Molecular Characterization of Small Ruminant Lentiviruses Isolated from Polish Goats with Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Serology and Tissue Sample Collection
2.2. Histopathology and Immunohistochemistry
2.3. DNA Extraction, Amplification and Sequencing
2.4. Proviral Load Quantification
3. Results
3.1. Histopathology and Immunohistochemistry (IHC)
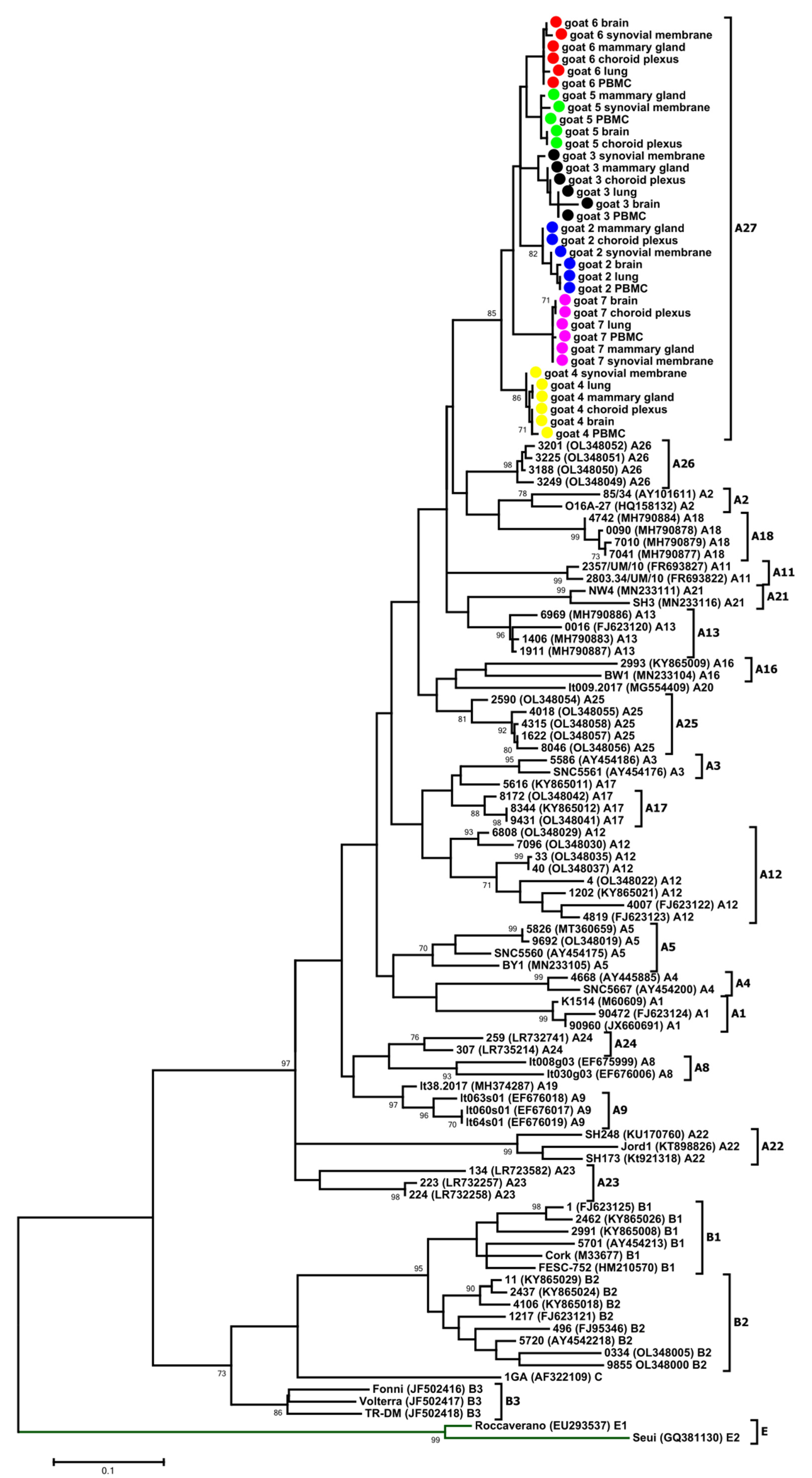
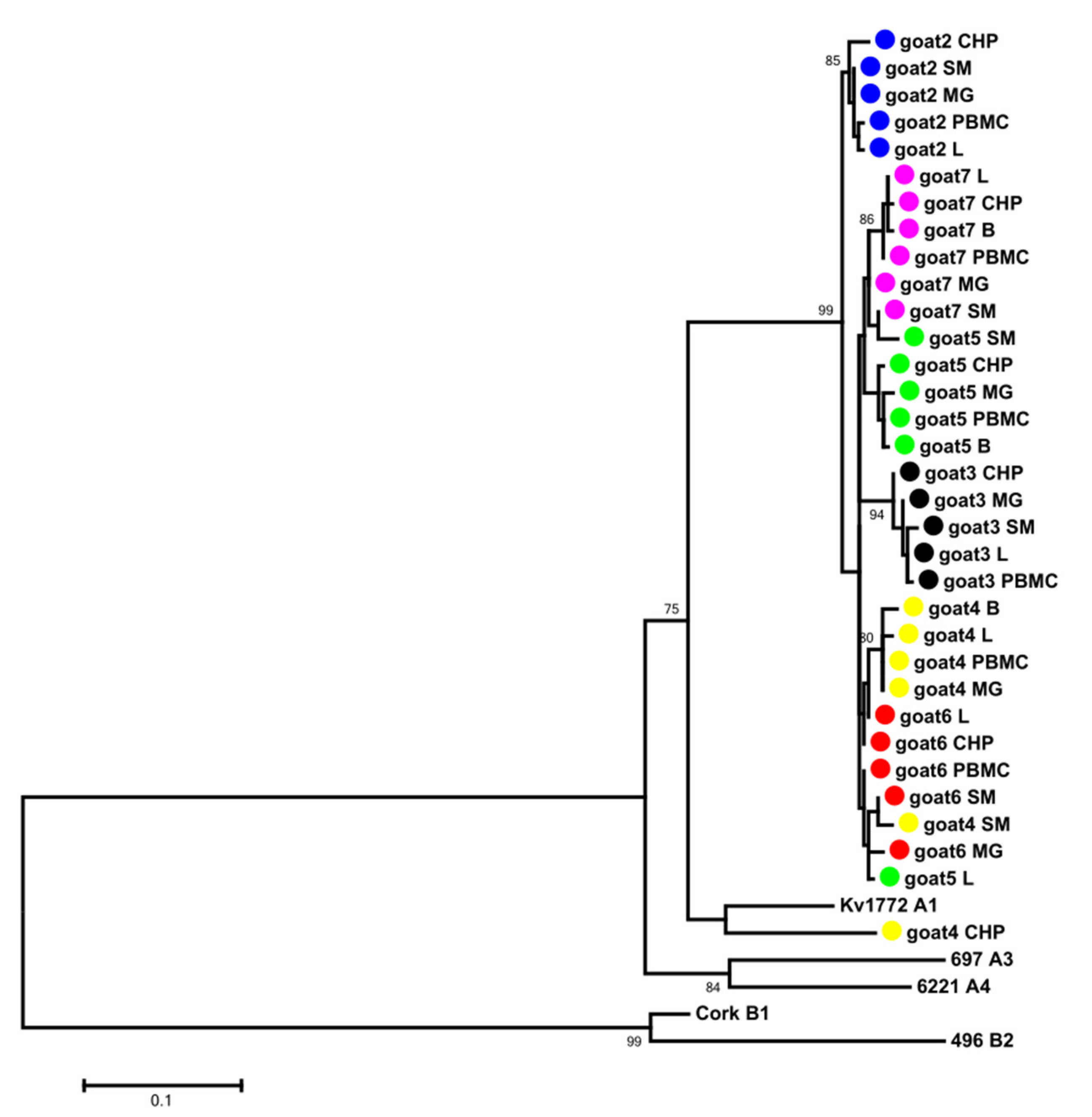
3.2. Phylogenetic Analysis
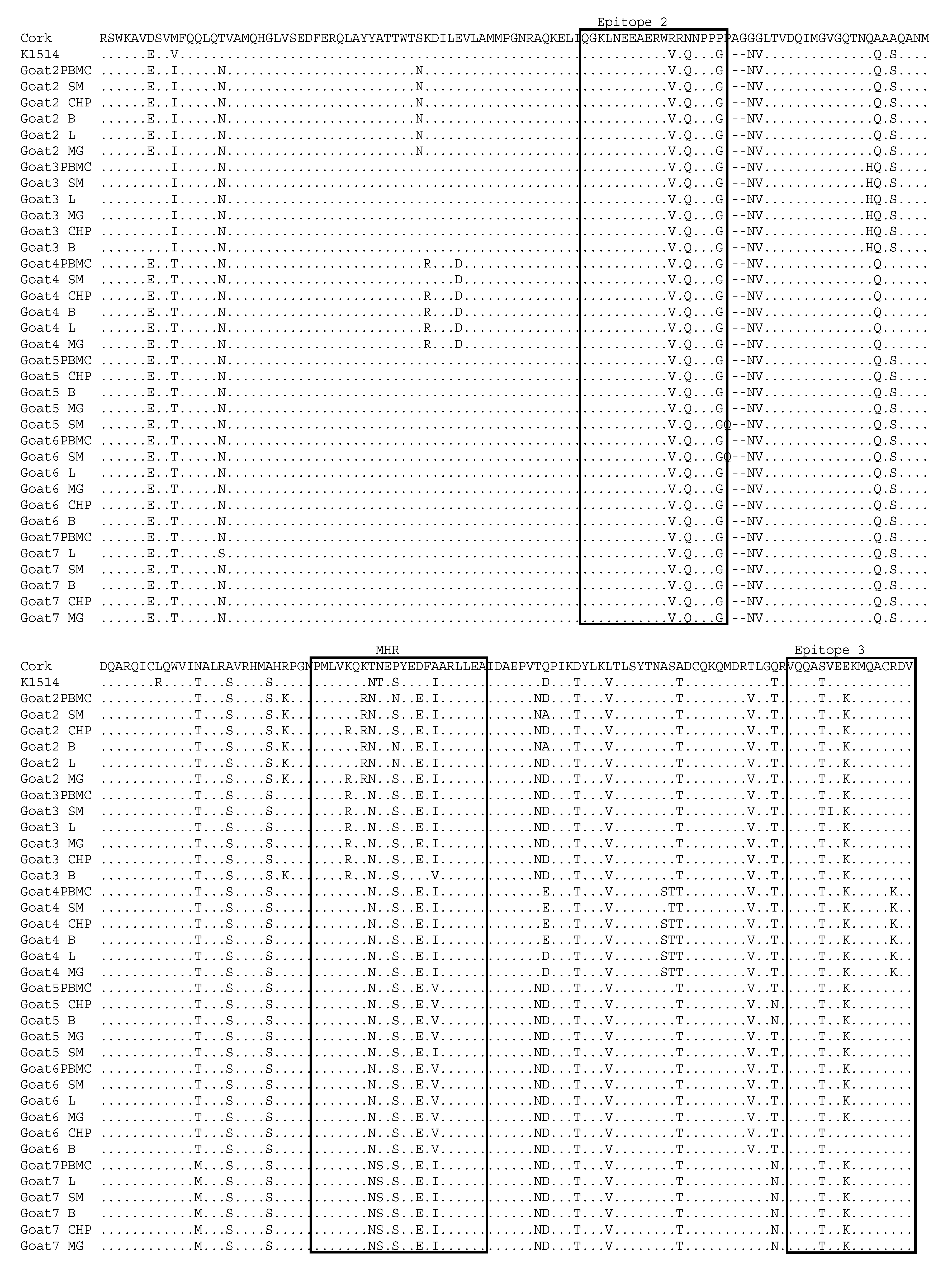
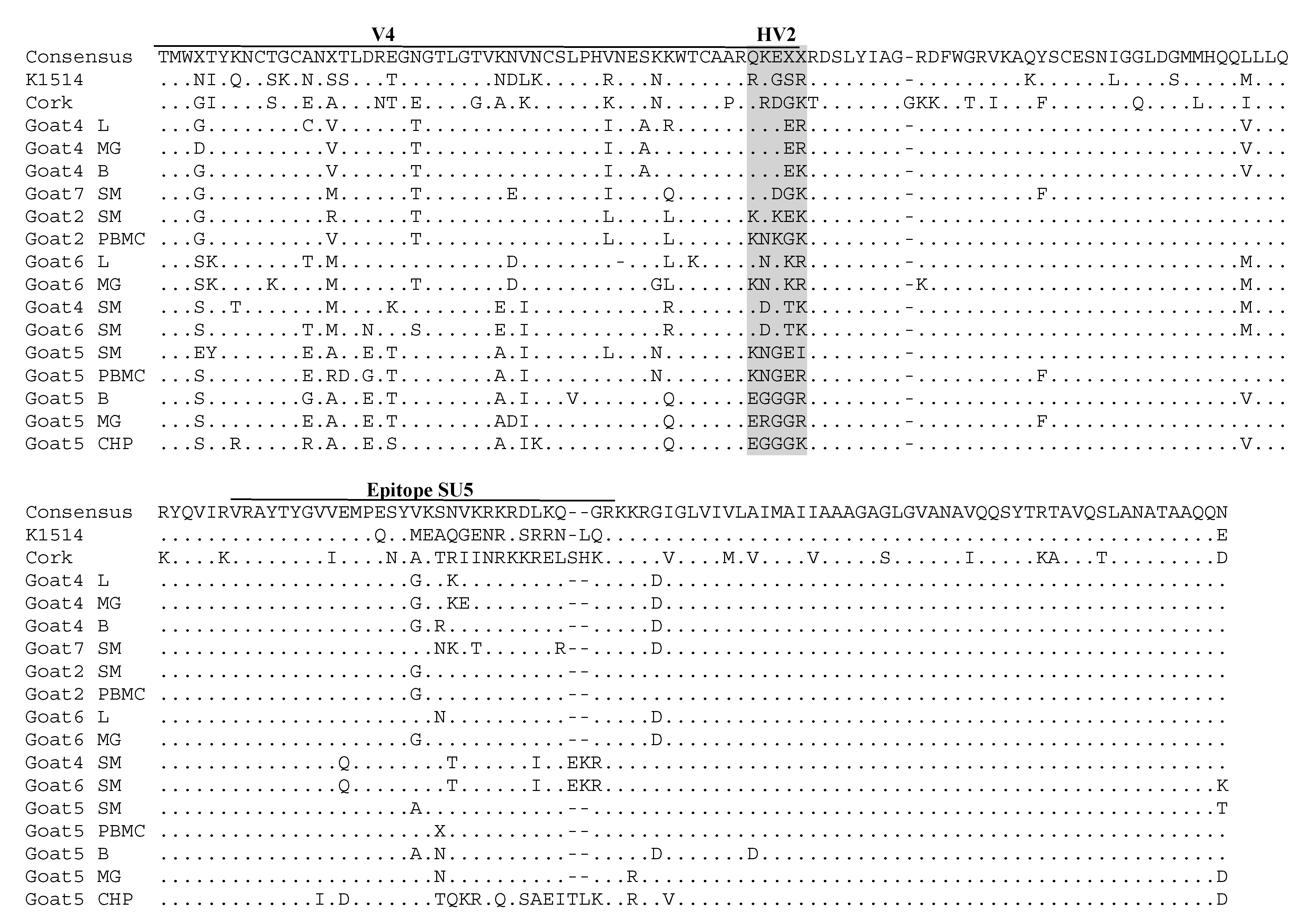
3.3. Comparative Analysis of Immunodominant Regions
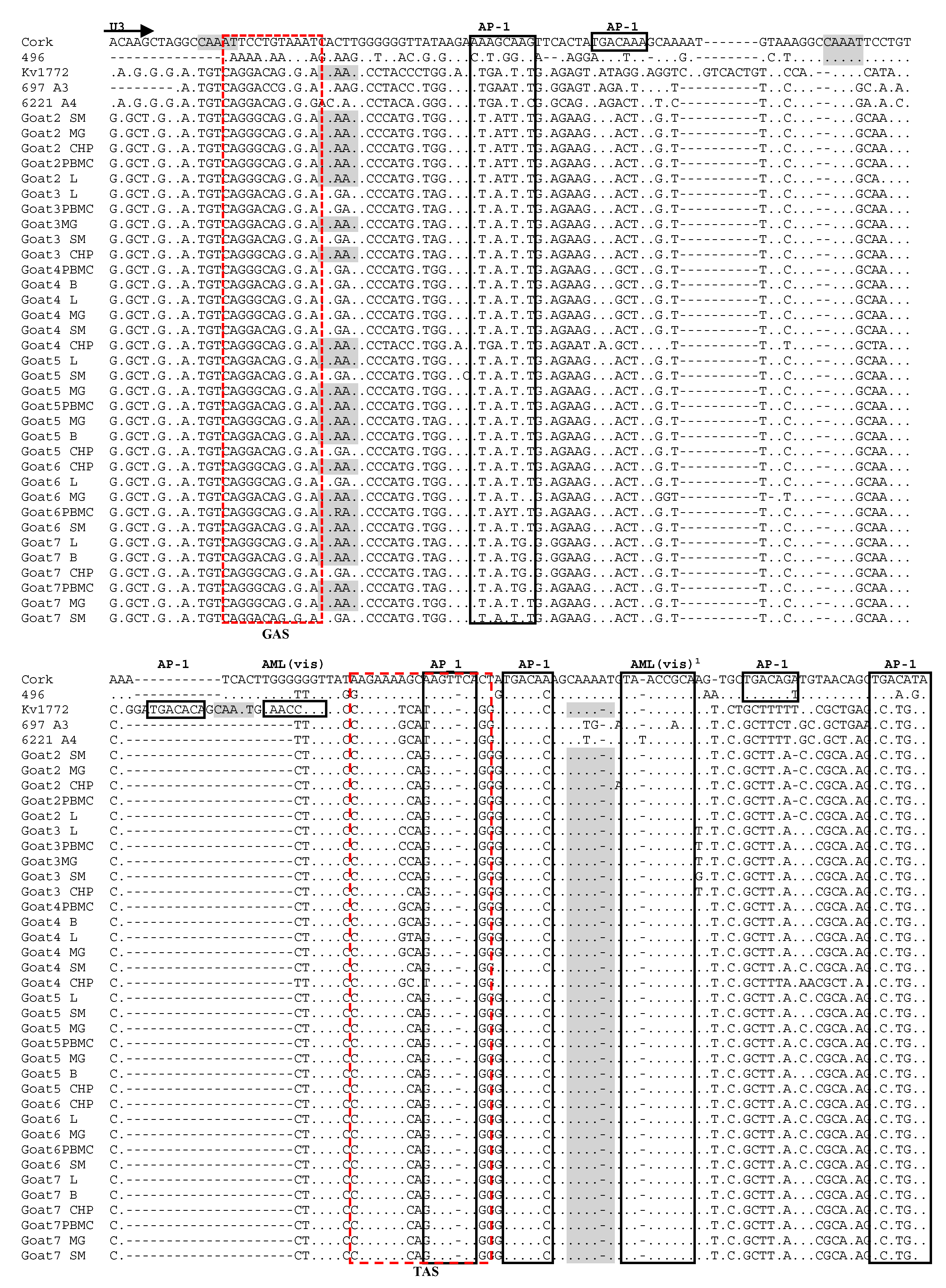
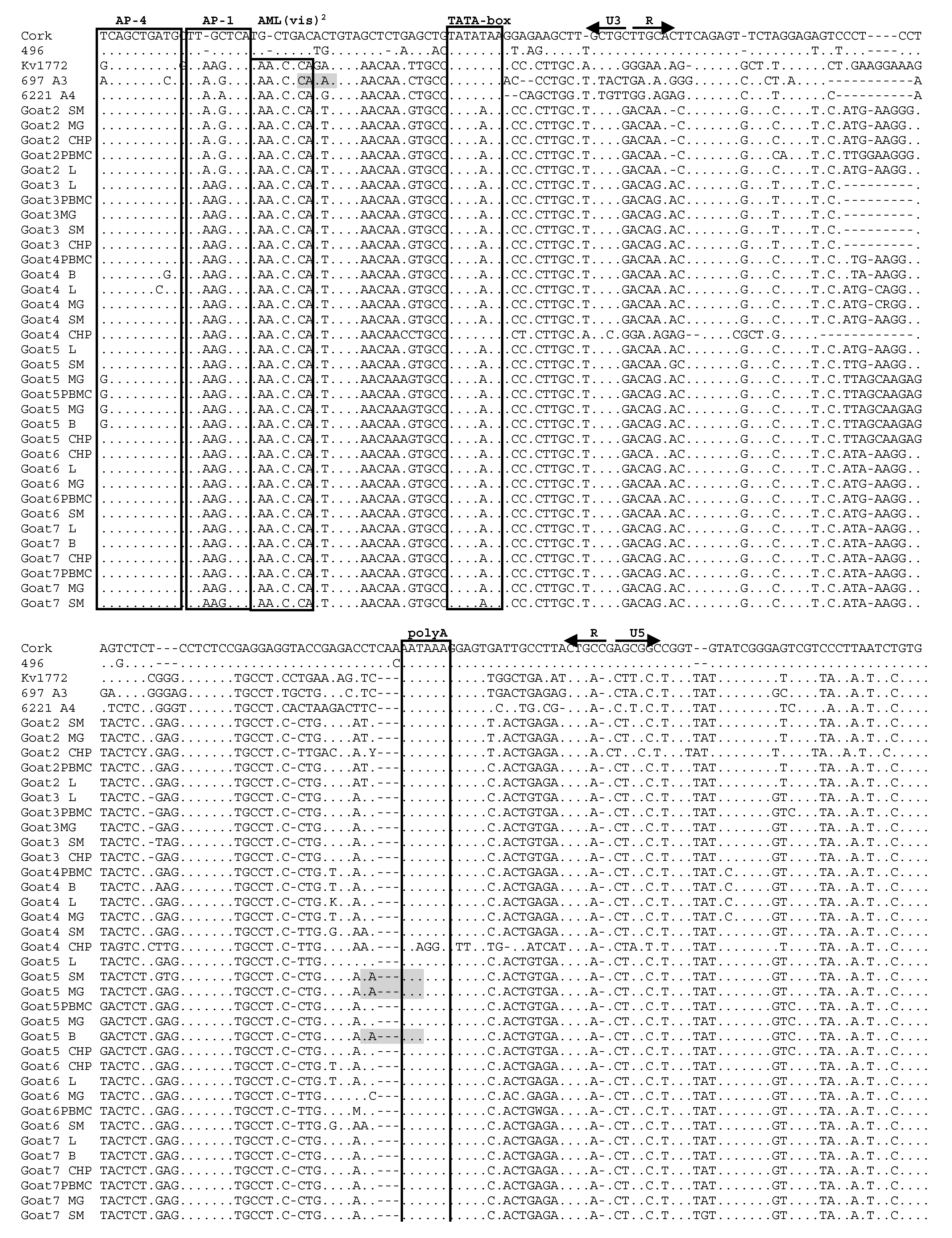
3.4. Analysis of LTR Sequences
3.5. Provirus Detection and Quantification
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blacklaws, B.A. Small ruminant lentiviruses: Immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Narayan, O.; Wolinsky, J.S.; Clements, J.E.; Strandberg, J.D.; Grin, D.E.; Cork, L.C. Slow virus replication: The role of macrophages in the persistence and expression of visna viruses of sheep and goats. J. Gen. Virol. 1982, 59, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Tiley, L.; McConnell, I.; Blacklaws, B. Infection of dendritic cells by the Maedi-Visna lentivirus. J. Virol. 2000, 74, 10096–10103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaton, M.P.; Clawson, M.L.; Chitko-Mckown, C.G.; Leymaster, K.A.; Smith, T.P.; Harhay, G.P.; White, S.N.; Herrmann-Hoesing, L.M.; Mousel, M.R.; Lewis, G.S.; et al. Reduced lentivirus susceptibility in sheep with TMEM154 mutations. PLoS Genet. 2012, 8, e1002467. [Google Scholar] [CrossRef] [Green Version]
- Sider, L.H.; Heaton, M.P.; Chitko-McKown, C.G.; Harhay, G.P.; Smith, T.P.; Leymaster, K.A.; Laegreid, W.W.; Clawson, M.L. Small ruminant lentivirus genetic subgroups associate with sheep TMEM154 genotypes. Vet. Res. 2013, 44, 64. [Google Scholar] [CrossRef] [Green Version]
- Ravazzolo, A.P.; Nenci, C.; Vogt, H.R.; Waldvogel, A.; Obexer-Ruff, G.; Peterhans, E.; Bertoni, G. Viral load, organ distribution, histopathological lesions, and cytokine mRNA expression in goats infected with a molecular clone of the caprine arthritis encephalitis virus. Virology 2006, 350, 116–127. [Google Scholar] [CrossRef]
