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
F.B.: Advisory Board and Speakers Bureau for Abbott, DiaPro, DiaSorin, Echosens, Fujirebio, and Roche. M.R.B.: Advisory Board and Speakers’ Bureau AbbVie, Gilead, Janssen, EISAI-MSD, Roche.
References
- Gerber, M.A.; Hadziyannis, S.; Vissoulis, C.; Schaffner, F.; Paronetto, F.; Popper, H. Electron microscopy and immune-electron-microscopy of cytoplasmic hepatitis B antigen in hepatocytes. Am. J. Pathol. 1974, 75, 489–502. [Google Scholar] [PubMed]
- Gudat, F.; Bianchi, L.; Sonnabend, W.; Thiel, G.; Aenishaenslin, W.; Stadler, G.A. Pattern of core and surface expression in liver tissue reflects state of specific immune response in hepatitis. Lab. Investig. 1975, 32, 1–9. [Google Scholar] [PubMed]
- Magnius, L.O.; Espmark, J.A. New specificities in Australia antigen positive sera distinct from the Le Bouvier determinants. J. Immunol. 1972, 109, 1017–1021. [Google Scholar] [PubMed]
- Trepo, C.; Magnius, L.O.; Schacfer, R.A.; Prince, A.M. Detection of “e” antigen and antibody: Correlations with hepatitis B surface and hepatitis B core antigens, liver diseases, and outcome in hepatitis B infections. Gastroenterology 1976, 71, 804–808. [Google Scholar] [CrossRef]
- Rizzetto, M.; Canese, M.G.; Aricò, S.; Crivelli, O.; Trepo, C.; Bonino, F.; Verme, G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977, 18, 997–1003. [Google Scholar] [CrossRef]
- Bonino, F.; Hoyer, B.; Nelson, J.; Engle, R.; Verme, G.; Gerin, J. Hepatitis B virus DNA in the sera of HBsAg carriers: A marker of active hepatitis B virus replication in the liver. Hepatology 1981, 1, 386–391. [Google Scholar] [CrossRef]
- Brechot, C.; Hadchouel, M.; Scotto, J.; Degos, F.; Charnay, P.; Trepo, C.; Tiollais, P. Detection of hepatitis B virus DNA in liver and serum: A direct appraisal of the chronic carrier state. Lancet 1981, 2, 765–768. [Google Scholar] [CrossRef]
- Weller, I.V.; Fowler, M.J.; Monjardino, J.; Thomas, H.C. The detection of HBV-DNA in serum by molecular hybridization: A more sensitive method for the detection of complete HBV particles. J. Med. Virol. 1982, 9, 273–280. [Google Scholar] [CrossRef]
- Kam, W.; Rall, L.B.; Smuckler, E.A.; Schmid, R.; Rutter, W.J. Hepatitis B viral DNA in liver and serum of asymptomatic carriers. Proc. Natl. Acad. Sci. USA 1982, 79, 7522–7526. [Google Scholar] [CrossRef]
- Lieberman, H.M.; LaBrecque, D.R.; Kew, M.C.; Hadziyannis, S.J.; Shafritz, D.A. Detection of hepatitis B virus DNA directly in human serum by a simplified molecular hybridization test: Comparison to HBeAg/anti-HBe status in HBsAg carriers. Hepatology 1983, 3, 285–291. [Google Scholar] [CrossRef]
- Hadziyannis, S.J.; Lieberman, H.M.; Karvountzis, G.G.; Shafritz, D.A. Analysis of liver disease, nuclear HBcAg, viral replication, and hepatitis B virus DNA in liver and serum of HBeAg vs. anti-HBe positive carriers of hepatitis B virus. Hepatology 1983, 3, 656–662. [Google Scholar] [CrossRef]
- Lok, A.S.; Hadziyannis, S.J.; Weller, I.V.; Karvountzis, M.G.; Monjardino, J.; Karayiannis, P.; Montano, L.; Thomas, H.C. Contribution of low level HBV replication to continuing inflammatory activity in patients with anti-HBe positive chronic hepatitis B virus infection. Gut 1984, 25, 1283–1287. [Google Scholar] [CrossRef]
- Karayiannis, P.; Fowler, M.J.; Lok, A.S.; Greenfield, C.; Monjardino, J.; Thomas, H.C. Detection of serum HBV-DNA by molecular hybridization. Correlation with HBeAg/anti-HBe status, racial origin, liver histology and hepatocellular carcinoma. J. Hepatol. 1985, 1, 99–106. [Google Scholar] [CrossRef]
