Abstract
Providencia rettgeri is an emerging opportunistic Gram-negative pathogen with reports of increasing antibiotic resistance. Pan-drug resistant (PDR) P. rettgeri infections are a growing concern, demonstrating a need for the development of alternative treatment options which is fueling a renewed interest in bacteriophage (phage) therapy. Here, we identify and characterize phage vB_PreP_EPr2 (EPr2) with lytic activity against PDR P. rettgeri MRSN 845308, a clinical isolate that carries multiple antibiotic resistance genes. EPr2 was isolated from an environmental water sample and belongs to the family Autographiviridae, subfamily Studiervirinae and genus Kayfunavirus, with a genome size of 41,261 base pairs. Additional phenotypic characterization showed an optimal MOI of 1 and a burst size of 12.3 ± 3.4 PFU per bacterium. EPr2 was determined to have a narrow host range against a panel of clinical P. rettgeri strains. Despite this fact, EPr2 is a promising lytic phage with potential for use as an alternative therapeutic for treatment of PDR P. rettgeri infections.
1. Introduction
Providencia rettgeri is a Gram-negative bacterial pathogen from the Enterobacteriaceae family and is an emerging cause of nosocomial infections [1,2]. Infections caused by P. rettgeri are typically linked to catheter placements and travelers’ diarrhea, but cellulitis, sepsis, and meningitis have also been reported, predominantly in immunocompromised individuals [2,3,4]. P. rettgeri is intrinsically resistant to the antibiotics colistin and tigecycline and has the ability to acquire additional drug resistance against antibiotic classes commonly used to treat infections [5]. Several studies have identified P. rettgeri strains that produce extended spectrum β-lactamases (ESBLs) and carbapenemases [6,7,8,9,10]. Increasing incidence of multidrug-resistant (MDR), extensively drug-resistant (XDR) and pan-drug resistant (PDR) P. rettgeri has been reported, severely limiting available treatment options in the clinical setting [7,8,9].
Bacteriophage (phage) therapy is an alternative treatment option being developed against bacterial infections, including those caused by drug-resistant strains. Phage mechanisms of action are distinct from those of antibiotics, and phages have been shown to target and kill MDR strains of both Gram-negative and Gram-positive bacterial species [11,12,13]. Recent publications have also demonstrated the potential benefit of phage-induced resensitization of MDR bacterial strains to antibiotics [14,15,16,17]. Additionally, the specificity of phages allows targeted treatment of bacterial infections without compromising patient’s normal microflora [12]. There are a limited number of lytic phages isolated and characterized with activity against P. rettgeri strains [18,19]. Identification of additional phages that target P. rettgeri will provide the opportunity to develop robust phage therapeutics against this emerging pathogen.
PDR P. rettgeri MRSN 845308 was isolated from a patient receiving care for COVID-19 in Texas, USA in December 2020 [9]. The patient’s symptoms did not improve with antibiotic therapy (vancomycin and cefepime), so the strain underwent further characterization by the Multidrug-Resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research (WRAIR). P. rettgeri MRSN 845308 was found to be non-susceptible to all available antibiotics and whole genome sequencing analysis showed that the strain possessed multiple antibiotic resistance genes, including blaNDM-1, blaPER-1, and rmtB2 [9]. In this study, we report the isolation and characterization of phage vB_PreP_EPr2 (EPr2) from an environmental water sample with lytic activity against P. rettgeri MRSN 845308.
2. Materials and Methods
2.1. Bacterial Strains
Bacterial strains used in this study are listed in Table 1. P. rettgeri were obtained from the MRSN and the American Type Culture Collection (ATCC, Manassas, VA, USA). Bacteria were grown in Heart Infusion Broth (HIB) with shaking (200 rpm) or on HIB agar plates at 37 °C overnight.

Table 1.
Host Range of EPr2.