- Herrmann-Hoesing, L.M.; Noh, S.M.; White, S.N.; Snekvik, K.R.; Truscott, T.; Knowles, D.P. Peripheral ovine progressive pneumonia provirus levels correlate with and predict histological tissue lesion severity in naturally infected sheep. Clin. Vaccine Immunol. 2009, 16, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Olech, M.; Kuźmak, J. Molecular Characterization of Small Ruminant Lentiviruses in Polish Mixed Flocks Supports Evidence of Cross Species Transmission, Dual Infection, a Recombination Event, and Reveals the Existence of New Subtypes within Group A. Viruses 2021, 13, 2529. [Google Scholar] [CrossRef]
- Bazzucchi, M.; Pierini, I.; Gobbi, P.; Pirani, S.; Torresi, C.; Iscaro, C.; Feliziani, F.; Giammarioli, M. Genomic Epidemiology and Heterogeneity of SRLV in Italy from 1998 to 2019. Viruses 2021, 13, 2338. [Google Scholar] [CrossRef]
- Gomez-Lucia, E.; Rowe, J.; Collar, C.; Murphy, B. Diversity of caprine arthritis-encephalitis virus promoters isolated from goat milk and passaged in vitro. Vet. J. 2013, 196, 431–438. [Google Scholar] [CrossRef]
- Gomez-Lucia, E.; Barquero, N.; Domenech, A. Maedi-Visna virus: Current perspectives. Vet. Med. 2018, 9, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Angelopoulou, K.; Brellou, G.D.; Greenland, T.; Vlemmas, I. A novel deletion in the LTR region of a Greek small ruminant lentivirus may be associated with low pathogenicity. Virus Res. 2006, 118, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Hreggvidsdottir, H.S.; Agnarsdottir, G.; Matthiasdottir, S.; Ogmundsdottir, M.H.; Jónsson, S.R.; Georgsson, G.; Ingvarsson, S.; Andrésson, O.S.; Andrésdóttir, V. Duplicated sequence motif in the long terminal repeat of maedi-visna virus extends cell tropism and is associated with neurovirulence. J. Virol. 2007, 81, 4052–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnarsdóttir, G.; Thorsteinsdóttir, H.; Óskarsson, T.; Matthíasdóttir, S.; St Haflidadóttir, B.; Andrésson, Ó.S.; Andrésdóttir, V. The long terminal repeat is a determinant of cell tropism of maedi-visna virus. J. Gen. Virol. 2000, 81, 1901–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adedeji, A.O.; Barr, B.; Gomez-Lucia, E.; Murphy, B. A polytropic caprine arthritis encephalitis virus promoter isolated from multiple tissues from a sheep with multisystemic lentivirus-associated inflammatory disease. Viruses 2013, 5, 2005–2018. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.; McElliott, V.; Vapniarsky, N.; Oliver, A.; Rowe, J. Tissue tropism and promoter sequence variation in caprine arthritis encephalitis virus infected goats. Virus Res. 2010, 151, 177–184. [Google Scholar] [CrossRef]
