Abstract
Since September 2020, Germany has experienced the first ever outbreak of African swine fever (ASF). The first known cases occurred exclusively in wild boar in forest areas in Brandenburg and Saxony; in July 2021, infected domestic pigs were also confirmed for the first time. As wild boar are considered the main reservoir for the virus in the European region, an effective interruption of this infection chain is essential. In particular, the removal and safe disposal of infected carcasses and the direct disinfection of contaminated, unpaved ground are priorities in this regard. For the disinfection, highly potent as well as environmentally compatible disinfectants must be used, which are neither influenced in their effectiveness by the soil condition nor by increased organic contamination. Thus, in this study, slaked lime, milk of lime and quicklime (1% to 10% solutions) were selected for efficacy testing against the test virus recommended by the German Veterinary Society (DVG), Modified Vaccinia Ankara virus (MVAV), and ASF virus (ASFV) in conjunction with six different forest soils from Saxony in two different soil layers (top soil and mineral soil) each. In summary, 10% of any tested lime type is able to inactivate both MVAV and ASFV under conditions of high organic load and independent of the water content of the soil. At least a 4 log reduction of the virus titer in all tested forest soil types and layers and by all applied lime types was observed. In conclusion, the high efficacy and suitability of all tested lime products against both viruses and in the presence of high organic load in forest soil can be confirmed and will help to control ASF spread.
1. Introduction
African swine fever virus (ASFV) is one of the most important emerging animal diseases, which spreads rapidly worldwide. From 2007, ASFV spread through Georgia and Russia to the EU in the Baltic states. From there, it continuously spread westwards and in September 2020 the first case in Germany was diagnosed in the state of Brandenburg [1]. Within one year, the cases of the haemorrhagic disease of pigs spread across the states of Brandenburg and Saxony, with more than 2000 cases reported in wild boar one year later [2]. Moreover, three ASF outbreaks were confirmed in Brandenburg domestic pigs in July 2021 [2]. In all cases, local virus variants were also found to be circulated in the wild boar population [3]. Thus, the epidemic in the local wild boar population was the most likely source of these outbreaks. To prevent further spread and economic losses, the German government has implemented strict emergency and hygiene plans in accordance with European legislation (e.g., Commission Implementing Regulation (EU) 2021/605). The control strategy relies on reducing the number of wild boar, fencing of affected areas, monitoring of the susceptible population and proper disposal of infected carcasses [4,5,6]. Furthermore, the disinfection of potentially contaminated soil beneath and around the ASFV-positive carcasses contributed to the active control measures.
The stability of ASFV in the environment is well documented [7,8,9]. ASFV belongs to the family of Asfarviridae, which is enveloped and can be easily inactivated by commonly used disinfectants like NaOH or formaldehyde [8]. Peracetic acid and citric acid were shown to be highly effective with ASFV contaminated soil [10], but may be of limited efficacy in the presence of blood [11,12]. Nevertheless, screening the effectiveness of other disinfectants is necessary. Various lime products have been used for decades [13,14,15,16] and are considered to be promising solutions because of their availability in powdery form and their environmentally friendly characteristics. The approval of the use of disinfectants against specific animal diseases in Germany is nationally regulated and linked to an efficacy testing according to test protocols of the German Veterinary Society (DVG). The DVG has recommended the Orthopoxvirus Modified Vaccinia Ankara virus (MVAV) as a representative of enveloped viruses [17]. The aim of the study was to test the disinfection potency of lime according to the guidelines of the DVG on MVAV and try to reproduce the results using ASFV.
In order to determine effective concentrations to inactivate enveloped viruses in contaminated soil, various concentrations of slaked lime (Ca(OH)2), quicklime (CaO) and lime milk (Ca(OH)2 in water) were tested. We have focused on six soil types in one of the most affected states in Germany, Saxony, and followed the guidelines of the DVG of using MVAV for screening the disinfectants. The most effective concentration was then tested on ASFV contaminated soil under appropriate high containment conditions. In addition, the best water/lime ratio was determined. The experimental layout is presented in Table 1.

Table 1.
Experimental layout.