2.2. Phage Isolation and Propagation
The phage EPr2 was isolated from environmental water collected from Rock Creek in Bethesda, MD, USA. The environmental water was filtered using sterile 0.22 µm filters (MilliporeSigma, Bollington, MA, USA) prior to phage enrichment. To enrich for P. rettgeri phages, 5 × HIB was mixed with the filter-sterilized environmental water sample at a 1:5 ratio and overnight broth culture of P. rettgeri MRSN 845308 was added. The enrichment mixture was incubated overnight at 37 °C with shaking at 200 rpm, after which the supernatant was sterilized using a 0.22 µm filter. The resulting lysate was assessed for phage activity against P. rettgeri MRSN 845308 through plating on double-layer HIB agar plates [20]. Phage purification was performed by three rounds of sequential single plaque isolation until uniform plaque morphology was achieved. Plaque morphology was analyzed using ImageJ software v. 1.53 (National Institutes of Health, Bethesda, MD, USA).
High titer phage lysates were obtained by liquid culture propagation of the phage in HIB supplemented with 0.5 mM CaCl2, 2 mM MgCl2, and 0.1% glucose. Phages were harvested after supernatant filtration with a 0.22 µm filter and centrifugation and stored in SM buffer (50 mM Tris-HCl, pH 7.5, 99 mM NaCl, 8 mM MgSO4, 0.01% gelatin) (Teknova, Hollister, CA, USA) at 4 °C protected from light. Plaque-forming units (PFUs) per ml were determined through 10-fold dilution in SM buffer and plating on double-layer agar plates.
2.3. Host Range Testing
The host range was determined against the P. rettgeri strains listed in Table 1 as described previously using a micro-spot dilution assay on square grid double-layer agar plates [21]. Lytic activity was determined positive if plaque formation was observed after overnight plate incubation at 37 °C. Host range testing was performed in two separate replicates with representative results reported.
2.4. Transmission Electron Microscopy
EPr2 was prepared for transmission electron microscopy (TEM) imaging as previously described [22]. Briefly, phages were washed twice with 0.1% ammonium acetate using high-speed centrifugation. Prepared phage particles were stained with 2% uranyl acetate for 1 min after being deposited on 300 mesh carbon-coated copper grids (Electron Microscopy Sciences, Hatfield, PA, USA). Samples were examined in a JEOL JEM-1400 electron microscope at 80 kv. Image analysis was conducted using Image J software v. 1.53 (National Institutes of Health, Bethesda, MD, USA).
2.5. Bacterial Strains Lytic Properties Assay
The dynamics of EPr2 lysis against P. rettgeri MRSN 845308 was determined as previously described [23]. Briefly, phage were mixed with a mid-log phase bacterial culture (OD600 = 0.5) at a multiplicity of infection (MOI) of 1, 0.1, 0.01, 0.001, and 0. The mixed culture was incubated at 37 °C with shaking at 200 rpm for the duration of the experiment. OD600 readings were taken every 10–20 min for 3 h. Experiments were repeated independently three times and analyzed using GraphPad Prism v. 9 (GraphPad Software, San Diego, CA, USA).
2.6. Determination of Optimal Multiplicity of Infection
The optimal MOI of EPr2 was determined as previously described [24]. Briefly, phage particles at various MOIs were added to mid-log phase bacterial culture (OD600 = 0.5) and incubated for 4 h at 37 °C with shaking at 200 rpm. The samples were centrifuged for 10 min and the supernatant was filtered using a 0.22 µm filter. Phage titer for each sample was determined by plating on double-layer agar plates. Experiments were repeated independently three times and analyzed using GraphPad Prism v. 9 (GraphPad Software, San Diego, CA, USA).