- Valas, S.; Benoit, C.; Baudry, C.; Perrin, G.; Mamoun, R.Z. Variability and immunogenicity of caprine arthritis encephalitis virus surface glycoprotein. J. Virol. 2000, 74, 6178–6185. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, H.; Reina, R.; Bertolotti, L.; Cenoz, A.; Hernández, M.M.; San Román, B.; Glaria, I.; de Andrés, X.; Crespo, H.; Jáuregui, P.; et al. Study of compartmentalization in the visna clinical form of small ruminant lentivirus infection in sheep. BMC Vet. Res. 2012, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Pisoni, G.; Moroni, P.; Turin, L.; Bertoni, G. Compartmentalization of small ruminant lentivirus between blood and colostrum in infected goats. Virology 2007, 369, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Olech, M.; Kuźmak, J. Compartmentalization of Subtype A17 of Small Ruminant Lentiviruses between Blood and Colostrum in Infected Goats Is Not Exclusively Associated to the env Gene. Viruses 2019, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Cheevers, W.P.; Snekvik, K.R.; Trujillo, J.D.; Kumpula-McWhirter, N.M.; Top, K.P.O.; Knowles, D.P. Prime-boost vaccination with plasmid DNA encoding caprine-arthritis encephalitis lentivirus env and viral SU suppresses challenge virus and development of arthritis. Virology 2003, 306, 116–125. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colitti, B.; Coradduzza, E.; Puggioni, G.; Capucchio, M.T.; Reina, R.; Bertolotti, L.; Rosati, S. A new approach for Small Ruminant Lentivirus full genome characterization revealed the circulation of divergent strains. PLoS ONE 2019, 14, e0212585. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.L.; Pyper, J.M.; Clements, J.E. Nucleotide sequence and transcriptional activity of the caprine arthritis-encephalitis virus long terminal repeat. J. Virol. 1986, 60, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Glaria, I.; Reina, R.; Ramírez, H.; de Andrés, X.; Crespo, H.; Jauregui, P.; Salazar, E.; Luján, L.; Pérez, M.M.; Benavides, J.; et al. Visna/Maedi virus genetic characterization and serological diagnosis of infection in sheep from a neurological outbreak. Vet. Microbiol. 2012, 155, 137–146. [Google Scholar] [CrossRef]
- Glaria, I.; Reina, R.; Crespo, H.; de Andrés, X.; Ramírez, H.; Biescas, E.; Pérez, M.M.; Badiola, J.; Luján, L.; Amorena, B.; et al. Phylogenetic analysis of SRLV sequences from an arthritic sheep outbreak demonstrates the introduction of CAEV-like viruses among Spanish sheep. Vet. Microbiol. 2009, 138, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Cardinaux, L.; Zahno, M.L.; Deubelbeiss, M.; Zanoni, R.; Vogt, H.R.; Bertoni, G. Virological and phylogenetic characterization of attenuated small ruminant lentivirus isolates eluding eluding efficient serological detection. Vet. Microbiol. 2013, 162, 572–581. [Google Scholar] [CrossRef]
