Susceptibility of Type I Interferon Receptor Knock-Out Mice to Heartland Bandavirus (HRTV) Infection and Efficacy of Favipiravir and Ribavirin in the Treatment of the Mice Infected with HRTV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Animals
2.3. Evaluation of Susceptibility of IFNAR−/− Mice to HRTV Infection
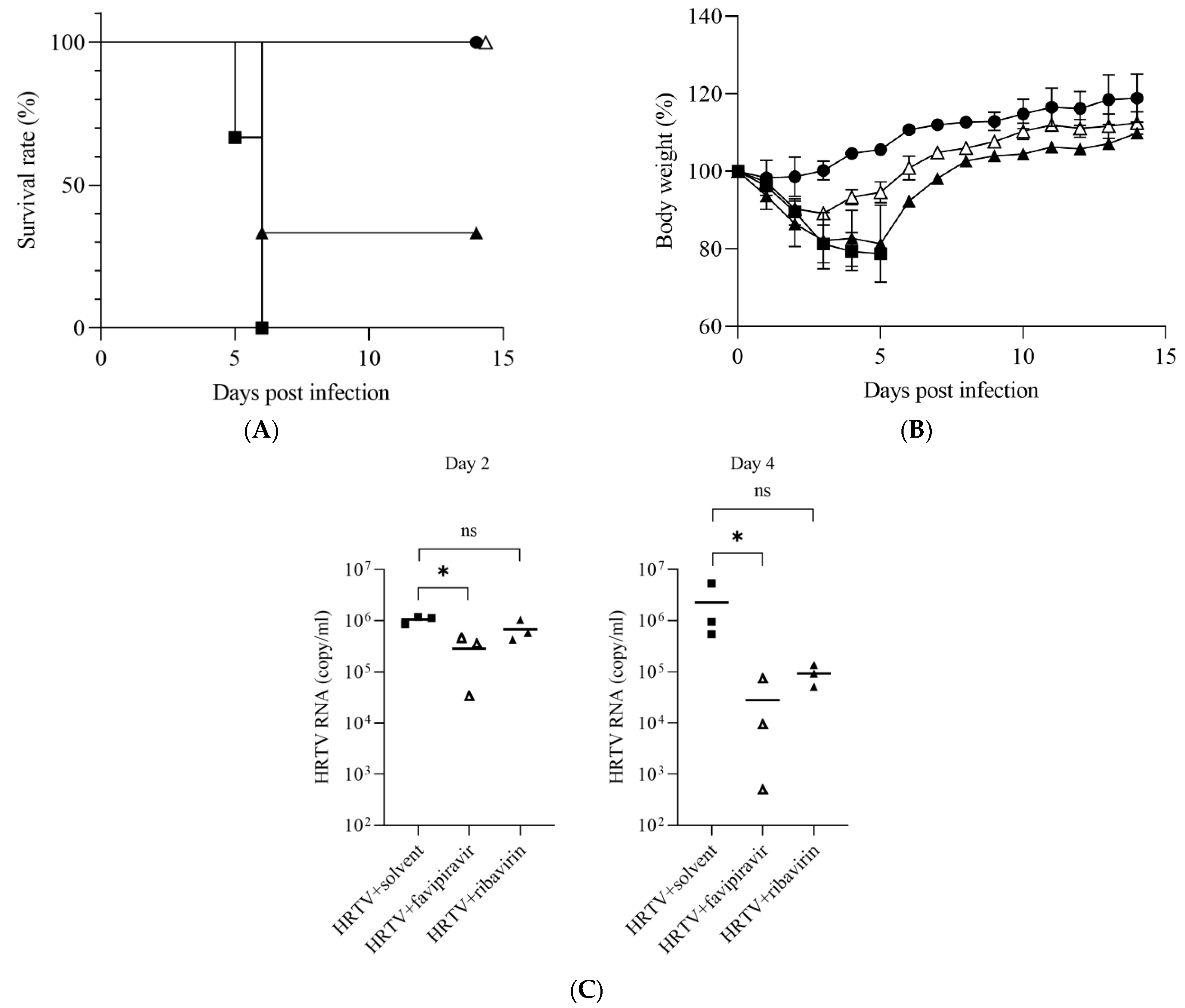
2.4. Evaluation of Antiviral Efficacy against HRTV Using IFNAR−/− Mice
2.5. Measurement of Viral RNA Levels in Blood
3. Results
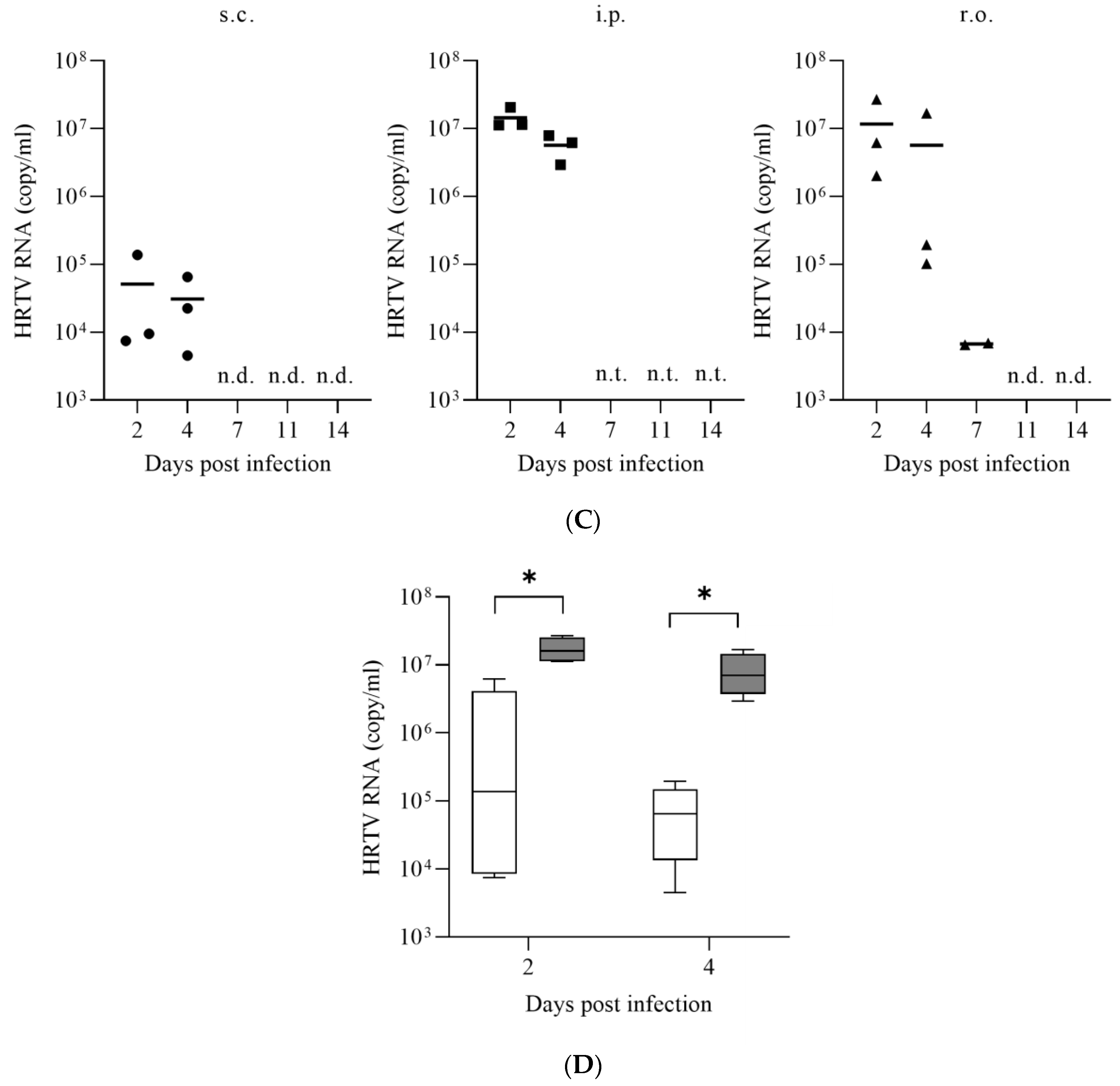
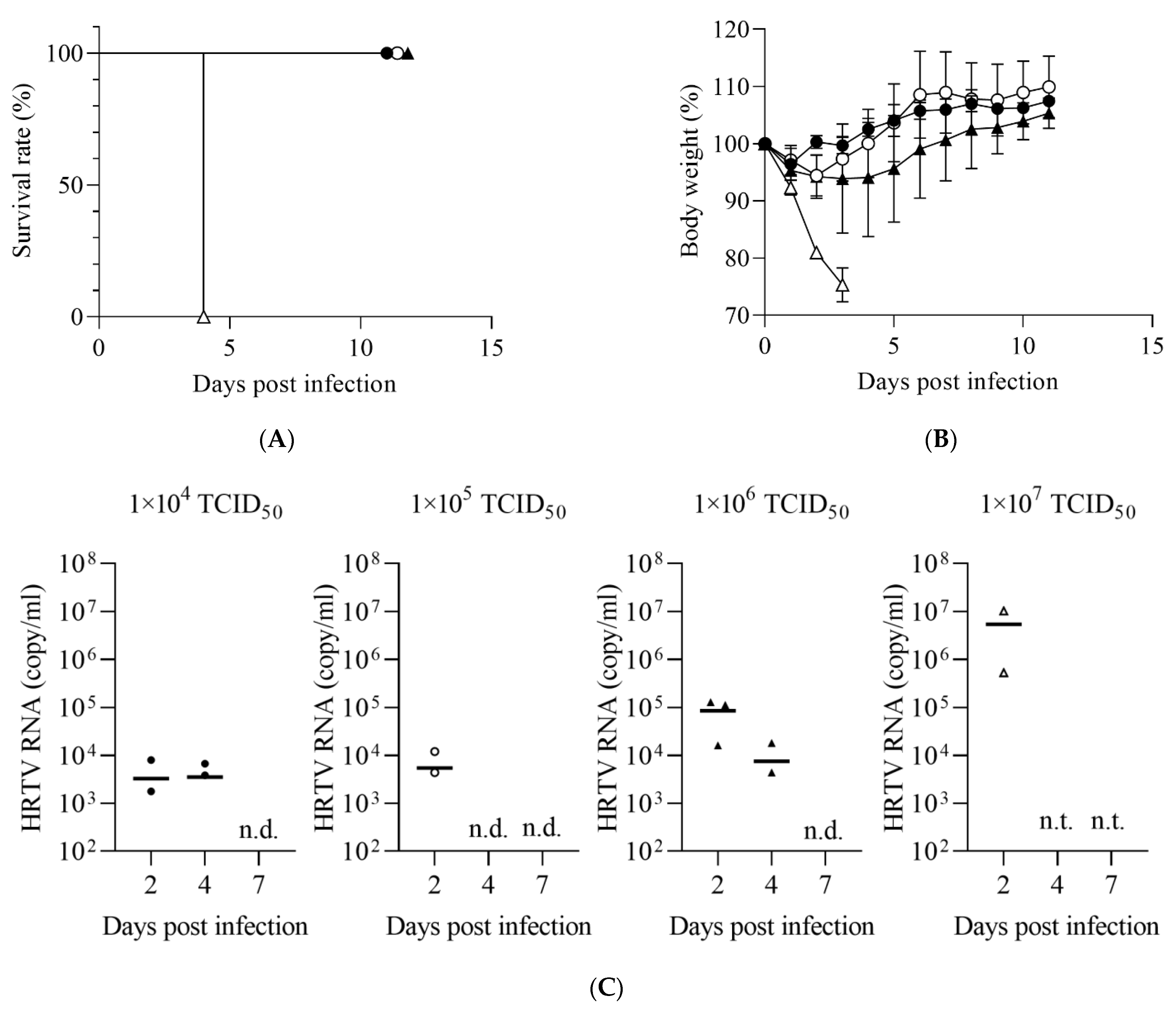
3.1. Susceptibility of IFNAR−/− Mice to HRTV
3.2. Antiviral Activities of Favipiravir and Ribavirin against HRTV In Vivo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhn, J.H.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Amarasinghe, G.K.; Anthony, S.J.; Avsic-Zupanc, T.; Ayllon, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2020, 165, 3023–3072. [Google Scholar] [CrossRef]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, N.J.C.; Han, H.J.; Park, S.J.; Choi, Y.K. Epidemiology of severe fever and thrombocytopenia syndrome virus infection and the need for therapeutics for the prevention. Clin. Exp. Vaccine Res. 2018, 7, 43–50. [Google Scholar] [CrossRef]
- Kim, K.H.; Yi, J.; Kim, G.; Choi, S.J.; Jun, K.I.; Kim, N.H.; Choe, P.G.; Kim, N.J.; Lee, J.K.; Oh, M.D. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg. Infect. Dis. 2013, 19, 1892–1894. [Google Scholar] [CrossRef]
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T.; et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef]
- Xu, B.; Liu, L.; Huang, X.; Ma, H.; Zhang, Y.; Du, Y.; Wang, P.; Tang, X.; Wang, H.; Kang, K.; et al. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: Discovery of a new bunyavirus. PLoS Pathog. 2011, 7, e1002369. [Google Scholar] [CrossRef]