2.7. One Step Growth Curve
One-step growth curve experiments were performed as previously described [25]. Briefly, 1 mL of 5 × 106 PFU/mL EPr2 stock was mixed with 1 mL of mid-log phase bacterial culture in HIB and allowed to adsorb for 5 min at 37 °C. The mixture was then diluted with fresh HIB and incubated at 37 °C for the duration of the experiment. Samples were taken every 5 min and plated using the double-layer agar plate method to determine phage titer. Experiments were repeated independently three times and analyzed using GraphPad Prism v. 9 (GraphPad Software, San Diego, CA, USA).
2.8. DNA Extraction and Whole Genome Sequencing
DNA extraction was performed using a modified QIAamp DNA Mini Kit (Qiagen, Germantown, MD, USA) protocol as described previously [26]. Briefly, a high-titer phage sample was treated with 2.5 U/mL DNase and 0.7 mg/mL RNase for 1.5 h at 37 °C, after which 20 mM EDTA was added to the sample. Next, the sample was treated with 3 µL of proteinase K and incubated for 1.5 h at 56 °C. An equal volume of Buffer AL was added to the treated phage lysate and QIAamp DNA Mini Kit instructions were followed to complete the DNA extraction.
Whole genome sequencing libraries were constructed from extracted EPr2 DNA using the KAPA HyperPlus Library preparation kit (Roche Diagnostics, Indianapolis, IN, USA). The prepared library was sequenced using MiSeq Reagent Kit v3 (600 cycle; 2 × 300 bp) (Illumina, San Diego, CA, USA). Whole genome sequencing analysis and phylogenetic analysis was performed as described in Supplementary Material Document S1. Briefly, paired end sequences were evaluated for quality then used for assembly with Unicycler [27]. The phage genome termini were determined using PhageTerm [28], and protein coding sequences (CDSs) were predicted and annotated using Prodigal [29]. The sequencing data is available in GenBank (accession number OM256482).
3. Results
3.1. Morphological Characterization of EPr2
The phage EPr2 was isolated from an environmental water sample from Rock Creek in Bethesda, Maryland, USA against the host strain P. rettgeri MRSN 845308. EPr2 forms large plaques of 2.4 ± 0.6 mm diameter with a halo that further extends 2.7 ± 0.3 mm from the edge of the plaque (Figure 1A). The phage particle morphology was observed using transmission electron microscopy (TEM). The TEM images showed that phage EPr2 has an icosahedral structure head with an approximate diameter of 43.7 ± 2.2 nm with a short non-contractile tail (Figure 1B). Based on these characteristics and in accordance with the International Committee on Taxonomy of Viruses (ICTV), EPr2 is morphologically classified as a podophage.
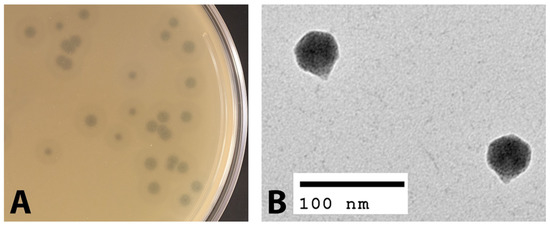
Figure 1.
Morphological characterization of phage EPr2. (A) EPr2 plaque morphology on P. rettgeri MRSN 845308 and (B) TEM image.
3.2. Host Range of EPr2
The host range of EPr2 was evaluated against 14 strains of P. rettgeri, 13 of which were clinical isolates collected by the MRSN from 2012–2021, and one strain was acquired from ATCC (Table 1). The results indicated that EPr2 has a narrow host range against the strains tested, only exhibiting lysis against three PDR isolates from the same patient within a short time period, including the initial host strain (Table 1). EPr2 was not active against other P. rettgeri strains collected from different sources.
3.3. Lytic Properties of EPr2
The dynamics of host strain lysis were measured at various MOIs of EPr2. The most rapid and robust lytic effect was demonstrated with an MOI of 1 starting after 30 min incubation and reaching full lysis at 90 min. Reduced MOIs resulted in increasing delay in and completeness of observed lysis (Figure 2A).