- Negro, F.; Chiaberge, E.; Oliviero, S.; Hammer, M.; Berninger, M.; Canese, M.G.; Bonino, F. Hepatitis B virus DNA (HBV-DNA) in anti-HBe positive sera. Liver 1984, 4, 177–183. [Google Scholar] [CrossRef]
- Bonino, F.; Rosina, F.; Rizzetto, M.; Rizzi, R.; Chiaberge, E.; Tardanico, R.; Callea, F.; Verme, G. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology 1986, 90 Pt 1, 1268–1273. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Stemler, M.; Schodel, F.; Will, H.; Ottobrelli, A.; Rizzetto, M.; Verme, G.; Bonino, F. Identification of HBV variants which cannot produce precore derived HBeAg and may be responsible for severe hepatitis. Ital. J. Gastroenterol. 1989, 21, 151–154. [Google Scholar]
- Carman, W.F.; Jacyna, M.R.; Hadziyannis, S.; Karayiannis, P.; McGarvey, M.J.; Makris, A.; Thomas, H.C. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet 1989, 2, 588–591. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Stemler, M.; Bonino, F.; Schodel, F.; Oliveri, F.; Rizzetto, M.; Verme, G.; Will, H. A new hepatitis B virus strain in patients with severe anti-HBe positive chronic hepatitis B. J. Hepatol. 1990, 10, 258–261. [Google Scholar] [CrossRef]
- Bonino, F.; Brunetto, M.R.; Rizzetto, M.; Will, H. Hepatitis B virus unable to secrete e antigen. Gastroenterology 1991, 100, 1138–1141. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Giarin, M.M.; Oliveri, F.; Chiaberge, E.; Baldi, M.; Alfarano, A.; Serra, A.; Saracco, G.; Verme, G.; Will, H.; et al. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc. Natl. Acad. Sci. USA 1991, 88, 4186–4190. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Oliveri, F.; Coco, B.; Leandro, G.; Colombatto, P.; Gorin, J.M.; Bonino, F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: A long term cohort study. J. Hepatol. 2002, 36, 263–270. [Google Scholar] [CrossRef]
- Chisari, F.V.; Isogawa, M.; Wieland, S.F. Pathogenesis of hepatitis B virus infection. Pathol. Biol. (Paris) 2010, 58, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Seeger, C.; Zoulim, F.; Mason, W.S. Hepadnaviruses. In Field’s Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2014; pp. 3376–3436. [Google Scholar]
- Lamontagne, R.J.; Bagga, S.; Bouchard, M.J. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016, 2, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.T.; Billaud, J.N.; Sällberg, M.; Guidotti, L.G.; Chisari, F.V.; Jones, J.; Hughes, J.; Milich, D.R. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl. Acad. Sci. USA 2004, 101, 14913–14918. [Google Scholar] [CrossRef]
- Zlotnick, A.; Venkatakrishnan, B.; Tan, Z.; Lewellyn, E.; Turner, W.; Francis, S. Core Protein: A Pleiotropic Keystone in the HBV Lifecycle. Antivir. Res. 2015, 121, 82–93. [Google Scholar] [CrossRef]
- Chong, C.K.; Cheng, C.Y.S.; Tsoi, S.Y.J.; Huang, F.Y.; Liu, F.; Seto, W.K.; Lai, C.-L.; Yuen, M.-F.; Wong, D.K.-H. Role of Hepatitis B Core Protein in HBV Transcription and Recruitment of Histone Acetyltransferases to cccDNA Mini-chromosome. Antivir. Res. 2017, 144, 1–7. [Google Scholar] [CrossRef]
- Milich, D.R. Is the function of the HBeAg really unknown? Hum. Vaccines Immunother. 2019, 15, 2187–2191. [Google Scholar] [CrossRef]