- Olech, M.; Rachid, A.; Croisé, B.; Kuźmak, J.; Valas, S. Genetic and antigenic characterization of small ruminant lentiviruses circulating in Poland. Virus Res. 2012, 163, 528–536. [Google Scholar] [CrossRef]
- Olech, M.; Valas, S.; Kuźmak, J. Epidemiological survey in single-species flocks from Poland reveals expanded genetic and antigenic diversity of small ruminant lentiviruses. PLoS ONE 2018, 13, e193892. [Google Scholar] [CrossRef] [Green Version]
- Olech, M.; Murawski, M.; Kuźmak, J. Molecular analysis of small-ruminant lentiviruses in Polish flocks reveals the existence of a novel subtype in sheep. Arch. Virol. 2019, 164, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Olech, M.; Kuźmak, J. Molecular Characterization of Small Ruminant Lentiviruses of Subtype A5 Detected in Naturally Infected but Clinically Healthy Goats of Carpathian Breed. Pathogens 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Rosati, S.; Mannelli, A.; Merlo, T.; Ponti, N. Characterization of the immunodominant cross-reacting epitope of visna maedi virus and caprine arthritis-encephalitis virus capsid antigen. Virus Res. 1999, 61, 177–183. [Google Scholar] [CrossRef]
- Grego, E.; Profiti, M.; Giammarioli, M.; Giannino, L.; Rutili, D.; Woodall, C.; Rosati, S. Genetic heterogeneity of small ruminant lentiviruses involves immunodominant epitope of capsid antigen and affects sensitivity of single-strain-based immunoassay. Clin. Diagn. Lab. Immunol. 2002, 9, 828–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayo, E.; Cuteri, V.; Polledo, L.; Rossi, G.; García Marín, J.F.; Preziuso, S. Genetic Characterization and Phylogenetic Analysis of Small Ruminant Lentiviruses Detected in Spanish Assaf Sheep with Different Mammary Lesions. Viruses 2018, 10, 315. [Google Scholar] [CrossRef] [Green Version]
- Hötzel, I.; Kumpula-McWhirter, N.; Cheevers, W.P. Rapid evolution of two discrete regions of the caprine arthritis-encephalitis virus envelope surface glycoprotein during persistent infection. Virus Res. 2002, 84, 17–25. [Google Scholar] [CrossRef]