2. Materials and Methods
2.1. Study Sites and Soil Types
As illustrated in Figure 1, six different soil types across Saxony were selected. For each type, top soil (TS) and mineral soil layer (MS) were collected (Figure 2). For all soil samples, quantitative polymerase chain reaction (real-time PCR) was performed to proof their freedom of both ASFV and MVAV.
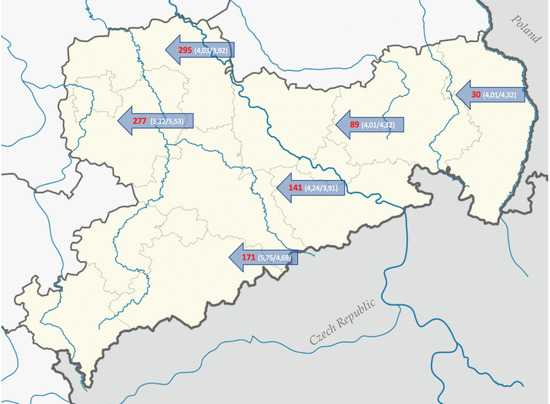
Figure 1.
Collection spots of the soil samples. Official names by the authority of Sachsenforst, Pirna, Germany (arrows, red), the numbers in brackets describe the pH of top soil (first number) and mineral soil (second number). Forest stand of soil 277 and 89 is deciduous forest, of 171 and 141 spruce forest and of 295 and 30 pine forest. Map credit [18] (Original Author: TUBS; license link: https://commons.wikimedia.org/wiki/Commons:GNU_Free_Documentation_License,_version_1.2).

Figure 2.
Exemplary representation of a sampled pine forest (left) with the top soil (a) and upper mineral soil (b).
2.2. Disinfection Experiment with Slaked Lime, Lime Milk and Quicklime (Lime Experiments)
Three different lime products were examined in a suspension form: powdery slaked lime (Chemdiscount/WHC, Hilgertshausen, Germany), powdery quicklime (Carl Roth, Karlsruhe, Germany) and lime milk (made by mixing the slaked lime with water at various concentrations). The lime milk was freshly prepared to a stock solution before using in the experiment, representing 3.4-fold the desired final concentration of either 1%, 5% or 10%. A volume of 2.7 (diameter) + 1.5 (height) cm of TS and MS of each of the 6 soil types corresponding to about 3 mL soil were filled in 50-mL centrifuge tubes, and 3 mL of virus (108 TCID50/mL for MVAV or 106.75 TCID50/mL for ASFV Armenia ∆258L GFP huCD4 [19]) and 4 mL of fetal calf serum (FCS) were added and mixed for 5 s. To obtain the end concentrations for the powdery disinfectants (slaked lime or powdery quicklime) in the suspension, 0.7 g for 10%, 0.35 g for 5% or 0.07 g for 1% of were added to the mixture of soil, virus and FCS (Supplementary Table S5). In the case of lime milk, 2.9 mL of each of 3.4-fold concentrated lime milk was added to the centrifuge tubes (Supplementary Table S6). As an example, for a final working solution of 1%, 3.4% of the disinfectant was added. Thereafter, the complete mixture was vortexed for 5 s. After incubation at 10 °C for 2 h, ice-cold PBS was added to generate a final virus dilution of 1:4. The mixture was then sonicated in an ultrasound bath (Bandelin Sonorex Super RK 103H, Berlin, Germany) at 4 °C for 5 min and centrifuged at 4500 rpm at 4 °C for 5 min. The supernatant, approximately, 5 mL was collected and filtrated (Filtropour 0.45 µm, Sarstedt, Nuembrecht, Germany). To determine the loss of infectivity, ten-fold dilutions were prepared and cultivated as described previously [10]. To avoid cell toxicity, in some cases, 25 µL was applied to either 24- or 96-well plates.
The experiment for each concentration of the lime products in each soil type and layer were done in duplicates. For each control, the experimental setup remained the same but without the disinfectant. The final virus dilution of the control suspensions at the end of the experiment corresponds to that of the suspensions with disinfectant. Quantitative real-time PCR to determine the total amount of viral DNA for both MVAV and ASFV were done in all disinfection experiments as described previously [10]. This was done to rule out adhesion of infectious virus particles to sand particles, which would have potentially led to false positive disinfection results due to such reduced recoverability. A true disinfection will lead to a decrease in infectivity (virus titer in TCID50) at a constant DNA genome copy number concentration.
