Hepatitis B Virus Chronic Infection in Blood Donors from Asian and African High or Medium Prevalence Areas: Comparison According to Sex
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. HBsAg Testing
2.3. HBV DNA Testing
2.4. Anti-HBs Quantification
2.5. Ethical Approval
2.6. Study Design
2.7. Statistical Analysis
3. Results
3.1. HBV Viral Load in Children below 5 Years of Age
3.2. Viral Load according to Sex in Adult Blood Donors
3.3. Sex Distribution in Blood Donors with Occult HBV Infection (OBI)
3.4. Distribution of HBsAg Prevalence According to Age and Sex of Random Blood Donors
3.5. Humoral Immune Response to Natural HBV Infection or HBV Vaccination at Birth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, S.; Klein, R.S. Sex Drives Dimorphic Immune Responses to Viral Infections. J. Immunol. 2017, 198, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Giefing-Kroll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [PubMed]
- van Lunzen, J.; Altfeld, M. Sex differences in infectious diseases-common but neglected. J. Infect. Dis. 2014, 209, S79–S80. [Google Scholar] [CrossRef]
- Chu, C.M.; Liaw, Y.F.; Sheen, I.S.; Lin, D.Y.; Huang, M.J. Sex difference in chronic hepatitis B virus infection: An appraisal based on the status of hepatitis B e antigen and antibody. Hepatology 1983, 3, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, P.J.; Yeh, S.H. Gender disparity in chronic hepatitis B: Mechanisms of sex hormones. J. Gastroenterol. Hepatol. 2015, 30, 1237–1245. [Google Scholar] [CrossRef]
- Shimizu, I.; Kohno, N.; Tamaki, K.; Shono, M.; Huang, H.W.; He, J.H.; Yao, D.F. Female hepatology: Favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J. Gastroenterol. 2007, 13, 4295–4305. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, H.I.; Iloeje, U.H.; Group, R.-H.S. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 2009, 49, S72–S84. [Google Scholar] [CrossRef]
- Su, F.H.; Chen, J.D.; Cheng, S.H.; Lin, C.H.; Liu, Y.H.; Chu, F.Y. Seroprevalence of Hepatitis-B infection amongst Taiwanese university students 18 years following the commencement of a national Hepatitis-B vaccination program. J. Med. Virol. 2007, 79, 138–143. [Google Scholar] [CrossRef]