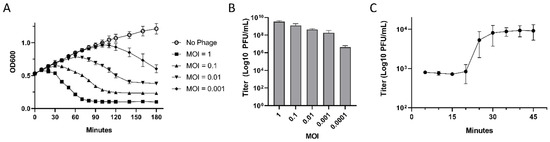
Figure 2.
Lytic properties of phage EPr2 (A) EPr2 lysis dynamics against P. rettgeri MRSN 845308, (B) optimal MOI, and (C) one-step growth curve.
To determine optimal MOI of EPr2, mid log-phase cultures of P. rettgeri MRSN 845308 were incubated with phage MOIs of 0.0001 to 1. The highest phage titer achieved was 3.5 × 109 PFU/mL with a starting MOI of 1 (Figure 2B). Lower starting MOIs resulted in decreasing total phage titer.
A one-step growth curve of EPr2 was also performed. The latent phase was estimated to be 20 min, followed by a rise phase of 10 min, and a plateau phase reached 30 min after initial infection (Figure 2C). The average burst size of EPr2 was estimated to be 12.3 ± 3.4 PFU per infected cell.
3.4. Genomic Analysis of EPr2
Phage EPr2 has a 41,261 base pair (bp) genome with a G + C content of 50.2% (GenBank accession no. OM256482). It has short 179 bp direct terminal repeats that were identified using PhageTerm [28]. Annotation of EPr2 resulted in the prediction of 69 CDSs. No antibiotic resistance genes, toxin genes, or other bacterial virulence genes were identified. BLASTn analysis against the NCBI non-redundant (NR) database assigned EPr2 to the family Autographiviridae, subfamily Studiervirinae and genus Kayfunavirus.
The BLASTn results showed that EPr2 does not share high similarity with any known Providencia sp. phages. The most closely related phages to EPr2 targeted diverse bacterial host species, predominantly Escherichia, Salmonella, and Citrobacter. A phylogenetic tree was constructed with EPr2, 12 phages included in the top BLASTn results, 22 Providencia sp. phages, and 5 reference phages related to Providencia phages (Figure 3) [19]. Phage EPr2 was shown to share the most similarity with Escherichia coli phages through the phylogenetic analysis.
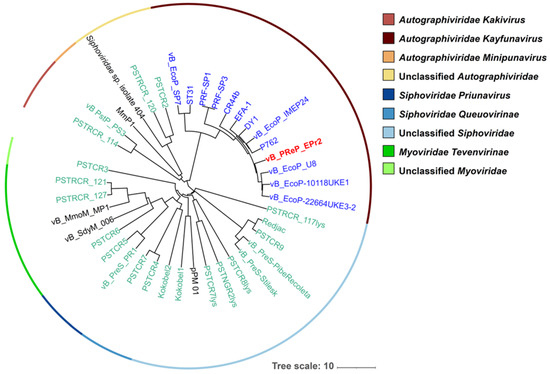
Figure 3.
Phylogenetic tree of Providencia phage EPr2. A whole-genome average nucleotide distance tree was constructed for 40 total phage genomes (22 phages that infect Providencia species (green leaves), 5 phages related to known Providencia phages (black leaves), 12 with top BLASTn matches (blue leaves) and vB_PReP_EPr2 (bold red leaf) with MASH [30] (sketch size of s = 5000, k-mer size of k = 12 and GGRaSP [31] (see Supplementary Materials and Methods). Color strips denote taxonomic assignments (see key). The scale bar represents percent average nucleotide divergence.
4. Discussion
P. rettgeri is an important emerging pathogen associated with drug-resistant hospital-associated infections. Several reports have identified P. rettgeri strains that harbor ESBL and carbapenemase genes, resulting in difficult to treat infections in a clinical setting, including the recent report of PDR P. rettgeri MRSN 845308 isolated from a patient being treated for COVID-19 [2,9]. Phage therapy offers a potential alternative treatment method for these MDR, XDR, and PDR strains [11,12].