2.3. Lime/Water Ratio
To determine the amount of water needed to activate the powdery lime products (quicklime and slaked lime), various water contents of soil were tested. The same experimental layout for MVAV was performed as above for one MS and one TS with little modifications (Table 2). Briefly, the volume of MVAV was reduced to 1 mL to decrease the water content by using a high titer virus stock. Similarly, FCS was replaced by 0.24 g bovine serum albumin (BSA) in a powder form. The powdery disinfectants were added to the centrifuge tubes containing an equivalent to 3 mL soil in a mass of 0.42 g. After vortexing of the mix for 5 s, one tube was left without adding more water and 420, 840, 1680 and 3360 µL of water with standardized hardness level (WSH) [19,20] was added to reach a ratio of disinfectant of 0 (no further dilution), 1:2; 1:3; 1:5 and 1:9 in the experimental solution. Furthermore, one control tube without disinfectant was prepared (Table 2). The mixture was vortexed again and incubated at 10 °C for 2 h. Subsequently, ice-cold PBS was added to give a final virus dilution of 1:8 in the tubes to stop the disinfectant activity. The supernatant was collected and immediately inoculated into the cell culture system as described previously [10]. DNA extraction and real-time PCR was performed for samples as published previously [10]. Each experiment was performed twice.

Table 2.
Setup and results of lime/water ratio experiments. WSH (water with standardized hardness level) was used in the experiments. The water content has no influence on the efficacy of the liming. No infectious virus was detected after disinfection in any soil type, independent of the water content. The limit of detection is 1.7 Log10 TCID50/mL. Control value is the average of virus concentrations in TS and MS of 277.
2.4. Avoidance of Cell Toxicity
The experimental use of highly potent disinfectants in conjunction with cell cultures can lead to pronounced cell-toxic effects that can severely compromise the validity of results, especially for the exclusive evaluation via cytopathogenic effects (CPE) like in the case of MVA.
While the CPE of MVA appears in the form of swollen, blistered and rounded cell bodies, toxic effects present themselves in the form of black staining (necrosis) and detachment of the cells. Only in case of high-grade cell toxicity, no more healthy cells remain to identify the CPE, which would make correct evaluation impossible.
Extensive preliminary tests were carried out to investigate the toxicity of the different types and amounts of lime on the corresponding cell cultures.
For this purpose, the respective lime concentrations (1%, 5%, 10%) in the final dilutions used in the experiment were titrated without virus and added to the corresponding cell culture in 96-well plates. The cell culture was incubated and tested for toxicity over 2 h. If cell toxicity (necrosis/detachment of cells) was observed during this time in one or more dilution levels, the respective dilution level was added in addition to a 24-well plate containing cell culture. Again, incubation and observation of toxicity was performed for 2 h. If toxicity was no longer detectable here in the respective dilutions, inoculation of the critical dilution levels could be performed in larger cell culture plates in the main experiment (containing disinfectant and virus). Throughout the experiments, at least one control was titrated per experiment (tube with regular experimental setup and virus but without addition of disinfectant) to ensure a visual comparison between potential cell toxicity and pure CPE. This allowed optimal discrimination between toxic effects and CPE.
3. Results
3.1. Disinfection of MVAV and ASFV with Lime (Lime Experiments)
Various types and concentrations of lime were used to deactivate MVAV in the presence of high organic contents (FCS). For lime milk, quicklime and slaked lime, 10% was sufficient to reduce the viral titer of MVAV by at least 4 logs (Figure 3). Same efficacy was seen against ASFV, as a concentration 10% of all lime types revealed a complete inactivation of ASFV in all soil types tested (Figure 4). The viral load as determined by real-time PCR for both the control and the experimental set revealed similar values (Supplementary Tables S1–S3).