- Voysey, M.; Barker, C.I.; Snape, M.D.; Kelly, D.F.; Trück, J.; Pollard, A.J. Sex-dependent immune responses to infant vaccination: An individual participant data meta-analysis of antibody and memory B cells. Vaccine 2016, 34, 1657–1664. [Google Scholar] [CrossRef]
- Alward, W.L.; McMahon, B.J.; Hall, D.B.; Heyward, W.L.; Francis, D.P.; Bender, T.R. The long-term serological course of asymptomatic hepatitis B virus carriers and the development of primary hepatocellular carcinoma. J. Infect. Dis. 1985, 151, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ye, X.; Du, P.; Zeng, J.; Zhu, W.; Yang, B.; Li, C.; Allain, J.P. High prevalence of anti-hepatitis B core antigen in hepatitis B virus-vaccinated Chinese blood donors suggests insufficient protection but little threat to the blood supply. Transfusion 2015, 55, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Eko Mba, J.M.; Bisseye, C.; Ntsame Ndong, J.M.; Mombo, L.E.; Bengone, C.; Mouelet Migolet, G.; M’Batchi, B.; Kosiorek, H.E.; Butterfield, R.J.; Roberts, L.R.; et al. Prevalent hepatitis B surface antigen among first-time blood donors in Gabon. PLoS ONE 2018, 13, e0194285. [Google Scholar] [CrossRef]
- Candotti, D.; Diarra, B.; Bisseye, C.; Tao, I.; Pham Quang, K.; Sanou, M.; Laperche, S.; Sanogo, R.; Allain, J.P.; Simpore, J. Molecular characterization of hepatitis B virus in blood donors from Burkina Faso: Prevalence of quasi-subgenotype A3, genotype E, and mixed infections. J. Med. Virol. 2016, 88, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- El Chaar, M.; El Jisr, T.; Allain, J.P. Hepatitis B virus DNA splicing in Lebanese blood donors and genotype A to E strains: Implications for hepatitis B virus DNA quantification and infectivity. J. Clin. Microbiol. 2012, 50, 3159–3167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garmiri, P.; Rezvan, H.; Abolghasemi, H.; Allain, J.P. Full genome characterization of hepatitis B virus strains from blood donors in Iran. J. Med. Virol. 2011, 83, 948–952. [Google Scholar] [CrossRef]
- Zheng, X.; Ye, X.; Zhang, L.; Wang, W.; Shuai, L.; Wang, A.; Zeng, J.; Candotti, D.; Allain, J.P.; Li, C. Characterization of occult hepatitis B virus infection from blood donors in China. J. Clin. Microbiol. 2011, 49, 1730–1737. [Google Scholar] [CrossRef]