In this study, we report the phenotypic and genomic characteristics of phage EPr2 with lytic activity against P. rettgeri MRSN 845308. Morphologically, EPr2 has an icosahedral head and short, non-contractile tail consistent with classic podovirus morphology. EPr2 forms large plaques with a halo, indicating production of a polysaccharide depolymerase and potential antibiofilm activity [32]. It has an optimal MOI of 1 and a relatively low burst size of 12.3 ± 3.4 PFU per bacterium. EPr2 belongs to the family Autographiviridae, subfamily Studiervirinae and genus Kayfunavirus, which include phages with short, non-contractile tails. To our knowledge, no other Autographiviridae Kayfunavirus phages that target Providencia sp. have been reported to date. Genomic analysis showed a 41,261 bp genome that appears to not carry any genes that would disqualify EPr2 from therapeutic use. The P. rettgeri strains tested are representative isolates collected from US military treatment facilities from 2012–2021. EPr2 has a narrow host range against this set, with lytic activity specific to the original PDR strains isolated within a short time period from the same patient. Interestingly, genome comparisons using NCBI BLASTn and phylogenetic analysis showed that EPr2 is the most similar to Autographiviridae Kayfunavirus phages that target E. coli and other Enterobacteriaceae, highlighting the lack of similar published P. rettgeri phages and an opportunity to further study potential cross-species lytic activity of EPr2.
Ideally, multiple phages would be used together in a cocktail with activity against a P. rettgeri infection to improve host range and minimize the opportunity for resistance to arise during therapy. However, there are currently limited P. rettgeri phages reported in the literature that may be suitable for therapeutic development. Oliveira et al. reported phage vB_PreS_PR1, a Siphoviridae phage with a plaque size of under 0.1 mm and broad host range activity against MDR P. rettgeri clinical isolates [18]. A study by Rakov et al. identified 12 phages with lytic activity against Providencia stuartii and P. rettgeri as part of the Israeli Phage Bank (IPB), including activity against biofilms [19]. The phages in that study represented Siphoviridae, Myoviridae, and Autographiviridae phylogenetic families and 39/41 Providencia sp. strains showed susceptibility to at least one phage [19]. In comparison, EPr2 forms large plaques with a halo, belongs to the Autographiviridae family, and has a narrow host range. This indicates a broad diversity of P. rettgeri phages and suggests that discovery of additional phages is required for the development of a broad-coverage therapeutic phage cocktail.
In summary, we report the isolation and characterization of EPr2, a lytic phage with activity against the PDR clinical isolate P. rettgeri MRSN 845308. This study provides evidence that phage treatment is a viable alternative therapeutic option for PDR P. rettgeri infections. Importantly, EPr2 was discovered and isolated in one week from a single environmental source, indicating phages that target clinical P. rettgeri strains can be identified rapidly and on demand. EPr2 demonstrates desirable characteristics for a therapeutic phage, including strong lytic activity against a PDR strain of interest and no genes indicative of a lysogenic lifestyle, drug resistance, or virulence. It is important to continue phage hunting for Providencia sp. phages as infections rise and drug resistance profiles continue to evolve, rendering most or all available antibiotics ineffective.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14040708/s1, Supplemental Materials and Methods Document S1. References [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] are cited in the supplementary materials.