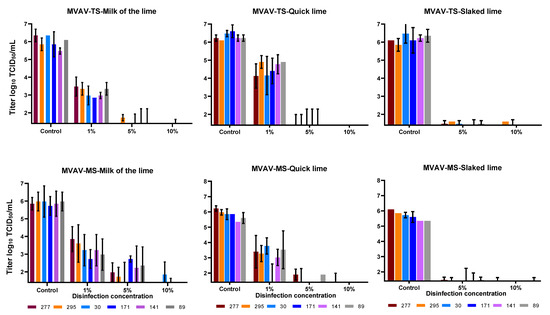
Figure 3.
Comparison of the disinfection of MVAV with three different types of lime. Top three panels represent soil (TS) and lower three panels mineral soil (MS). The mean value and standard deviation of at least duplicate tests are shown. Virus titers were calculated by Spearman–Kaerber method. Limit of detection was 1.4 log10 TCID50/mL.
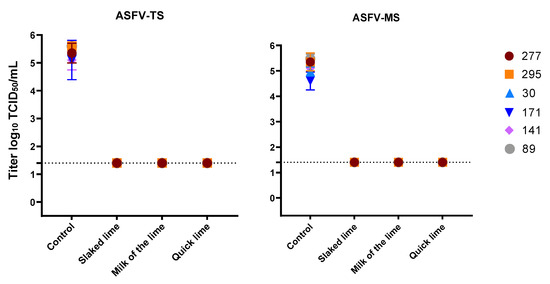
Figure 4.
Disinfection of ASFV with three different types of lime in a concentration of 10%. The mean value and standard deviation of at least duplicate tests are shown. Virus titers were calculated by Spearman–Kaerber method. Limit of detection was 1.4 log10 TCID50/mL.
3.2. Lime/Water Ratio
The influence of water contents on the disinfectant’s potency was tested. A complete inactivation was observed, when no extra water or up to 16 times the volume of the lime powder extra water was added. The amount of added water had no influence on the inactivation of MVAV in soil (Table 2). The viral load as determined by real-time PCR for both the control and the experimental set (Supplementary Table S4 behaved similar to the viral loads of the lime experiments).
4. Discussion
Decontamination of soil beneath and around carcasses can play an important role in preventing spread of ASFV. Many studies were conducted on the efficacy of the disinfectants on contaminated solid floor and walls of stables [21,22,23], but few have been performed on forest soil, which is considered to contribute to the spread of the virus within the wild boar population in Germany. The World Organization for Animal Health (OIE) and the German Federal Ministry of Food and Agriculture (BMEL) recommended the removal of the soil beneath the carcasses under biosecurity measurements [6,24,25,26]. To avoid further spread of ASFV during handling and transportation of infected soils, the easier and quicker alternative is an effective soil disinfection onsite. In this study, the virucidal effectivity of slaked lime, quicklime and lime milk were examined. The disinfectants were tested first with MVAV as recommended by DVG on six soil types under BSL-2 conditions and applied to the ASFV in a high-containment facilities. At least a 4 log10 TCID50/mL inactivation was achieved with 10% for all three tested lime types at 10 °C and an exposure time of two hours. To simulate a field situation, the experiments were done with high organic soiling. In a previous study, 0.1% peracetic acid completely inactivated ASFV in various soil types, while citric acid had only limited efficacy [10]. While blood may reduce the efficacy on peracetic acid performance [11], lime was shown to be effective in in the presence of blood, decomposition material or other disruptive substances [27]. Powdery slaked lime and powdery quicklime are commonly used for the disinfection of poultry farms in case of avian influenza virus [28,29]. Lime milk is the disinfectant of choice in the elimination of the infectivity of contaminated slurry [30,31] or ponds [14,31].
The powder form of lime has an advantage of ease of transportation to the affected area and to the target zone in forest, where carcasses are found. Ambient temperature has little to no effect on lime decontamination characteristics [27]. Less or extensive water contents of the matrix can reduce the virucidal properties of powdery lime products [32]. In contrast, in our experiment, water contents did not influence the inactivation of MVAV in soil by powdery lime. Our experiment was designed to reduce the water content of the mixtures as much as possible by using dry soil, BSA in powder form, and a high virus stock in a small volume). However, our study was performed on different soils collected from Saxony, Germany, from areas with varying annual rainfall (656–1146 mm). Therefore, it is highly recommended to test the system before widespread application in a particular environment, country or soil type.