- You, E.; Wang, L.; Zhang, L.; Wu, J.; Zhao, K.; Huang, F. Epidemiological characteristics of severe fever with thrombocytopenia syndrome in Hefei of Anhui Province: A population-based surveillance study from 2011 to 2018. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 929–939. [Google Scholar] [CrossRef]
- Tran, X.C.; Yun, Y.; van An, L.; Kim, S.H.; Thao, N.T.P.; Man, P.K.C.; Yoo, J.R.; Heo, S.T.; Cho, N.H.; Lee, K.H. Endemic Severe Fever with Thrombocytopenia Syndrome, Vietnam. Emerg. Infect. Dis. 2019, 25, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Ou, S.C.; Maeda, K.; Shimoda, H.; Chan, J.P.; Tu, W.C.; Hsu, W.L.; Chou, C.C. The first discovery of severe fever with thrombocytopenia syndrome virus in Taiwan. Emerg. Microbes Infect. 2020, 9, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/heartland-virus/statistics/index.html (accessed on 28 June 2022).
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albarino, C.G.; Zaki, S.R.; Rollin, P.E.; et al. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Muehlenbachs, A.; Fata, C.R.; Lambert, A.J.; Paddock, C.D.; Velez, J.O.; Blau, D.M.; Staples, J.E.; Karlekar, M.B.; Bhatnagar, J.; Nasci, R.S.; et al. Heartland virus-associated death in tennessee. Clin. Infect. Dis. 2014, 59, 845–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, N.P.; Menitove, J.E.; Biggerstaff, B.J.; Turabelidze, G.; Parton, P.; Peck, K.; Basile, A.J.; Kosoy, O.I.; Fischer, M.; Staples, J.E. Seroprevalence of Heartland Virus Antibodies in Blood Donors, Northwestern Missouri, USA. Emerg. Infect. Dis. 2019, 25, 358–360. [Google Scholar] [CrossRef]
- Silvas, J.A.; Aguilar, P.V. The Emergence of Severe Fever with Thrombocytopenia Syndrome Virus. Am. J. Trop. Med. Hyg. 2017, 97, 992–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brault, A.C.; Savage, H.M.; Duggal, N.K.; Eisen, R.J.; Staples, J.E. Heartland Virus Epidemiology, Vector Association, and Disease Potential. Viruses 