- Skraban, R.; Matthíasdóttir, S.; Torsteinsdóttir, S.; Agnarsdóttir, G.; Gudmundsson, B.; Georgsson, G.; Meloen, R.H.; Andrésson, Ó.S.; Staskus, K.A.; Thormar, H.; et al. Naturally occurring mutations within 39 amino acids in the envelope glycoprotein of maedi-visna virus alter the neutralization phenotype. J. Virol. 1999, 73, 8064–8072. [Google Scholar] [CrossRef] [Green Version]
- González Méndez, A.S.; Cerón Téllez, F.; Tórtora Pérez, J.L.; Martínez Rodríguez, H.A.; García Flores, M.M.; Ramírez Álvarez, H. Signature patterns in region V4 of small ruminant lentivirus surface protein in sheep and goats. Virus Res. 2020, 280, 197900. [Google Scholar] [CrossRef]
- Ramírez, H.; Reina, R.; Amorena, B.; de Andrés, D.; Martínez, H.A. Small ruminant lentiviruses: Genetic variability, tropism and diagnosis. Viruses 2013, 5, 1175–1207. [Google Scholar] [CrossRef] [Green Version]
- Maury, W.; Thompson, R.J.; Jones, Q.; Bradley, S.; Denke, T.; Baccam, P.; Smazik, M.; Oaks, J.L. Evolution of the equine infectious anemia virus long terminal repeat during the alteration of cell tropism. J. Virol. 2005, 79, 5653–5664. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.; Hillman, C.; Castillo, D.; Vapniarsky, N.; Rowe, J. The presence or absence of the gamma-activated site determines IFN gamma-mediated transcriptional activation in CAEV promoters cloned from the mammary gland and joint synovium of a single CAEV-infected goat. Virus Res. 2012, 163, 537–545. [Google Scholar] [CrossRef]
- Angelopoulou, K.; Poutahidis, T.; Brellou, G.D.; Greenland, T.; Vlemmas, I. A deletion in the R region of long terminal repeats in small ruminant lentiviruses is associated with decreased pathology in the lung. Vet. J. 2008, 175, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Blatti-Cardinaux, L.; Sanjosé, L.; Zahno, M.L.; Zanoni, R.; Reina, R.; Bertoni, G. Detailed analysis of the promoter activity of an attenuated lentivirus. J. Gen. Virol. 2016, 97, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Deubelbeiss, M.; Blatti-Cardinaux, L.; Zahno, M.L.; Zanoni, R.; Vogt, H.R.; Posthaus, H.; Bertoni, G. Characterization of small ruminant lentivirus A4 subtype isolates and assessment of their pathogenic potential in naturally infected goats. Virol. J. 2014, 11, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grego, E.; Reina, R.; Lanfredini, S.; Tursi, M.; Favole, A.; Profiti, M.; Lungu, M.M.; Perona, G.; Gay, L.; Stella, M.C.; et al. Viral load, tissue distribution and histopathological lesions in goats naturally and experimentally infected with the Small Ruminant Lentivirus Genotype E (subtype E1 Roccaverano strain). Res. Vet. Sci. 2018, 118, 107–114. [Google Scholar] [CrossRef]