The mechanism behind this class of disinfectants is the alkalization of the matrix (pH up to 12 or higher) [31,33]. This pH level causes the denaturation and coagulation of proteins [31]. Despite the high acid content of soil in Saxony, Germany [10], the pH of the soil after adding the lime was raised to pH 11 to 12 [34]. Furthermore, in the case of quicklime, exothermal reaction with water with a possible heating up to 80 °C will enforce the microbiocidal potency [31]. However, the increased risk of forest fires must be taken into account here, especially in summer. Additionally, special precautions to protect the health of workers must be taken to prevent the potential caustic effect on skin, eyes, lungs and exposed mucosa [31].
DVG in Germany has recommended the MVAV as a representative for enveloped viruses for testing the efficacy of disinfection solutions [17]. A previous study, comparing the efficacy of peracetic and citric acids to inactivate both MVAV and ASFV, has shown only little inconsistency between both viruses [10]. In the experiments described here with the lime products, both viruses are once again showing a very similar behaviour during disinfection under mentioned conditions. Compared to peracetic acid and citric acid, lime is not affected by low ambient temperature or any kind of organic load. This fact makes lime a highly efficient as well as simultaneously cost-effective means of combating ASF.
5. Conclusions
In conclusion, lime (quicklime, slaked lime, lime milk) are effective for the disinfection of forest soil contaminated with ASFV, especially in the presence of high organic soiling.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14040734/s1. The Supplementary File contains the real-time PCR results. Table S1: qPCR—lime experiments—MVA—Of (top soil) of soil samples 277, 295, 30, 171, 141, 89; Table S2: qPCR—lime experiments—MVA—A (mineral soil) of soil samples 277, 295, 30, 171, 141, 89; Table S3: qPCR—ASFV—lime experiments—Of and A (top soil and mineral soil) of soil samples 277, 295, 30, 171, 141, 89; Table S4: qPCR—lime/water-ratio—MVA—Of and A (top soil and mineral soil) of soil sample 277; Table S5: Final concentrations and added amount of quicklime and slaked lime in experimental layout for lime experiments; Table S6: Stock dilution and final concentrations of lime milk in experimental layout for lime experiments.
Author Contributions
Conceptualization, U.T.; methodology, F.T., A.A.E.W., S.B., U.T.; validation, F.T., A.A.E.W., M.F., P.D., H.R., T.C., S.B., U.T.; formal analysis, F.T., A.A.E.W., S.B., U.T.; investigation, F.T., A.A.E.W., M.F., P.D., H.R., T.C., S.B., U.T.; resources, S.B., U.T.; data curation, F.T., A.A.E.W., M.F., P.D., H.R., T.C., S.B., U.T.; writing—original draft preparation, F.T., A.A.E.W., U.T.; writing—review and editing, F.T., A.A.E.W., M.F., S.B., U.T.; supervision, A.A.E.W., U.T.; project administration, U.T.; funding acquisition, U.T. All authors have read and agreed to the published version of the manuscript.
Funding
There is no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript and the Supplementary Materials.
Acknowledgments
We acknowledge the financial support by the Saxon State Ministry for Social Affairs and Cohesion and the help with the characterization and definition of the soil types by the state—owned enterprise Sachsenforst. We thank Nadja Leinecker, Dana Rüster, Mario Reinhardt and Robert Küchler for the expert technical support. The authors thank Saxon State Ministry for Social Affairs and Cohesion for collaboration and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Goldner, M.; Schatz, J.; et al. Joining the club: First detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 2020, 68, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Loeffler-Institute (FLI). Karten: ASP in Deutschland, 08.01.2021 bis 22.12.2021. Available online: https://www.openagrar.de/receive/openagrar_mods_00076885 (accessed on 7 January 2022).
- Friedrich-Loeffler-Institute (FLI). Karten zur Afrikanischen Schweinepest. Available online: https://www.fli.de/de/aktuelles/tierseuchengeschehen/afrikanische-schweinepest/karten-zur-afrikanischen-schweinepest/ (accessed on 17 September 2021).
- Forth, J.H.; (Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Greifswald, Germany). African Swine Fever Virus—Variants on the Rise. Personal communication, 2022. in press.