2018, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Fill, M.A.; Compton, M.L.; McDonald, E.C.; Moncayo, A.C.; Dunn, J.R.; Schaffner, W.; Bhatnagar, J.; Zaki, S.R.; Jones, T.F.; Shieh, W.J. Novel Clinical and Pathologic Findings in a Heartland Virus-Associated Death. Clin. Infect. Dis. 2017, 64, 510–512. [Google Scholar] [PubMed]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013, 100, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Beaucourt, S.; Vignuzzi, M. Ribavirin: A drug active against many viruses with multiple effects on virus replication and propagation. Molecular basis of ribavirin resistance. Curr. Opin. Virol. 2014, 8, 10–15. [Google Scholar] [CrossRef]
- Shimojima, M.; Fukushi, S.; Tani, H.; Yoshikawa, T.; Fukuma, A.; Taniguchi, S.; Suda, Y.; Maeda, K.; Takahashi, T.; Morikawa, S.; et al. Effects of ribavirin on severe fever with thrombocytopenia syndrome virus in vitro. Jpn. J. Infect. Dis. 2014, 67, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Westover, J.B.; Rigas, J.D.; van Wettere, A.J.; Li, R.; Hickerson, B.T.; Jung, K.H.; Miao, J.; Reynolds, E.S.; Conrad, B.L.; Nielson, S.; et al. Heartland virus infection in hamsters deficient in type I interferon signaling: Protracted disease course ameliorated by favipiravir. Virology 2017, 511, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Fukuma, A.; Fukushi, S.; Taniguchi, S.; Yoshikawa, T.; Iwata-Yoshikawa, N.; Sato, Y.; Suzuki, T.; Nagata, N.; Hasegawa, H.; et al. Efficacy of T-705 (Favipiravir) in the Treatment of Infections with Lethal Severe Fever with Thrombocytopenia Syndrome Virus. mSphere 2016, 1, e00061-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Posadas-Herrera, G.; Aoki, K.; Morita, K.; Hayasaka, D. Therapeutic effect of post-exposure treatment with antiserum on severe fever with thrombocytopenia syndrome (SFTS) in a mouse model of SFTS virus infection. Virology 2015, 482, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tani, H.; Komeno, T.; Fukuma, A.; Fukushi, S.; Taniguchi, S.; Shimojima, M.; Uda, A.; Morikawa, S.; Nakajima, N.; Furuta, Y.; et al. Therapeutic effects of favipiravir against severe fever with thrombocytopenia syndrome virus infection in a lethal mouse model: Dose-efficacy studies upon oral administration. PLoS ONE 2018, 13, e0206416. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, B.; Paessler, S.; Walker, D.H.; Tesh, R.B.; Yu, X.J. The pathogenesis of severe fever with thrombocytopenia syndrome virus infection in alpha/beta interferon knockout mice: Insights into the pathologic mechanisms of a new viral hemorrhagic fever. J. Virol. 2014, 88, 1781–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, K.; Orba, Y.; Maede-White, K.; Scott, D.; Feldmann, F.; Liang, M.; Ebihara, H. Animal Models of Emerging Tick-Borne Phleboviruses: Determining Target Cells in a Lethal Model of SFTSV Infection. Front. Microbiol. 2017, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shimojima, M.; Narita, R.; Tsukamoto, Y.; Kato, H.; Saijo, M.; Fujita, T. RIG-I-Like Receptor and Toll-Like Receptor Signaling Pathways Cause Aberrant Production of Inflammatory Cytokines/Chemokines in a Severe Fever with Thrombocytopenia Syndrome Virus Infection Mouse Model. J. Virol. 2018, 92, e02246-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco-Lauth, A.M.; Calvert, A.E.; Root, J.J.; Gidlewski, T.; Bird, B.H.; Bowen, R.A.; Muehlenbachs, A.; Zaki, S.R.; Brault, A.C. Vertebrate Host Susceptibility to Heartland Virus. Emerg. Infect. Dis. 2016, 22, 2070–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, S.; Inagaki, T.; Tajima, S.; Suzuki, T.; Yoshikawa, T.; Fukushi, S.; Park, E.S.; Fujii, H.; Morikawa, S.; Tani, H.; et al. Reverse Genetics System for Heartland Bandavirus: NSs Protein Contributes to Heartland Bandavirus Virulence. J. Virol. 2022, 96, e0004922. [Google Scholar] [CrossRef]