- Pisoni, G.; Bertoni, G.; Manarolla, G.; Vogt, H.R.; Scaccabarozzi, L.; Locatelli, C.; Moroni, P. Genetic analysis of small ruminant lentiviruses following lactogenic transmission. Virology 2010, 407, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Mankowski, J.L.; Flaherty, M.T.; Spelman, J.P.; Hauer, D.A.; Didier, P.J.; Amedee, A.M.; Murphey-Corb, M.; Kirstein, L.M.; Muñoz, A.; Clements, J.E.; et al. Pathogenesis of simian immunodeficiency virus encephalitis: Viral determinants of neurovirulence. J. Virol. 1997, 71, 6055–6060. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.; Biescas, E.; Reina, R.; Glaria, I.; Marín, B.; Marquina, A.; Salazar, E.; Álvarez, N.; de Andrés, D.; Fantova, E.; et al. Small ruminant lentivirus-induced arthritis: Clinicopathologic findings in sheep infected by a highly replicative SRLV B2 genotype. Vet. Pathol. 2015, 52, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Larruskain, A.; Jugo, B.M. Retroviral infections in sheep and goats: Small ruminant lentiviruses and host interaction. Viruses 2013, 5, 2043–2061. [Google Scholar] [CrossRef] [Green Version]
- Herrmann-Hoesing, L.M.; White, S.N.; Mousel, M.R.; Lewis, G.S.; Knowles, D.P. Ovine progressive pneumonia provirus levels associate with breed and Ovar-DRB1. Immunogenetics 2008, 60, 749–758. [Google Scholar] [CrossRef]
- Larruskain, A.; Bernales, I.; Luján, L.; de Andrés, D.; Amorena, B.; Jugo, B.M. Expression analysis of 13 ovine immune response candidate genes in Visna/Maedi disease progression. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 405–413. [Google Scholar] [CrossRef] [Green Version]
- White, S.N.; Knowles, D.P. Expanding possibilities for intervention against small ruminant lentiviruses through genetic markerassisted selective breeding. Viruses 2013, 5, 1466–1499. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Dadousis, C.; Bozzi, R.; Fratini, F.; Russo, C.; Bandecchi, P.; Cantile, C.; Mazzei, M. Genome scan for the possibility of identifying candidate resistance genes for goat lentiviral infections in the Italian Garfagnina goat breed. Trop. Anim. Health Prod. 2019, 51, 729–733. [Google Scholar] [CrossRef] [PubMed]