- European Food Safety Authority (EFSA). Epidemiological Analyses of African Swine Fever in the European Union (November 2018 to October 2019). Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.5996 (accessed on 30 January 2020).
- European Food Safety Authority (EFSA). Epidemiological Analyses of African Swine fever in the European Union (November 2017 until November 2018). Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2018.5494 (accessed on 29 November 2018).
- Bundesministerium für Gesundheit und Konsumentenschutz GZ 39.505/6-III/A/4b/96. Mittel und Verfahren für die Durchführung der Desinfektion bei Anzeigepflichtigen Tierseuchen; Durchführungsbestimmungen des Bundesministeriums für Gesundheit und Konsumentenschutz GZ 39.505/6-III/A/4b/96. Available online: https://www.verbrauchergesundheit.gv.at/tiere/tierseuchenuebungen/tierseuchen_desinfektionserlass.pdf?7vj8ky (accessed on 5 March 2021).
- Kalmar, I.D.; Cay, A.B.; Tignon, M. Sensitivity of African swine fever virus (ASFV) to heat, alkalinity and peroxide treatment in presence or absence of porcine plasma. Vet. Microbiol. 2018, 219, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Panasiuk, N.; Zmudzki, J.; Wozniakowski, G. African Swine Fever Virus—Persistence in Different Environmental Conditions and the Possibility of its Indirect Transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanneberger, F.; Abd El Wahed, A.; Fischer, M.; Blome, S.; Truyen, U. The efficacy of disinfection on modified vaccinia Ankara and African Swine fever Virus in various forest soil types. Viruses 2021, 14, 2173. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Loeffler-Institute (FLI). Empfehlungen zur Desinfektion bei Tierseuchen—Peressigsäure. Greifswald—Insel Riems, Friedrich-Loeffler-Inst. Available online: https://www.openagrar.de/servlets/MCRFileNodeServlet/openagrar_derivate_00025821/FLI-V3-3-6-Chemische-Desinfektionsmittel-Peressigsaeure-RL-Desinfektion-V02-2020-07-30.pdf (accessed on 30 July 2020).
- European Food Safety Authority (EFSA). Available data on notified biocides efficacy under field conditions. EFSA J. 2009, 2009, 34. [Google Scholar]
- Grabow, W.O.; Middendorff, I.G.; Basson, N.C. Role of lime treatment in the removal of bacteria, enteric viruses, and coliphages in a wastewater reclamation plant. Appl. Environ. Microbiol. 1978, 35, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G. Removal of viruses from sewage, effluents, and waters. I. A review. Bull. World Health Organ. 1973, 49, 451–460. [Google Scholar] [PubMed]
- Matsuzaki, S.; Azuma, K.; Lin, X.; Kuragano, M.; Uwai, K.; Yamanaka, S.; Tokuraku, K. Farm use of calcium hydroxide as an effective barrier against pathogens. Sci. Rep. 2021, 11, 7941. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Veterinärmedizinische Gesellschaft (DVG). DVG-Prüfrichtlinien, V. Tierhaltung Viruzidie—Methoden der PrüEfung von Chemischen Desinfektionsmitteln für die Tierhaltung. Available online: https://www.desinfektion-d.dvg.de/fileadmin/FG_Desinfektion/Dokumente/Fuer_Gutachter/Pruefrichtlinien/V.4_Viruzidie_7Nov2017.pdf (accessed on 7 November 2017).
- Location Map Saxony, Germany (Modified for Experimental Background). 2009. Available online: https://de.wikipedia.org/wiki/Datei:Saxony_location_map.svg (accessed on 29 August 2012).
- Hübner, A.; Keßler, C.; Pannhorst, K.; Forth, J.H.; Kabuuka, T.; Karger, A.; Mettenleiter, T.C.; Fuchs, W. Identification and characterization of the 285L and K145R proteins of African swine fever virus. J. Gen. Virol. 2019, 100, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Leitlinie der Deutschen Vereinigung zur Bekämpfung der Viruskrankheiten. Leitlinie der Deutschen Vereinigung zur Bekämpfung der Viruskrankheiten (DVV) e.V.2 und des Robert Koch-Instituts (RKI) zur Prüfung von Chemischen Desinfektionsmitteln auf Wirksamkeit Gegen Viren in der Humanmedizin Fassung vom 1. August 2008. Available online: https://www.springermedizin.de/leitlinie1-der-deutschen-vereinigung-zur-bekaempfung-der-viruskr/8010706 (accessed on 1 August 2008).