Author Contributions
Conceptualization, J.L.M., D.G., D.W.E. and K.R.M.; methodology, J.L.M., Y.H., A.A.F., M.P.N., A.T.B., D.E.F. and K.R.M.; software, A.T.B.; formal analysis, J.L.M., A.A.F., M.P.N., A.T.B., D.E.F. and K.R.M.; resources, A.A.F., M.P.N., P.T.M., B.E.S., D.G., D.W.E. and K.R.M.; data curation, J.L.M., A.T.B. and K.R.M.; writing—original draft preparation, J.L.M. and K.R.M.; writing—review and editing, J.L.M., Y.H., A.A.F., M.P.N., A.T.B., D.E.F., P.T.M., B.E.S., D.G., D.W.E. and K.R.M.; visualization, J.L.M., D.E.F. and K.R.M.; supervision, A.A.F., M.P.N., B.E.S., D.G., D.W.E. and K.R.M.; project administration, A.A.F., M.P.N., B.E.S., D.G., D.W.E. and K.R.M.; funding acquisition, A.A.F., M.P.N., P.T.M., B.E.S., D.G., D.W.E. and K.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Peer Reviewed Medical Research Program, Focused Program Award PR182667.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The whole genome sequence of vB_PreP_EPr2 has been deposited in GenBank under the accession number OM256482.
Acknowledgments
We acknowledge and thank the staff at the Walter Reed Army Institute of Research-Multidrug-Resistant Organism Repository and Surveillance Network (WRAIR/MRSN) for the provision of P. rettgeri strains and whole genome sequencing of EPr2. We also thank Brittney Kociuba and staff at the WRAIR Diagnostic Pathology Branch for capturing the phage electron microscopy images. Material has been reviewed by the Walter Reed Army Institute of Research and the Uniformed Services University of the Health Sciences. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The opinions and assertions expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.M.; Brenner, F.W.; Miller, J.M. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Wie, S.H. Clinical significance of Providencia bacteremia or bacteriuria. Korean J. Intern. Med. 2015, 30, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Yoh, M.; Matsuyama, J.; Ohnishi, M.; Takagi, K.; Miyagi, H.; Mori, K.; Park, K.S.; Ono, T.; Honda, T. Importance of Providencia species as a major cause of travellers’ diarrhoea. J. Med. Microbiol. 2005, 54 Pt 11, 1077–1082. [Google Scholar] [CrossRef]
- Abdallah, M.; Balshi, A. First literature review of carbapenem-resistant Providencia. New Microbes New Infect. 2018, 25, 16–23. [Google Scholar] [CrossRef]
- Iwata, S.; Tada, T.; Hishinuma, T.; Tohya, M.; Oshiro, S.; Kuwahara-Arai, K.; Ogawa, M.; Shimojima, M.; Kirikae, T. Emergence of carbapenem-resistant Providencia rettgeri and Providencia stuartii producing IMP-Type Metallo-beta-Lactamase in Japan. Antimicrob. Agents Chemother. 2020, 64, e00382-20. [Google Scholar] [CrossRef]
- Shin, S.; Jeong, S.H.; Lee, H.; Hong, J.S.; Park, M.J.; Song, W. Emergence of multidrug-resistant Providencia rettgeri isolates co-producing NDM-1 carbapenemase and PER-1 extended-spectrum beta-lactamase causing a first outbreak in Korea. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 20. [Google Scholar] [CrossRef]
- Piza-Buitrago, A.; Rincon, V.; Donato, J.; Saavedra, S.Y.; Duarte, C.; Morero, J.; Falquet, L.; Reguero, M.T.; Barreto-Hernandez, E. Genome-based characterization of two Colombian clinical Providencia rettgeri isolates co-harboring NDM-1, VIM-2, and other beta-lactamases. BMC Microbiol. 2020, 20, 345. [Google Scholar] [CrossRef]
- Mc Gann, P.; Geringer, M.R.; Hall, L.R.; Lebreton, F.; Markelz, E.; Kwak, Y.I.; Johnson, S.; Ong, A.C.; Powell, A.; Tekle, T.; et al. Pan-drug resistant Providencia rettgeri contributing to a fatal case of COVID-19. J. Med. Microbiol. 2021, 70, 001406. [Google Scholar] [CrossRef]
