Spillover of a Tobamovirus from the Australian Indigenous Flora to Invasive Weeds
Abstract
:1. Introduction
2. Materials and Methods
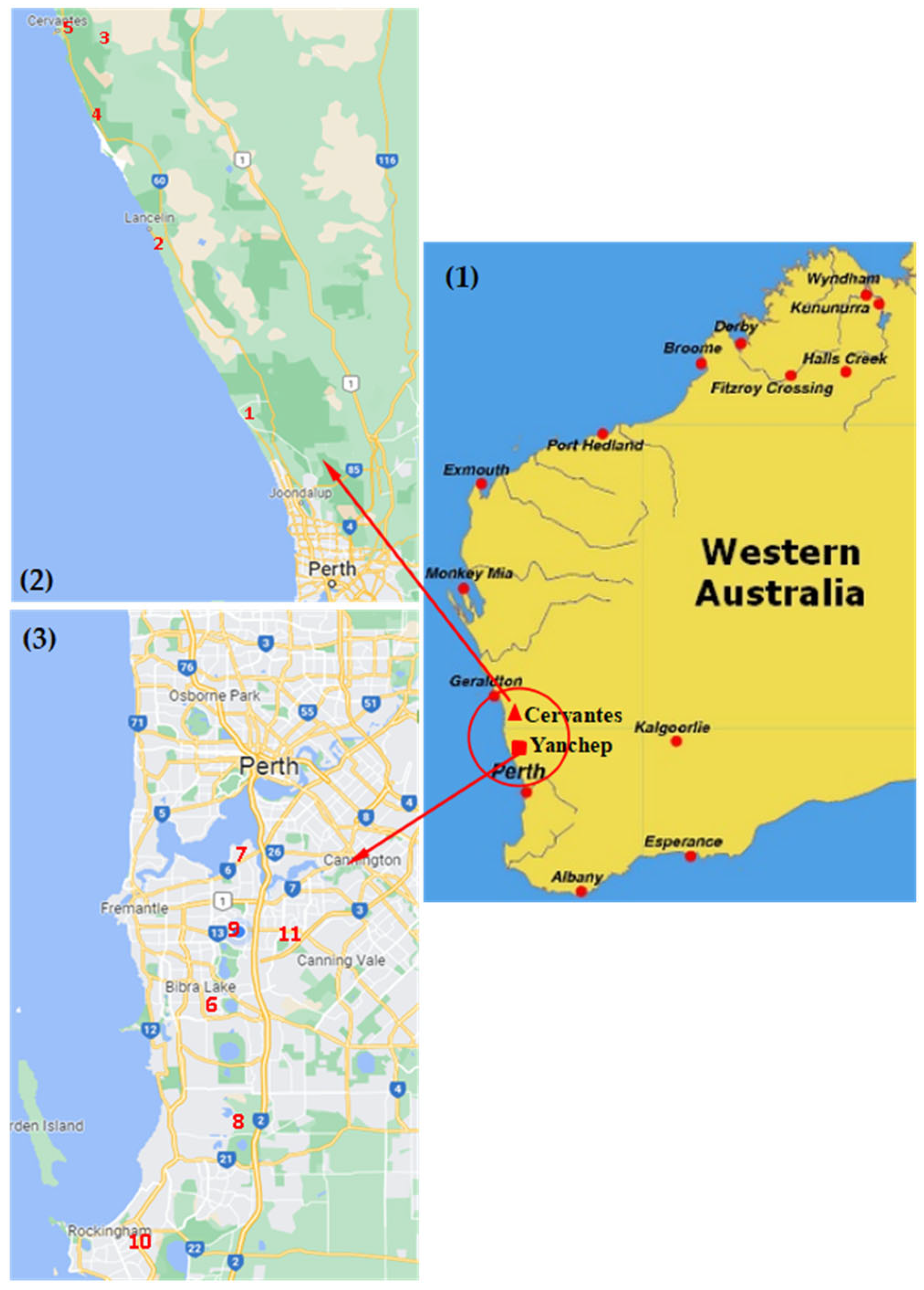
2.1. Sample Collection
2.2. RNA Extraction, Amplification, and Sequencing
2.3. Mechanical Inoculation of YTMMV
2.4. Vertical Transmission of YTMMV in Seed
3. Results
3.1. YTMMV Spillover
3.2. Sequences
3.3. Vertical Transmission of YTMMV in S. nigrum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alishiri, A.; Rakhshandehroo, F.; Zamanizadeh, H.R.; Palukaitis, P. Prevalence of tobacco mosaic virus in Iran and evolutionary analyses of the coat protein gene. Plant Pathol. J. 2013, 29, 260–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylie, S.J.; Li, H.; Jones, M.G. Yellow tailflower mild mottle virus: A new tobamovirus described from Anthocercis littorea (Solanaceae) in Western Australia. Arch. Virol. 2014, 159, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Wylie, S.; Li, H. A survey of natural distribution of yellow tailflower mild mottle virus in south-western Australia reveals new indigenous and exotic hosts. Australasian Plant Dis. Notes 2017, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Wylie, S.J.; Zhang, C.; Long, V.; Roossinck, M.J.; Koh, S.H.; Jones, M.G.; Iqbal, S.; Li, H. Differential responses to virus challenge of laboratory and wild accessions of Australian species of Nicotiana, and comparative analysis of RDR1 gene sequences. PLoS ONE 2015, 10, e0121787. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, C.; Luo, H.; Jones, M.G.K.; Sivasithamparam, K.; Koh, S.H.; Ong, J.W.L.; Wylie, S.J. Yellow tailflower mild mottle virus and pelargonium zonate spot virus co-infect a wild plant of red-striped tailflower in Australia. Plant Pathol. 2016, 65, 503–509. [Google Scholar] [CrossRef]
- Uehara-Ichiki, T.; Uke, A.; Hanada, K.; Hishida, A.; Nakazono-Nagaoka, E.; Kodaira, E. Scopolia mild mottle virus: A new tobamovirus isolated from a Scopolia japonica plant in Japan. Arch. Virol. 2022, 167, 947–951. [Google Scholar] [CrossRef]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 1, 2–3. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for MacOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Särkinen, T.; Poczai, P.; Barboza, G.E.; van der Weerden, G.M.; Baden, M.; Knapp, S. A revision of the Old World black nightshades (Morelloid clade of Solanum L., Solanaceae). PhytoKeys 2018, 106, 1–223. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.A.; Karl, B.J. Fleshy fruits of indigenous and adventive plants in the diet of birds in forest remnants, Nelson, New Zealand. N. Zeal. J. Ecol. 1996, 20, 127–145. [Google Scholar]
- Edmonds, J.M.; Chweya, J.A. Black Nightshades: Solanum nigrum L. and Related Species; Biodiversity International: Roma, Italy, 1997; Volume 15. [Google Scholar]
- Bravo, C.; Velilla, S.; Bautista, L.M.; Peco, B. Effects of great bustard (Otis tarda) gut passage on black nightshade (Solanum nigrum) seed germination. Seed Sci. Res. 2014, 24, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Reingold, V.; Lachman, O.; Blaosov, E.; Dombrovsky, A. Seed disinfection treatments do not sufficiently eliminate the infectivity of cucumber green mottle mosaic virus (CGMMV) on cucurbit seeds. Plant Pathol. 2015, 64, 245–255. [Google Scholar] [CrossRef]