- Fenglin, Z.; Xiaoyu, X.; Xu, T.; Hongli, Y.; Miaomiao, T.; Huanran, L.; Chengyong, Q.; Jianni, Q.; Qiang, Z. The Functions of Hepatitis B Virus Encoding Proteins: Viral Persistence and Liver Pathogenesis. Front. Immunol. 2021, 12, 691766. [Google Scholar] [CrossRef]
- Revill, P.; Yuen, L.; Walsh, R.; Perrault, M.; Locarnini, S.; Kramvis, A. Bioinformatic analysis of the hepadnavirus e-antigen and its precursor identifies remarkable sequence conservation in all orthohepadnaviruses. J. Med. Virol. 2010, 82, 104–115. [Google Scholar] [CrossRef]
- Magnius, L.O.; Lindholm, A.; Lundin, P.; Iwarson, S.J. A new antigen-antibody system: Clinical significance in long-term carriers of hepatitis B surface antigen. JAMA 1975, 231, 356–359. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Chang, M.H.; Hsieh, K.H.; Lee, C.Y.; Lin, H.H.; Hwang, L.H.; Chen, P.J.; Chen, D.S. Cellular immune response to HBcAg in mother-to-infant transmission of hepatitis B virus. Hepatology 1992, 15, 770–776. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Yang, H.; Li, X.; Wen, S.; Guo, Y.; Sun, J.; Hou, J. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J. Med. Virol. 2003, 71, 360–366. [Google Scholar] [CrossRef]
- Milich, D.R.; Jones, J.E.; Hughes, J.L.; Price, J.; Raney, A.K.; McLachlan, A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc. Natl. Acad. Sci. USA 1990, 87, 6599–6603. [Google Scholar] [CrossRef]
- Chen, M.; Sällberg, M.; Hughes, J.; Jones, J.; Guidotti, L.G.; Chisari, F.V.; Billaud, J.N.; Milich, D.R. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 2005, 79, 3016–3027. [Google Scholar] [CrossRef]
- Tian, Y.; Kuo, C.-F.; Akbari, O.; Ou, J.-H.J. Maternal-derived hepatitis B virus e antigen alters macrophage function in offspring to drive viral persistence after vertical transmission. Immunity 2016, 44, 1204–1214. [Google Scholar] [CrossRef]
- Hadziyannis, S.J.; Papatheodoridis, G.V. Hepatitis B e antigen-negative chronic hepatitis B: Natural history and treatment. Semin. Liver. Dis. 2006, 26, 130–141. [Google Scholar] [CrossRef]
- Hasegawa, K.; Huang, J.K.; Wands, J.R.; Obata, H.; Liang, T.J. Association of hepatitis B viral precore mutations with fulminant hepatitis B in Japan. Virology 1991, 185, 460–463. [Google Scholar] [CrossRef]
- Liang, T.J.; Hasegawa, K.; Rimon, N.; Wands, J.R.; Ben-Porath, E. A hepatitis B virus mutant associated with an epidemic of fulminant hepatitis. N. Engl. J. Med. 1991, 324, 1705–1709. [Google Scholar] [CrossRef]
- Sato, S.; Suzuki, K.; Akahane, Y.; Akamatsu, K.; Akiyama, K.; Yunomura, K.; Tsuda, F.; Tanaka, T.; Okamoto, H.; Miyakawa, Y.; et al. Hepatitis B virus strains with mutations in the core promoter in patients with fulminant hepatitis. Ann. Intern. Med. 1995, 122, 241–248. [Google Scholar] [CrossRef]
- Friedt, M.; Gerner, P.; Wintermeyer, P.; Wirth, S. Complete hepatitis B virus genome analysis in HBsAg positive mothers and their infants with fulminant hepatitis B. BMC Gastroenterol. 2004, 4, 11. [Google Scholar] [CrossRef][Green Version]
- Fagan, E.A.; Smith, P.M.; Davison, F.; Williams, R. Fulminant hepatitis B in successive female sexual partners of two anti-HBe-positive males. Lancet 1986, 2, 538–540. [Google Scholar] [CrossRef]