- Candotti, D.; Opare-Sem, O.; Rezvan, H.; Sarkodie, F.; Allain, J.P. Molecular and serological characterization of hepatitis B virus in deferred Ghanaian blood donors with and without elevated alanine aminotransferase. J. Viral Hepat. 2006, 13, 715–724. [Google Scholar] [CrossRef]
- Owusu-Ofori, S.; Temple, J.; Sarkodie, F.; Anokwa, M.; Candotti, D.; Allain, J.P. Predonation screening of blood donors with rapid tests: Implementation and efficacy of a novel approach to blood safety in resource-poor settings. Transfusion 2005, 45, 133–140. [Google Scholar] [CrossRef]
- Allain, J.P.; Candotti, D.; Soldan, K.; Sarkodie, F.; Phelps, B.; Giachetti, C.; Shyamala, V.; Yeboah, F.; Anokwa, M.; Owusu-Ofori, S.; et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood 2003, 101, 2419–2425. [Google Scholar] [CrossRef]
- Meldal, B.H.M.; Moula, N.M.; Barnes, I.H.A.; Boukef, K.; Allain, J.P. A novel hepatitis B virus subgenotype, D7, in Tunisian blood donors. J. Gen. Virol. 2009, 90, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Nagalo, B.M.; Bisseye, C.; Sanou, M.; Kienou, K.; Nebie, Y.K.; Kiba, A.; Dahourou, H.; Ouattara, S.; Nikiema, J.B.; Moret, R.; et al. Seroprevalence and incidence of transfusion-transmitted infectious diseases among blood donors from regional blood transfusion centres in Burkina Faso, West Africa. Trop. Med. Int. Health 2012, 17, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Parsyan, A.; Addo-Yobo, E.; Owusu-Ofori, S.; Akpene, H.; Sarkodie, F.; Allain, J.P. Effects of transfusion on human erythrovirus B19-susceptible or -infected pediatric recipients in a genotype 3-endemic area. Transfusion 2006, 46, 1593–1600. [Google Scholar] [CrossRef]
- Tang, X.; Allain, J.P.; Wang, H.; Rong, X.; Chen, J.; Huang, K.; Xu, R.; Wang, M.; Huang, J.; Liao, Q.; et al. Incidence of hepatitis B virus infection in young Chinese blood donors born after mandatory implementation of neonatal hepatitis B vaccination nationwide. J. Viral Hepat. 2018, 25, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Candotti, D.; Lin, C.K.; Belkhiri, D.; Sakuldamrongpanich, T.; Biswas, S.; Lin, S.; Teo, D.; Ayob, Y.; Allain, J.P. Occult hepatitis B infection in blood donors from South East Asia: Molecular characterisation and potential mechanisms of occurrence. Gut 2012, 61, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Candotti, D.; Grabarczyk, P.; Ghiazza, P.; Roig, R.; Casamitjana, N.; Iudicone, P.; Schmidt, M.; Bird, A.; Crookes, R.; Brojer, E.; et al. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. J. Hepatol. 2008, 49, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Allain, J.P.; Belkhiri, D.; Vermeulen, M.; Crookes, R.; Cable, R.; Amiri, A.; Reddy, R.; Bird, A.; Candotti, D. Characterization of occult hepatitis B virus strains in South African blood donors. Hepatology 2009, 49, 1868–1876. [Google Scholar] [CrossRef]
- Parkin, D.M.; Sitas, F.; Chirenje, M.; Stein, L.; Abratt, R.; Wabinga, H. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. The Lancet. Oncology 2008, 9, 683–692. [Google Scholar] [CrossRef]
- Fang, J.W.; Lai, C.L.; Chung, H.T.; Wu, P.C.; Lau, J.Y. Female children respond to recombinant hepatitis B vaccine with a higher titre than male. J. Trop. Pediatrics 1994, 40, 104–107. [Google Scholar] [CrossRef]
- Bock, H.L.; Kruppenbacher, J.; Sanger, R.; Hobel, W.; Clemens, R.; Jilg, W. Immunogenicity of a recombinant hepatitis B vaccine in adults. Arch. Intern. Med. 1996, 156, 2226–2231. [Google Scholar] [CrossRef]
- Mendy, M.E.; McConkey, S.J.; van der, M.A.S.; Crozier, S.; Kaye, S.; Jeffries, D.; Hall, A.J.; Whittle, H.C. Changes in viral load and HBsAg and HBeAg status with age in HBV chronic carriers in The Gambia. Virol. J. 2008, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Candotti, D.; Allain, J.P. Specific amino acid substitutions in the S protein prevent its excretion in vitro and may contribute to occult hepatitis B virus infection. J. Virol. 2013, 87, 7882–7892. [Google Scholar] [CrossRef] [PubMed]
- El Chaar, M.; Candotti, D.; Crowther, R.A.; Allain, J.P. Impact of hepatitis B virus surface protein mutations on the diagnosis of occult hepatitis B virus infection. Hepatology 2010, 52, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.J.; Stevens, G.A.; Groeger, J.; Wiersma, S.T. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012, 30, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xie, Y.; Deng, M.; Zhou, X.; Ruan, B. Prevalence of hepatitis B in the southeast of China: A population-based study with a large sample size. Eur. J. Gastroenterol. Hepatol. 2011, 23, 695–700. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet. Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Ruggieri, A.; Gagliardi, M.C.; Anticoli, S. Sex-Dependent Outcome of Hepatitis B and C Viruses Infections: Synergy of Sex Hormones and Immune Responses? Front. Immunol. 2018, 9, 2302. [Google Scholar] [CrossRef]