- Mims, C.A. Vertical transmission of viruses. Microbiol. Rev. 1981, 45, 267–286. [Google Scholar] [CrossRef]
- Antonovics, J. Plant venereal diseases: Insights from a messy metaphor. New Phytol. 2005, 165, 71–80. [Google Scholar] [CrossRef]
- Gundel, P.E.; Martínez-Ghersa, M.A.; Omacini, M.; Cuyeu, R.; Pagano, E.; Ríos, R.; Ghersa, C.M. Mutualism effectiveness and vertical transmission of symbiotic fungal endophytes in response to host genetic background. Evol. Appl. 2012, 5, 838–849. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.A.C. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Smith, E. Seed transmission of tobamoviruses: Aspects of global disease distribution. Adv. Seed Biol. 2017, 12, 233–260. [Google Scholar] [CrossRef] [Green Version]
- Darzi, E.; Smith, E.; Shargil, D.; Lachman, O.; Ganot, L.; Dombrovsky, A. The honeybee Apis mellifera contributes to cucumber green mottle mosaic virus spread via pollination. Plant Pathol. 2018, 67, 244–251. [Google Scholar] [CrossRef]
- Smith, E.; Dombrovsky, A. Aspects in tobamovirus management in intensive agriculture. In Plant Diseases-Current Threats and Management Trends; IntechOpen: London, UK, 2019. [Google Scholar]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef] [PubMed]
- Keeley, P.E.; Thullen, R.J. Biology and control of black nightshade (Solanum nigrum) in cotton (Gossypium hirsutum). Weed Technol. 1991, 5, 713–722. [Google Scholar] [CrossRef]
- Haapalainen, M. Biology and epidemics of Candidatus Liberibacter species, psyllid-transmitted plant-pathogenic bacteria. Ann. Appl. Biol. 2014, 165, 172–198. [Google Scholar] [CrossRef]
- Kroschel, J.; Mujica, N.; Okonya, J.; Alyokhin, A. Insect pests affecting potatoes in tropical, subtropical, and temperate regions. In The Potato Crop; Springer: Cham, Switzerland, 2020; pp. 251–306. [Google Scholar]
- Bedford, I.D.; Kelly, A.; Banks, G.K.; Briddon, R.W.; Cenis, J.L.; Markham, P.G. Solanum nigrum: An indigenous weed reservoir for a tomato yellow leaf curl geminivirus in southern Spain. Eur. J. Plant Pathol. 1998, 104, 221–222. [Google Scholar] [CrossRef]
- Jordá, C.; Pérez, A.L.; Martínez Culebras, P.V.; Lacasa, A. First report of pepino mosaic virus on natural hosts. Plant Dis. 2001, 85, 1292–1292. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Kumari, R.; Singh, B.; Hallan, V.; Nagpal, A.K. First report of potato virus M, potato virus Y and cucumber mosaic virus infection in Solanum nigrum in India. J. Plant Pathol. 2019, 101, 419. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Liang, Y.; Zhong, X.; Hu, X.; Zhang, P.; Yu, X. Nightshade curly top virus: A possible new virus of the genus Topocuvirus infecting Solanum nigrum in China. Plant Dis. 2021, 105, 1006–1012. [Google Scholar] [CrossRef]