- Terazawa, S.; Kojima, M.; Yamanaka, T.; Yotsumoto, S.; Okamoto, H.; Tsuda, F.; Miyakawa, Y.; Mayumi, M. Hepatitis B virus mutants with precore-region defects in two babies with fulminant hepatitis and their mothers positive for antibody to hepatitis B e antigen. Pediatr. Res. 1991, 29, 5–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, H.-L.; Chang, C.-J.; Kong, M.-S.; Huang, F.-C.; Lee, H.-C.; Lin, C.-C.; Liu, C.-C.; Lee, I.H.; Wu, T.-C.; Wu, S.-F.; et al. Pediatric fulminant hepatic failure in endemic areas of hepatitis B infection: 15 years after universal hepatitis B vaccination. Hepatology 2004, 39, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Sandalova, E.; Low, D.; Gehring, A.J.; Fieni, S.; Amadei, B.; Urbani, S.; Chong, Y.S.; Guccione, E.; Bertoletti, A. Trained immunity in newborn infants of HBV-infected mothers. Nat. Commun. 2015, 6, 6588. [Google Scholar] [CrossRef]
- Kennedy, P.T.F.; Sandalova, E.; Jo, J.; Gill, U.; Ushiro-Lumb, I.; Tan, A.T.; Naik, S.; Foster, G.R.; Bertoletti, A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012, 143, 637–645. [Google Scholar] [CrossRef]
- Bertoletti, A.; Kennedy, P. The immune tolerant phase of chronic HBV infection: New perspectives on an old concept. Cell. Mol. Immunol. 2015, 12, 258–263. [Google Scholar] [CrossRef]
- Bertoletti, A.; Hong, M. Age-Dependent Immune Events during HBV Infection from Birth to Adulthood: An Alternative Interpretation. Front. Immunol. 2014, 5, 441. [Google Scholar] [CrossRef]
- Chen, H.S.; Kew, M.C.; Hornbuckle, W.E.; Tennant, B.C.; Cote, P.J.; Gerin, J.L.; Purcell, R.H.; Miller, R.H. The precore gene of the woodchuck hepatitis virus genome is not essential for viral replication in the natural host. J. Virol. 1992, 66, 5682–5684. [Google Scholar] [CrossRef]
- Li, J.S.; Tong, S.P.; Wen, Y.M.; Vitvitski, L.; Zhang, Q.; Trépo, C. Hepatitis B virus genotype A rarely circulates as an HBe-minus mutant: Possible contribution of a single nucleotide in the precore region. J. Virol. 1993, 67, 5402–5410. [Google Scholar] [CrossRef]
- Okamoto, H.; Tsuda, F.; Akahane, Y.; Sugai, Y.; Yoshiba, M.; Moriyama, K.; Tanaka, T.; Miyakawa, Y.; Mayumi, M. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J. Virol. 1994, 68, 8102–8110. [Google Scholar] [CrossRef]
- Tong, S.P.; Li, J.S.; Vitvitski, L.; Trépo, C. Replication capacities of natural and artificial precore stop codon mutants of hepatitis B virus: Relevance of pregenome encapsidation signal. Virology 1992, 191, 237–245. [Google Scholar] [CrossRef]
- Lok, A.S.; Akarca, U.; Greene, S. Mutations in the pre-core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre-genome encapsidation signal. Proc. Natl. Acad. Sci. USA 1994, 91, 4077–4081. [Google Scholar] [CrossRef]
- Kramvis, A.; Arakawa, K.; Yu, M.C.; Nogueira, R.; Stram, D.O.; Kew, M.C. Relationship of serological subtype, basic core promoter and precore mutations to genotypes/subgenotypes of hepatitis B virus. J. Med. Virol. 2008, 80, 27–46. [Google Scholar] [CrossRef]
- Volz, T.; Lutgehetmann, M.; Wachtler, P.; Jacob, A.; Quaas, A.; Murray, J.M.; Dandri, M.; Petersen, J. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 2007, 133, 843–852. [Google Scholar] [CrossRef]
- Bozkaya, H.; Ayola, B.; Lok, A.S. High rate of mutations in the hepatitis B core gene during the immune clearance phase of chronic hepatitis B virus infection. Hepatology 1996, 24, 32–37. [Google Scholar] [CrossRef]
- Lim, S.G.; Cheng, Y.; Guindon, S.; Seet, B.L.; Lee, L.Y.; Hu, P.; Wasser, S.; Peter, F.J.; Tan, T.; Goode, M.; et al. Viral quasi-species evolution during hepatitis Be antigen seroconversion. Gastroenterology 2007, 133, 951–958. [Google Scholar] [CrossRef]