- Patel, N.B.; Jain, G.; Chandrakar, S.; Walikar, B.N. Ventilator-associated pneumonia due to carbapenem-resistant Providencia rettgeri. BMJ Case Rep. 2021, 14, e243908. [Google Scholar] [CrossRef]
- Chegini, Z.; Khoshbayan, A.; Vesal, S.; Moradabadi, A.; Hashemi, A.; Shariati, A. Bacteriophage therapy for inhibition of multi drug-resistant uropathogenic bacteria: A narrative review. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Oliveira, H.; Pinto, G.; Mendes, B.; Dias, O.; Hendrix, H.; Akturk, E.; Noben, J.P.; Gawor, J.; Łobocka, M.; Lavigne, R.; et al. A tailspike with exopolysaccharide depolymerase activity from a new Providencia stuartii phage makes multidrug-resistant bacteria susceptible to serum-mediated killing. Appl. Environ. Microbiol. 2020, 86, e00073-20. [Google Scholar] [CrossRef] [PubMed]
- Mangalea, M.R.; Duerkop, B.A. Fitness trade-offs resulting from bacteriophage resistance potentiate synergistic antibacterial strategies. Infect. Immun. 2020, 88, e00926-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, P.; Chen, L.; Guo, G.; Xiao, Y.; Chen, L.; Du, H.; Zhang, W. Identification of a phage-derived depolymerase specific for KL64 capsule of Klebsiella pneumoniae and its anti-biofilm effect. Virus Genes 2021, 57, 434–442. [Google Scholar] [CrossRef]
- Canfield, G.S.; Catterjee, A.; Espinosa, J.; Mangalea, M.R.; Sheriff, E.K.; Keidan, M.; McBride, S.W.; McCollister, B.D.; Hang, H.C.; Buerkop, B.A. Lytic bacteriophages facilitate antibiotic sensitization of Enterococcus faecium. Antimicrob. Agents Chemother. 2021, 65, e00143-21. [Google Scholar] [CrossRef]
- Engeman, E.; Freyberger, H.R.; Corey, B.W.; Ward, A.M.; He, Y.; Nikolich, M.P.; Filippov, A.A.; Tyner, S.D.; Jacobs, A.C. Synergistic killing and re-sensitization of Pseudomonas aeruginosa to antibiotics by phage-antibiotic combination treatment. Pharmaceuticals 2021, 14, 184. [Google Scholar] [CrossRef]
- Oliveira, H.; Pinto, G.; Hendrix, H.; Noben, J.P.; Gawor, J.; Kropinski, A.M.; Łobocka, M.; Lavigne, R.; Azeredo, J. A lytic Providencia rettgeri virus of potential therapeutic value is a deep-branching member of the T5 virus genus. Appl. Environ. Microbiol. 2017, 83, e01567-17. [Google Scholar] [CrossRef] [Green Version]
- Rakov, C.; Ben Porat, S.; Alkalay-Oren, S.; Yerushalmy, O.; Abdalrhman, M.; Gronovich, N.; Huang, L.; Pride, D.; Coppenhagen-Glazer, S.; Nir-Paz, R.; et al. Targeting biofilm of MDR Providencia stuartii by phages using a catheter model. Antibiotics 2021, 10, 375. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Sergueev, K.V.; Filippov, A.A.; Farlow, J.; Su, W.; Kvachadze, L.; Balarjishvili, N.; Kutateladze, M.; Nikolich, M.P. Correlation of host range expansion of therapeutic bacteriophage Sb-1 with allele state at a hypervariable repeat locus. Appl. Environ. Microbiol. 2019, 85, e0109-19. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, H.W. Basic phage electron microscopy. Methods Mol. Biol. 2009, 501, 113–126. [Google Scholar]
- Sergueev, K.V.; He, Y.; Borschel, R.H.; Nikolich, M.P.; Filippov, A.A. Rapid and sensitive detection of Yersinia pestis using amplification of plague diagnostic bacteriophages monitored by real-time PCR. PLoS ONE 2010, 5, e11337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Wu, D.; Hu, Y.; Luo, H.; Li, G. Characterization of an Enterococcus faecalis bacteriophage vB_EfaM_LG1 and its synergistic effect with antibiotic. Front. Cell Infect. Microbiol. 2021, 11, 698807. [Google Scholar] [CrossRef] [PubMed]
- Kropinski, A.M. Practical advice on the one-step growth curve. Methods Mol. Biol. 2018, 168, 41–47. [Google Scholar]