- Savage, H.M.; Godsey, M.S.; Lambert, A.; Panella, N.A.; Burkhalter, K.L.; Harmon, J.R.; Lash, R.R.; Ashley, D.C.; Nicholson, W.L. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg. 2013, 89, 445–452. [Google Scholar] [CrossRef]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Deng, F.; Hu, Z.; Wang, H.; Ning, Y.J. Heartland virus antagonizes type I and III interferon antiviral signaling by inhibiting phosphorylation and nuclear translocation of STAT2 and STAT1. J. Biol. Chem. 2019, 294, 9503–9517. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.M.; Safronetz, D.; Kobinger, G.P. Syrian Hamsters as a Small Animal Model for Emerging Infectious Diseases: Advances in Immunologic Methods. Adv. Exp. Med. Biol. 2017, 972, 87–101. [Google Scholar] [PubMed]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, M.C.; Cheung, C.Y.; Chui, W.H.; Tsao, S.W.; Nicholls, J.M.; Chan, Y.O.; Chan, R.W.; Long, H.T.; Poon, L.L.; Guan, Y.; et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005, 6, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuyama, S.; Kawaoka, Y. The pathogenesis of influenza virus infections: The contributions of virus and host factors. Curr. Opin. Immunol. 2011, 23, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, J.; Kato, H.; Fujita, T. The Role of Non-Structural Protein NSs in the Pathogenesis of Severe Fever with Thrombocytopenia Syndrome. Viruses 2021, 13, 876. [Google Scholar] [CrossRef]
- Carlson, A.L.; Pastula, D.M.; Lambert, A.J.; Staples, J.E.; Muehlenbachs, A.; Turabelidze, G.; Eby, C.S.; Keller, J.; Hess, B.; Buller, R.S.; et al. Heartland Virus and Hemophagocytic Lymphohistiocytosis in Immunocompromised Patient, Missouri, USA. Emerg. Infect. Dis. 2018, 24, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Pastula, D.M.; Turabelidze, G.; Yates, K.F.; Jones, T.F.; Lambert, A.J.; Panella, A.J.; Kosoy, O.I.; Velez, J.O.; Fisher, M.; Staples, E.; et al. Prevention, Notes from the field: Heartland virus disease—United States, 2012–2013. MMWR Morb. Mortal Wkly Rep. 2014, 63, 270–271. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, H.; Tani, H.; Egawa, K.; Taniguchi, S.; Yoshikawa, T.; Fukushi, S.; Yamada, S.; Harada, S.; Kurosu, T.; Shimojima, M.; et al. Susceptibility of Type I Interferon Receptor Knock-Out Mice to Heartland Bandavirus (HRTV) Infection and Efficacy of Favipiravir and Ribavirin in the Treatment of the Mice Infected with HRTV. Viruses 2022, 14, 1668. https://doi.org/10.3390/v14081668
Fujii H, Tani H, Egawa K, Taniguchi S, Yoshikawa T, Fukushi S, Yamada S, Harada S, Kurosu T, Shimojima M, et al. Susceptibility of Type I Interferon Receptor Knock-Out Mice to Heartland Bandavirus (HRTV) Infection and Efficacy of Favipiravir and Ribavirin in the Treatment of the Mice Infected with HRTV. Viruses. 2022; 14(8):1668. https://doi.org/10.3390/v14081668
Chicago/Turabian StyleFujii, Hikaru, Hideki Tani, Kazutaka Egawa, Satoshi Taniguchi, Tomoki Yoshikawa, Shuetsu Fukushi, Souichi Yamada, Shizuko Harada, Takeshi Kurosu, Masayuki Shimojima, and et al. 2022. "Susceptibility of Type I Interferon Receptor Knock-Out Mice to Heartland Bandavirus (HRTV) Infection and Efficacy of Favipiravir and Ribavirin in the Treatment of the Mice Infected with HRTV" Viruses 14, no. 8: 1668. https://doi.org/10.3390/v14081668
APA StyleFujii, H., Tani, H., Egawa, K., Taniguchi, S., Yoshikawa, T., Fukushi, S., Yamada, S., Harada, S., Kurosu, T., Shimojima, M., Maeki, T., Lim, C.-K., Takayama-Ito, M., Komeno, T., Nakajima, N., Furuta, Y., Uda, A., Morikawa, S., & Saijo, M. (2022). Susceptibility of Type I Interferon Receptor Knock-Out Mice to Heartland Bandavirus (HRTV) Infection and Efficacy of Favipiravir and Ribavirin in the Treatment of the Mice Infected with HRTV. Viruses, 14(8), 1668. https://doi.org/10.3390/v14081668