- Thompson, A.; Locarnini, S.; Visvanathan, K. The natural history and the staging of chronic hepatitis B: Time for revaluation of the virus-host relationship based on molecular virology and immunopathogenesis considerations? Gastroenterology 2007, 133, 1031–1035. [Google Scholar] [CrossRef]
- Croagh, C.M.; Desmond, P.V.; Bell, S.J. Genotypes and viral variants in chronic hepatitis B: A review of epidemiology and clinical relevance. World J. Hepatol. 2015, 7, 289–303. [Google Scholar] [CrossRef]
- Cavallone, D.; Ricco, G.; Oliveri, F.; Colombatto, P.; Moriconi, F.; Coco, B.; Romagnoli, V.; Salvati, A.; Surace, L.; Bonino, F.; et al. Do the circulating Pre-S/S quasispecies influence hepatitis B virus surface antigen levels in the HBeAg negative phase of HBV infection? Aliment Pharmacol. Ther. 2020, 51, 1406–1416. [Google Scholar] [CrossRef]
- McMahon, B.J. The influence of hepatitis B virus genotype and sub-genotype on the natural history of chronic hepatitis B. Hepatol. Int. 2009, 3, 334–342. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Brunetto, M.R.; Hadziyannis, S. The natural history of chronic- HBV infection and geographical differences. Antivir. Ther. 2010, 15, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kramvis, A.; Kostaki, E.-G.; Hatzakis, A.; Paraskevis, D. Immunomodulatory function of HBeAg related to short-sighted evolution, transmissibility, and clinical manifestation of hepatitis B virus. Front. Microbiol. 2018, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, A.; Tanaka, Y.; Orito, E.; Sugiyama, M.; Kang, J.-H.; Hige, S.; Kuramitsu, T.; Suzuki, K.; Tanaka, E.; Okada, S.; et al. Influence of genotypes and precore mutations on fulminant or chronic outcome of acute hepatitis B virus infection. Hepatology 2006, 44, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Farci, P.; Diaz, G.; Chen, Z.; Govindarajan, S.; Tice, A.; Agulto, L.; Pittaluga, S.; Boon, D.; Yu, C.; Engle, R.E.; et al. B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus-associated acute liver failure. Proc. Natl. Acad. Sci. USA 2010, 107, 8766–8771. [Google Scholar] [CrossRef]
- Chen, Z.; Diaz, G.; Pollicino, T.; Zhao, H.; Engle, R.E.; Schuck, P.; Shen, C.-H.; Zamboni, F.; Long, Z.; Kabat, J.; et al. Role of humoral immunity against hepatitis B virus core antigen in the pathogenesis of acute liver failure. Proc. Natl. Acad. Sci. USA 2018, 115, E11369–E11378. [Google Scholar] [CrossRef]
- Tsai, S.L.; Chen, P.J.; Lai, M.Y.; Yang, P.M.; Sung, J.L.; Huang, J.H.; Hwang, L.H.; Chang, T.H.; Chen, D.S. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J. Clin. Investig. 1992, 89, 87–96. [Google Scholar] [CrossRef]
- Martinet, J.; Leroy, V.; Dufeu-Duchesne, T.; Larrat, S.; Richard, M.J.; Zoulim, F.; Plumas, J.; Aspord, C. Plasmacytoid dendritic cells induce efficient stimulation of antiviral immunity in the context of chronic hepatitis B virus infection. Hepatology 2012, 56, 1706–1718. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Randone, A.; Ranki, M.; Jalanko, A.; Piantino, P.; Giarin, M.; Capra, G.; Calvo, P.L.; Oliveri, F.; Bonino, F. Quantitative analysis of wild-type and HBeAg minus hepatitis B viruses by a sequence-dependent primer extension assay. J. Med. Virol. 1994, 43, 310–315. [Google Scholar] [CrossRef]
- EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [CrossRef]
- Barbera, C.; Calvo, P.; Coscia, A.; Perugini, L.; Dastoli, G.; Randone, A.; Bonino, F.; Brunetto, M.R. Precore mutant hepatitis B virus and outcome of chronic infection and hepatitis in hepatitis B e antigen-positive children. Pediatr. Res. 1994, 36, 347–350. [Google Scholar] [CrossRef][Green Version]