- De Lorenzi, G.; Borella, L.; Alborali, G.L.; Prodanov-Radulovic, J.; Stukelj, M.; Bellini, S. African swine fever: A review of cleaning and disinfection procedures in commercial pig holdings. Res. Vet. Sci. 2020, 132, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Gabbert, L.R.; Neilan, J.G.; Rasmussen, M. Recovery and chemical disinfection of foot-and-mouth disease and African swine fever viruses from porous concrete surfaces. J. Appl. Microbiol. 2020, 129, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.W.; Davis, T.; O’Brien, C.; LaRocco, M.; Rodriguez, L.L. Disinfection of transboundary animal disease viruses on surfaces used in pork packing plants. Vet. Microbiol. 2018, 219, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.W.; Lee, L.J.; Eslami, A.C.; Larson, C.R.; Rodriguez, L. Chemical disinfection of high-consequence transboundary animal disease viruses on nonporous surfaces. Biologicals 2011, 39, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Franz, J.; Conraths, K.D.; Probst, C.; Sauter-Louis, C.; Beer, M.; Mettenleiter, T.; Blome, S. Maßnahmen im Falle Eines Ausbruchs von ASP bei Wildschweinen. Available online: https://www.bauernverband-mv.de/sites/default/files/2018-03/Vortrag2_Prof._Conr.raths.pdf (accessed on 8 March 2021).
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar—Ecology and Biosecurity. In FAO Animal Production and Health Manual No. 22; FAO: Rome, Italy, 2019; Available online: https://www.oie.int/app/uploads/2021/03/en-manual-asfinwildboar-2019-web.pdf (accessed on 19 March 2021).
- Friedrich-Loeffler-Institute (FLI). Empfehlungen zur Desinfektion bei Tierseuchen—Grundsätze zu Anforderungen und Anwendungen von Wirkstoffklassen; Friedrich-Loeffler-Institute: Riems, Germany, 2020. [Google Scholar]
- Anon. Infection Route of the 2007 Outbreak of Highly Pathogenic Avian Influenza in Japan; Food Safety and Consumer Affairs Bureau Ministry of Agriculture, Forestry & Fisheries: Tokyo, Japan, 2007. [Google Scholar]
- Ruenphet, S.; Punyadarsaniya, D.; Jantafong, T.; Takehara, K. Stability and virucidal efficacies using powder and liquid forms of fresh charcoal ash and slaked lime against Newcastle disease virus and Avian influenza virus. Vet. World 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Euler, B. Lime as Disinfactant für Pig Slurry Contaminated with Aujeszky’s Disease (Pseudorabies) Virus (ADV). Agric. Wastes 1984, 9, 289–297. [Google Scholar] [CrossRef]
- Schmitz, A.P.; Le Bouquin, M.S.; Rousset, N.; Ogor, K.; LeBras, M.O.; Martenot, C.; Daniel, P.; Belen Cepeda Hontecillas, A.; Scoizec, A.; Morin, H. Natural and Experimental Persistence of Highly Pathogenic H5 Influenza Viruses in Slurry of Domestic Ducks, with or without Lime Treatment. Appl. Environ. Microbiol. 2020, 86, e02288-20. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Loeffler-Institute (FLI). Empfehlungen zur Desinfektion bei Tierseuchen—Kalk; Friedrich-Loeffler-Institute: Riems, Germany, 2020. [Google Scholar]
- Schneider, T.; Strauch, B.R.D. Untersuchungen über den Einsatz von Branntkalk zur Desinfektion von Stallböden. Tierarztl. Umsch. 1992, 47, 534–538. [Google Scholar]
- Tanneberger, F.; Institute of Animal Hygiene and Veterinary Public Health. Survey Experiment on the Change in Soil pH after Disinfection with Lime Products; data not shown; Leipzig University: Leipzig, Germany, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).