- Jakočiūnė, D.; Moodley, A. A rapid bacteriophage DNA extraction method. Methods Protoc. 2018, 1, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [Green Version]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.H.; Brinkac, L.M.; Sutton, G.; Fouts, D.E. GGRaSP: A R-package for selecting representative genomes using Gaussian mixture models. Bioinformatics 2018, 34, 3032–3034. [Google Scholar] [CrossRef]
- Harper, D.R.; Parracho, H.; Walker, J.; Sharp, R.; Hughes, G.; Werthen, M.; Lehman, S.; Morales, S. Bacteriophages and biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilie, L.; Molnar, M. RACER: Rapid and accurate correction of errors in reads. Bioinformatics 2013, 29, 2490–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Pearson, W.R. Finding protein and nucleotide similarities with FASTA. Curr. Protoc. Bioinform. 2016, 53, 3.9.1–3.9.25. [Google Scholar] [CrossRef]
- Lueder, M.R.; Cer, R.Z.; Patrick, M.; Voegtly, L.J.; Long, K.A.; Rice, G.K.; Bishop-Lilly, K.A. Manual Annotation Studio (MAS): A collaborative platform for manual functional annotation of viral and microbial genomes. BMC Genom. 2021, 22, 733. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- McNair, K.; Zhou, C.; Dinsdale, E.A.; Souza, B.; Edwards, R.A. PHANOTATE: A novel approach to gene identification in phage genomes. Bioinformatics 2019, 35, 4537–4542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcais, G.; Kingsford, C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 2011, 27, 764–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laslett, D.; Canback, B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA genes in genomic sequences. Methods Mol. Biol. 2019, 1962, 1–14. [Google Scholar]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.H.; Davis, F.G.; et al. A tissue-mapped axolotl transcriptome enables identification of limb regeneration factors. Cell Rep. 2017, 18, 762–776. [Google Scholar] [CrossRef] [Green Version]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.M. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2014, 58, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Moller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [Green Version]
- Ingle, D.J.; Valcanis, M.; Kuzevski, A.; Tauschek, M.; Inouye, M.; Stinear, T.P.; Levine, M.M.; Robins-Browne, R.M.; Holt, K.E. In silico serotyping of E. coli from short read data identifies limited novel O-loci but extensive diversity of O:H serotype combinations within and between pathogenic lineages. Microb. Genom. 2016, 2, e000064. [Google Scholar] [CrossRef] [PubMed]
- Doster, E.; Lakin, S.M.; Dean, C.J.; Wolfe, C.; Young, J.G.; Boucher, C.; Belk, K.E.; Noyes, N.R.; Morley, P.S. MEGARes 2.0: A database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids Res. 2020, 48, D561–D569. [Google Scholar] [CrossRef]
- Zhou, C.E.; Smith, J.; Lam, M.; Zemla, A.; Dyer, M.D.; Slezak, T. MvirDB—A microbial database of protein toxins, virulence factors and antibiotic resistance genes for bio-defence applications. Nucleic Acids Res. 2007, 35, D391–D394. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Ghosh, S.; Chowdhary, G.; Maulik, U.; Chakrabarti, S. DBETH: A database of bacterial exotoxins for human. Nucleic Acids Res. 2012, 40, D615–D620. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [Green Version]
- Stothard, P.; Wishart, D.S. Circular genome visualization and exploration using CGView. Bioinformatics 2005, 21, 537–539. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, R.; Bernhart, S.H.; Höner zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).