- Colombatto, P.; Barbera, C.; Bortolotti, F.; Maina, A.M.; Moriconi, F.; Cavallone, D.; Calvo, P.; Oliveri, F.; Bonino, F.; Brunetto, M.R. HBV pre-core mutant in genotype-D infected children is selected during HBeAg/anti-HBe seroconversion and leads to HBeAg negative chronic hepatitis B in adulthood. J. Med. Virol. 2018, 90, 1232–1239. [Google Scholar] [CrossRef]
- Maruyama, T.; Mitsui, H.; Maekawa, H.; Yamada, H.; Hirayama, M.; Iino, S.; Yasuda, K.; Koike, K.; Kimura, S.; Milich, D.R. Emergence of the precore mutant late in chronic hepatitis B infection correlates with the severity of liver injury and mutations in the core region. Am. J. Gastroenterol. 2000, 95, 2894–2904. [Google Scholar] [CrossRef]
- Frelin, L.; Wahlström, T.; Tucker, A.E.; Jones, J.; Hughes, J.; Lee, B.O.; Billaud, J.-N.; Peters, C.; Whitacre, D.; Peterson, D.; et al. A mechanism to explain the selection of the hepatitis e antigen-negative mutant during chronic hepatitis B virus infection. J. Virol. 2009, 83, 1379–1392. [Google Scholar] [CrossRef]
- Ni, Y.H.; Chang, M.H.; Chen, P.J.; Tsai, K.S.; Hsu, H.Y.; Chen, H.L.; Tsuei, D.J.; Chen, D.S. Viremia profiles in children with chronic hepatitis B virus infection and spontaneous e antigen seroconversion. Gastroenterology 2007, 132, 2340–2345. [Google Scholar] [CrossRef]
- Colombatto, P.; Coco, B.; Bonino, F.; Brunetto, M.R. Management and Treatment of Patients with Chronic Hepatitis B: Towards Personalized Medicine. Viruses 2022, 14, 701. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Moriconi, F.; Bonino, F.; Lau, G.K.; Farci, P.; Yurdaydin, C.; Piratvisuth, T.; Luo, K.; Wang, Y.; Hadziyannis, S.; et al. Hepatitis B virus surface antigen levels: A guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009, 49, 1141–1150. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Marcellin, P.; Cherubini, B.; Yurdaydin, C.; Farci, P.; Hadziyannis, S.J.; Rothe, V.; Regep, L.; Bonino, F. Response to peginterferon alfa-2a (40KD) in HBeAg-negative CHB: On-treatment kinetics of HBsAg serum levels vary by HBV genotype. J. Hepatol. 2013, 59, 1153–1159. [Google Scholar] [CrossRef]
- Rijckborst, V.; Hansen, B.E.; Ferenci, P.; Brunetto, M.R.; Tabak, F.; Cakaloglu, Y.; Lanza, A.G.; Messina, V.; Iannacone, C.; Massetto, B.; et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J. Hepatol. 2012, 56, 1006–1011. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Oliveri, F.; Colombatto, P.; Moriconi, F.; Ciccorossi, P.; Coco, B.; Romagnoli, V.; Cherubini, B.; Moscato, G.; Maina, A.M.; et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology 2010, 139, 483–490. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Carey, I.; Maasoumy, B.; Marcos-Fosch, C.; Boonstra, A.; Caviglia, G.P.; Loglio, A.; Cavallone, D.; Scholtes, C.; Ricco, G.; et al. Incremental value of HBcrAg to classify 1582 HBeAg-negative individuals in chronic infection without liver disease or hepatitis. Aliment. Pharmacol. Ther. 2021, 53, 733–744. [Google Scholar] [CrossRef]
- Oliveri, F.; Surace, L.; Cavallone, D.; Colombatto, P.; Ricco, G.; Salvati, N.; Coco, B.; Romagnoli, V.; Gattai, R.; Salvati, A.; et al. Long-term outcome of inactive and active, low viremic HBeAg-negative-hepatitis B virus infection: Benign course towards HBsAg clearance. Liver. Int. 2017, 37, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).