- White, S.N.; Mousel, M.R.; Herrmann-Hoesing, L.M.; Reynolds, J.O.; Leymaster, K.A.; Neibergs, H.L.; Lewis, G.S.; Knowles, D.P. Genome-wide association identifies multiple genomic regions associated with susceptibility to and control of ovine lentivirus. PLoS ONE 2012, 7, e47829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colussi, S.; Desiato, R.; Beltramo, C.; Peletto, S.; Modesto, P.; Maniaci, M.G.; Campia, V.; Quasso, A.; Rosati, S.; Bertolotti, L.; et al. A single nucleotide variant in the promoter region of the CCR5 gene increases susceptibility to arthritis encephalitis virus in goats. BMC Vet. Res. 2019, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Molaee, V.; Eltanany, M.; Lühken, G. First survey on association of TMEM154 and CCR5 variants with serological maedi-visna status of sheep in German flocks. Vet. Res. 2018, 49, 36. [Google Scholar] [CrossRef] [Green Version]
- Molaee, V.; Otarod, V.; Abdollahi, D.; Lühken, G. Lentivirus Susceptibility in Iranian and German Sheep Assessed by Determination of TMEM154 E35K. Animals 2019, 9, 685. [Google Scholar] [CrossRef] [Green Version]
- Olech, M.; Ropka-Molik, K.; Szmatoła, T.; Piórkowska, K.; Kuźmak, J. Single Nucleotide Polymorphisms in Genes Encoding Toll-Like Receptors 7 and 8 and Their Association with Proviral Load of SRLVs in Goats of Polish Carpathian Breed. Animals 2021, 11, 1908. [Google Scholar] [CrossRef]
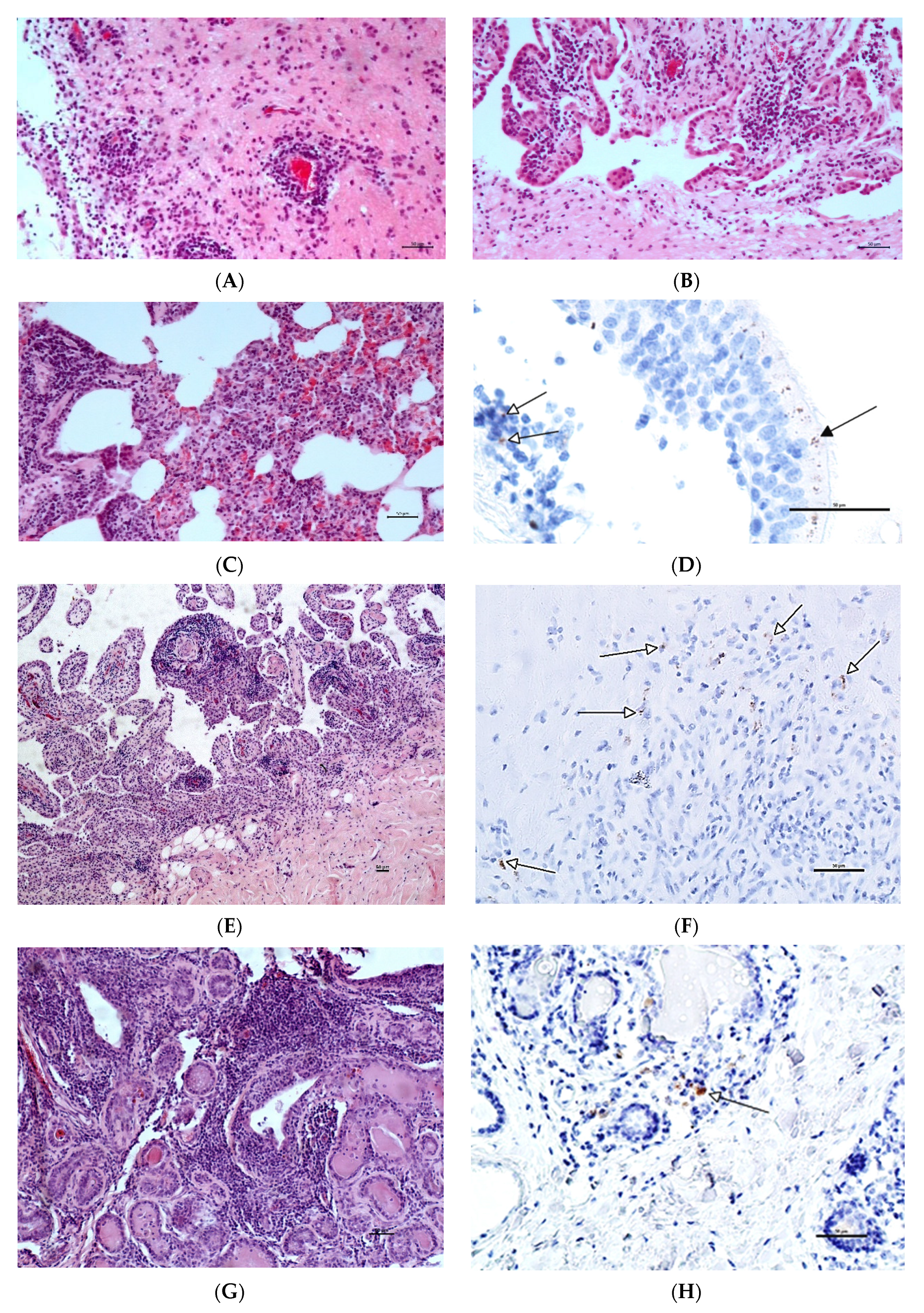



| Joints | Brains | Lung | Mammary Glands | |
|---|---|---|---|---|
| Goat 2 | 3 * | 0 | 1 | 2 |
| Goat 3 | 2 * | 0 | 1 | 2 |
| Goat 4 | 2 * | 0 | 1 | 3 |
| Goat 5 | 3 * | 2 | 1 | 2 |
| Goat 6 | 2 * | 0 | 1 | 2 |
| Goat 7 | 3 * | 2 | 2 | 2 |
| A1 | A2 | A3 | A4 | A5 | A8 | A9 | A11 | A12 | A13 | A16 | A17 | A18 | A19 | A20 | A21 | A22 | A23 | A24 | A25 | A26 | A27 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A2 | 16.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A3 | 13.8 | 11.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A4 | 14.7 | 16.1 | 15.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A5 | 15.7 | 14.7 | 11.9 | 15.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A8 | 16.2 | 17.1 | 15.3 | 17.3 | 17.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A9 | 15.2 | 14.5 | 12.2 | 16.5 | 14.3 | 15.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A11 | 15.9 | 14.6 | 14.6 | 18.4 | 15.9 | 15.9 | 13.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A12 | 16.2 | 12.7 | 10.9 | 16.6 | 13.5 | 16.7 | 15.8 | 14.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A13 | 16.0 | 10.8 | 11.9 | 14.9 | 13.8 | 17.5 | 16.0 | 14.7 | 13.3 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A16 | 17.9 | 15.9 | 17.2 | 15.7 | 16.4 | 19.6 | 20.6 | 18.0 | 16.1 | 16.3 | - | - | - | - | - | - | - | - | - | - | - | - |