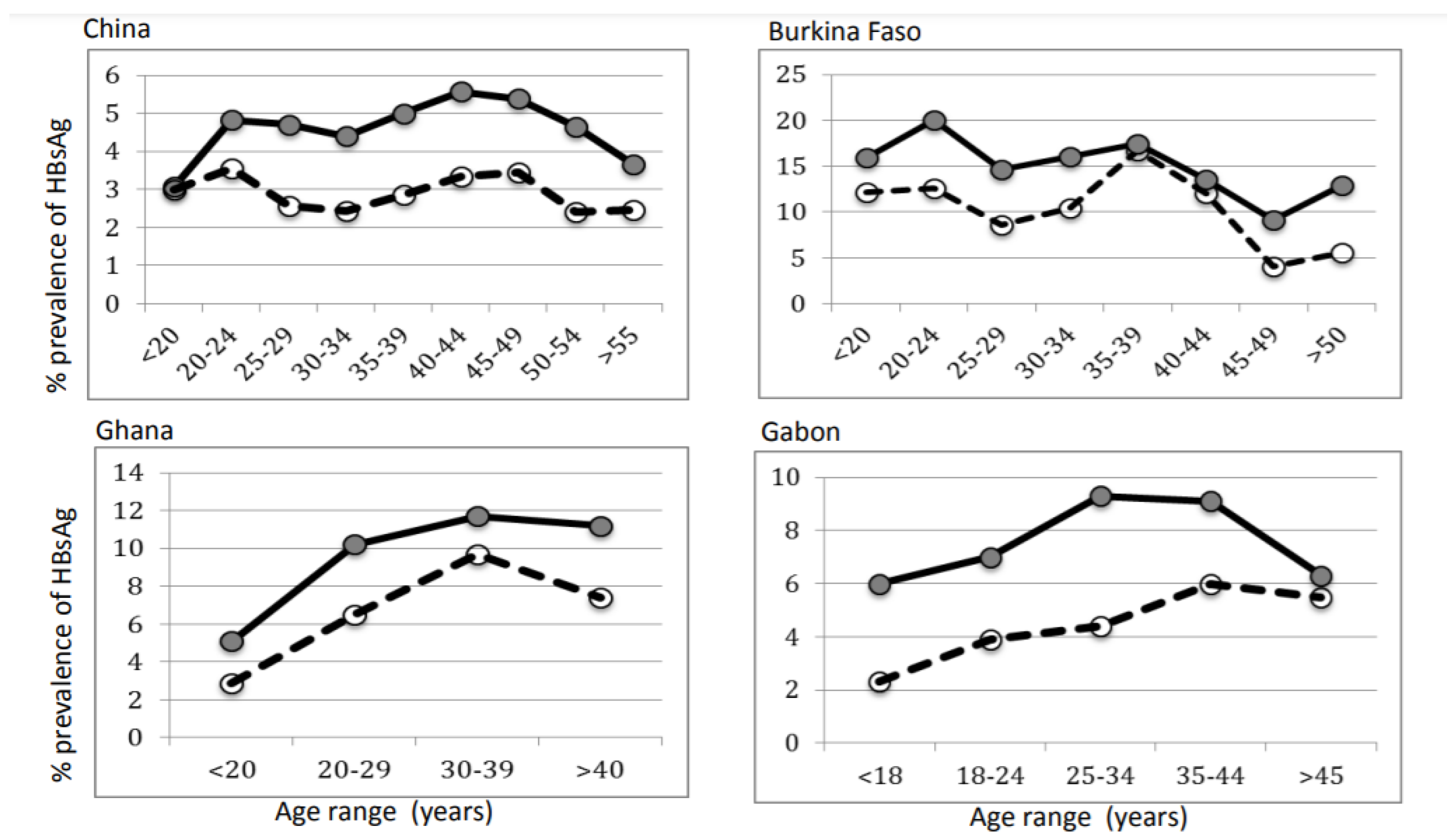
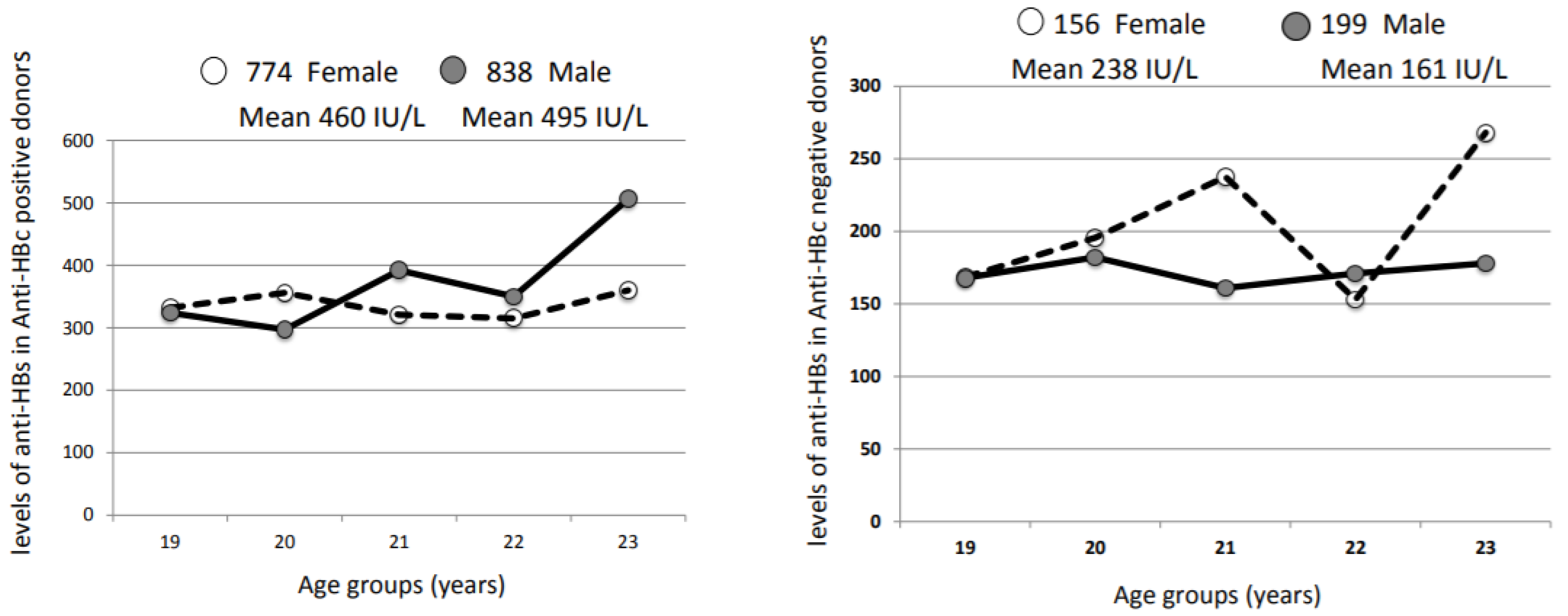
| Country | Reference | Dominant Genotype (s) | Male | Female | M/F OBI Ratio | M/F Donor Ratio | OBI Frequency |
|---|---|---|---|---|---|---|---|
| China | 12 | B/C | 28 | 6 | 4.7 | 1.7 | 1/7517 |
| Hong Kong | 25 | B | 56 | 21 | 2.7 | 0.6 | 1/4255 |
| Taiwan | 25 | B | 16 | 8 | 2 | 1.8 | 1/1280 |
| Thailand | 25 | C | 16 | 6 | 2.7 | 3.0 | 1/12,807 |
| Italy | 26 | D | 12 | 0 | 12 | 2.5 | 1/10,450 |
| Poland | 26 | D/A2 | 19 | 2 | 9.5 | 2.9 | 1/14,717 |
| Spain | 26 | A2/D | 15 | 2 | 7.5 | 1.2 | 1/6669 |
| Ghana | 20 | E | 28 | 6 | 4.7 | 3.2 | 1/78 |
| RSA 1 | 27 | A1 | 42 | 12 | 3.5 | 1.5 | 1/21,287 |
| Country | n Donors | n Male | % HBsAg+ | n Female | % HBsAg+ |
|---|---|---|---|---|---|
| Burkina Faso | 12,032 | 8854 | 17.0 | 3178 | 11.8 |
| Gabon | 75,864 | 58,637 | 8.28 | 17,227 | 4.46 |
| Ghana | 14,416 | 10,831 | 9.63 | 3585 | 4.74 |
| China | 244,275 | 147,507 | 4.67 | 96,768 | 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allain, J.-P.; Owusu-Ofori, S.; Ye, X.; Bisseye, C.; Chaar, M.E.; Li, C. Hepatitis B Virus Chronic Infection in Blood Donors from Asian and African High or Medium Prevalence Areas: Comparison According to Sex. Viruses 2022, 14, 673. https://doi.org/10.3390/v14040673
Allain J-P, Owusu-Ofori S, Ye X, Bisseye C, Chaar ME, Li C. Hepatitis B Virus Chronic Infection in Blood Donors from Asian and African High or Medium Prevalence Areas: Comparison According to Sex. Viruses. 2022; 14(4):673. https://doi.org/10.3390/v14040673
Chicago/Turabian StyleAllain, Jean-Pierre, Shirley Owusu-Ofori, Xianlin Ye, Cyrille Bisseye, Mira El Chaar, and Chengyao Li. 2022. "Hepatitis B Virus Chronic Infection in Blood Donors from Asian and African High or Medium Prevalence Areas: Comparison According to Sex" Viruses 14, no. 4: 673. https://doi.org/10.3390/v14040673
APA StyleAllain, J.-P., Owusu-Ofori, S., Ye, X., Bisseye, C., Chaar, M. E., & Li, C. (2022). Hepatitis B Virus Chronic Infection in Blood Donors from Asian and African High or Medium Prevalence Areas: Comparison According to Sex. Viruses, 14(4), 673. https://doi.org/10.3390/v14040673