- Rebenstorf, K.; Candresse, T.; Dulucq, M.J.; Büttner, C.; Obermeier, C. Host species-dependent population structure of a pollen-borne plant virus, Cherry leaf roll virus. J. Virol. 2006, 80, 2453–2462. [Google Scholar] [CrossRef] [Green Version]
- Aramburu, J.; Galipienso, L.; Aparicio, F.; Soler, S.; López, C. Mode of transmission of Parietaria mottle virus. J. Plant Pathol. 2010, 92, 679–684. [Google Scholar]
- Sdoodee, R.; Teakle, D.S. Studies on the mechanism of transmission of pollen-associated tobacco streak ilarvirus virus by Thrips tabaci. Plant Pathol. 1993, 42, 88–92. [Google Scholar] [CrossRef]
- Greber, R.S.; Teakle, D.S.; Mink, G.I. Thrips-facilitated transmission of prune dwarf and prunus necrotic ringspot viruses from cherry pollen to cucumber. Plant Dis. 1992, 76, 1039–1041. [Google Scholar] [CrossRef]
| Site/Species | A. illicifolia | A. littoria | P. peruviana | S. nigrum | S. lycopersicum |
|---|---|---|---|---|---|
| Yanchep | 8/26 | — | 6/8 | 15/26 | — |
| Ledge Point | 5/5 | 0/28 | — | — | — |
| Lake Thetis | 13/21 | — | — | — | — |
| Cervantes Dirt Track | — | 3/5 | — | — | — |
| Cervantes sandhills | — | 12/23 | — | — | — |
| Bibra Lake | — | — | — | 2/11 | 0/1 |
| Canning River | — | — | — | 0/4 | — |
| Bertram | — | — | — | 11/17 | — |
| Murdoch | — | — | — | 0/4 | — |
| Dixon Road | — | — | — | 4/6 | — |
| Leeming | — | — | — | 13/24 | — |
| Infected/tested | 26/52 | 15/56 | 6/8 | 45/92 | 0/1 |
| Plant/Virus Code | Virus Presence a | Seed Germination at 21 Days (%) | Seedling Stem Height at 21 Days (cm) b | Seedling Cotyledon Length at 21 Days (cm) b | Virus Transmission in 10 Seed |
|---|---|---|---|---|---|
| LM2 | − | 54/56 (96.4%) | 1.77 ± 0.04 b | 1.81 ± 0.04 b | 0/10 |
| LM8 | − | 50/55 (90.9%) | 2.20 ± 0.04 a | 1.98 ± 0.03 a | 0/10 |
| LM1 | + | 51/56 (91.1%) | 1.33 ± 0.05 c | 1.36 ± 0.05 d | 7/10 |
| LM3 | + | 50/55 (90.9%) | 1.31 ± 0.03 c | 1.16 ± 0.03 e | 7/10 |
| LM4 | + | 28/55 (50.1%) | 1.41 ± 0.02 c | 1.36 ± 0.05 d | 8/10 |
| LMB3 | + | 44/55 (80.0%) | 1.69 ± 0.03 b | 1.48 ± 0.02 c | 7/10 |
| BTA5 | + | 22/55 (40.0%) | 1.18 ± 0.05 d | 1.14 ± 0.02 e | 5/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, H.; Sivasithamparam, K.; Tran, D.T.; Jones, M.G.K.; Chen, X.; Wylie, S.J. Spillover of a Tobamovirus from the Australian Indigenous Flora to Invasive Weeds. Viruses 2022, 14, 1676. https://doi.org/10.3390/v14081676
Xu W, Li H, Sivasithamparam K, Tran DT, Jones MGK, Chen X, Wylie SJ. Spillover of a Tobamovirus from the Australian Indigenous Flora to Invasive Weeds. Viruses. 2022; 14(8):1676. https://doi.org/10.3390/v14081676
Chicago/Turabian StyleXu, Weinan, Hua Li, Krishnapillai Sivasithamparam, Dieu Thi Tran, Michael G. K. Jones, Xin Chen, and Stephen J. Wylie. 2022. "Spillover of a Tobamovirus from the Australian Indigenous Flora to Invasive Weeds" Viruses 14, no. 8: 1676. https://doi.org/10.3390/v14081676
APA StyleXu, W., Li, H., Sivasithamparam, K., Tran, D. T., Jones, M. G. K., Chen, X., & Wylie, S. J. (2022). Spillover of a Tobamovirus from the Australian Indigenous Flora to Invasive Weeds. Viruses, 14(8), 1676. https://doi.org/10.3390/v14081676