| A17 | 14.2 | 11.5 | 8.8 | 15.0 | 11.0 | 15.7 | 11.5 | 15.5 | 10.0 | 13.2 | 14.7 | - | - | - | - | - | - | - | - | - | - | - |
| A18 | 19.3 | 11.9 | 14.2 | 18.3 | 16.2 | 20.4 | 17.5 | 17.1 | 14.4 | 12.3 | 15.9 | 13.6 | - | - | - | - | - | - | - | - | - | - |
| A19 | 14.8 | 13.2 | 10.9 | 14.5 | 12.3 | 14.3 | 4.6 | 12.8 | 14.8 | 14.1 | 18.1 | 10.5 | 15.9 | - | - | - | - | - | - | - | - | - |
| A20 | 19.3 | 13.7 | 13.5 | 15.7 | 13.5 | 16.9 | 16.7 | 16.0 | 13.3 | 14.1 | 15.2 | 14.5 | 14.9 | 14.8 | - | - | - | - | - | - | - | - |
| A21 | 15.8 | 13.8 | 12.6 | 18.1 | 15.4 | 18.0 | 16.1 | 13.5 | 12.9 | 12.5 | 16.9 | 13.7 | 14.5 | 15.0 | 15.9 | - | - | - | - | - | - | - |
| A22 | 24.4 | 20.6 | 19.7 | 24.2 | 18.6 | 24.0 | 22.5 | 22.7 | 20.5 | 22.6 | 23.3 | 20.2 | 21.0 | 22.2 | 18.8 | 21.3 | - | - | - | - | - | - |
| A23 | 19.6 | 17.2 | 16.4 | 18.8 | 17.4 | 17.0 | 15.7 | 14.9 | 16.9 | 18.8 | 21.7 | 16.8 | 20.3 | 14.5 | 15.9 | 19.4 | 21.3 | - | - | - | - | - |
| A24 | 15.9 | 12.6 | 12.0 | 16.0 | 14.2 | 13.2 | 11.8 | 13.9 | 14.0 | 13.3 | 18.2 | 12.8 | 15.6 | 12.5 | 16.2 | 13.1 | 21.1 | 15.0 | - | - | - | - |
| A25 | 15.1 | 11.1 | 12.3 | 15.6 | 14.0 | 16.2 | 14.0 | 15.3 | 12.7 | 11.9 | 15.8 | 12.6 | 12.2 | 13.3 | 11.6 | 14.6 | 21.0 | 17.5 | 14.4 | - | - | - |
| A26 | 17.7 | 11.0 | 12.9 | 16.6 | 13.2 | 17.2 | 15.5 | 14.4 | 13.0 | 11.1 | 16.0 | 13.0 | 10.2 | 14.8 | 11.4 | 13.1 | 18.8 | 17.6 | 12.1 | 10.3 | - | - |
| A27 | 13.2 | 11.3 | 11.9 | 16.0 | 13.0 | 17.4 | 14.7 | 13.2 | 12.4 | 10.0 | 14.2 | 12.4 | 12.8 | 13.6 | 14.0 | 12.2 | 21.7 | 18.2 | 14.2 | 11.0 | 10.7 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olech, M.; Kycko, A.; Kuźmak, J. Molecular Characterization of Small Ruminant Lentiviruses Isolated from Polish Goats with Arthritis. Viruses 2022, 14, 735. https://doi.org/10.3390/v14040735
Olech M, Kycko A, Kuźmak J. Molecular Characterization of Small Ruminant Lentiviruses Isolated from Polish Goats with Arthritis. Viruses. 2022; 14(4):735. https://doi.org/10.3390/v14040735
Chicago/Turabian StyleOlech, Monika, Anna Kycko, and Jacek Kuźmak. 2022. "Molecular Characterization of Small Ruminant Lentiviruses Isolated from Polish Goats with Arthritis" Viruses 14, no. 4: 735. https://doi.org/10.3390/v14040735
APA StyleOlech, M., Kycko, A., & Kuźmak, J. (2022). Molecular Characterization of Small Ruminant Lentiviruses Isolated from Polish Goats with Arthritis. Viruses, 14(4), 735. https://doi.org/10.3390/v14040735






