DOLAVI Real-Life Study of Dolutegravir Plus Lamivudine in Naive HIV-1 Patients (48 Weeks)
Abstract
:1. Introduction
2. Patients and Methods
2.1. Intervention
2.2. Definition of Variables
2.3. Classification of Adverse Events
3. Results
3.1. Study Population
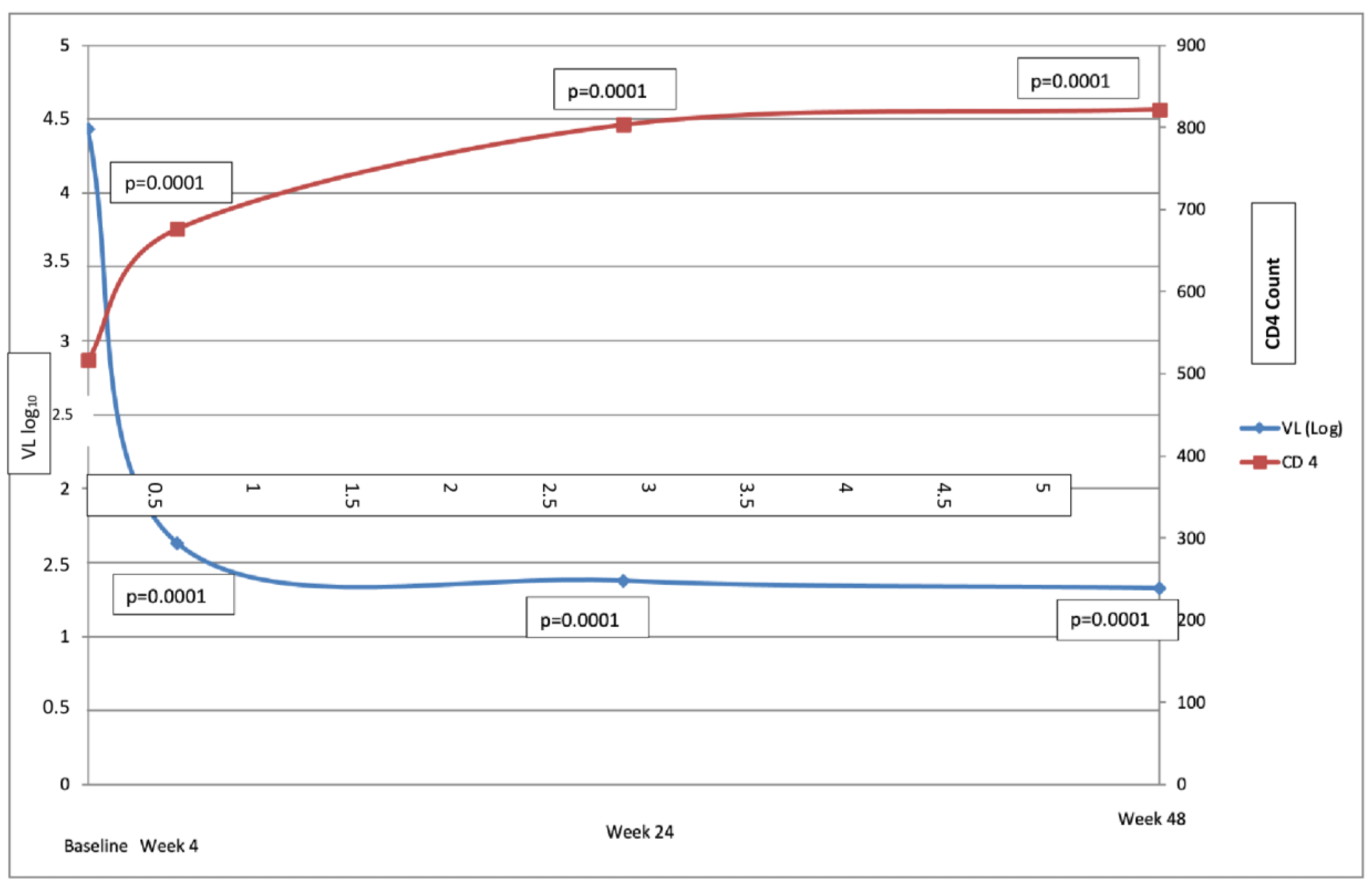
3.2. Effectiveness
3.3. Blood Analysis
3.4. Anthropometrics
3.5. Adverse Effects
3.6. Adherence and Satisfaction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquau, J.; Hidalgo-Tenorio, C. Nuke-Sparing Regimens for the Long-Term Care of HIV Infection. AIDS Rev. 2015, 17, 220–230. [Google Scholar]
- Pasquau, J.; Hidalgo-Tenorio, C.; Montes, M.L.; Romero-Palacios, A.; Vergas, J.; Sanjoaquín, I.; Hernández-Quero, J.; Aguirrebengoa, K.; Orihuela, F.; Imaz, A.; et al. High quality of life, treatment tolerability, safety and efficacy in HIV patients switching from triple therapy to lopinavir/ritonavir monotherapy: A randomized clinical trial. PLoS ONE 2018, 13, e0195068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiwo, B.O.; Zheng, L.; Stefanescu, A.; Nyaku, A.; Bezins, B.; Wallis, C.L.; Godfrey, C.; Sax, P.E.; Acosta, E.; Haas, D.; et al. ACTG A5353, A Pilot Study of Dolutegravir Plus Lamivudine for Initial Treatment of Human Immunodeficiency Virus-1 (HIV-1)-infected Participants With HIV-1 RNA < 500,000 Copies/mL. Clin. Infect. Dis. 2018, 66, 1689–1697. [Google Scholar] [PubMed] [Green Version]
- Cahn, P.; Rolon, M.J.; Figueroa, M.I.; Gun, A.; Patterson, P.; Sued, O. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J. Int. AIDS Soc. 2017, 20, 21678. [Google Scholar] [CrossRef]
- Llibre, J.M.; Hung, C.C.; Brinson, C.; Castelli, F.; Girard, P.M.; Kahl, L.P.; Blair, E.A.; Angelis, K.; Wynne, B.; Vandermeulen, K.; et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1, phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018, 391, 839–849. [Google Scholar] [CrossRef]
- Palacios, R.; Mayorga, M.; González-Domenech, C.M.; Hidalgo-Tenorio, C.; Gálvez, C.; Muñoz-Medina, L.; de la Torre, J.; Lozano, A.; Castaño, M.; Omar, M.; et al. Safety and Efficacy of Dolutegravir Plus Rilpivirine in Treatment-Experienced HIV-Infected Patients: The DORIVIR Study. J. Int. Assoc. Provid. AIDS Care 2018, 17, 2325958218760847. [Google Scholar] [CrossRef]
- Pasquau, J.; de Jesus, S.E.; Arazo, P.; Crusells, M.J.; Ríos, M.J.; Lozano, F.; de la Torre, J.; Galindo, M.J.; Carmena, J.; Santos, J.; et al. Effectiveness and safety of dual therapy with rilpivirine and boosted darunavir in treatment-experienced patients with advanced HIV infection: A preliminary 24 week analysis (RIDAR study). BMC Infect. Dis. 2019, 19, 207. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Tenorio, C.; Cortés, L.L.; Gutiérrez, A.; Santos, J.; Omar, M.; Gálvez, C.; Sequera, S.; Jesús, S.E.; Téllez, F.; Fernández, E.; et al. DOLAMA study: Effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients. Medicine 2019, 98, e16813. [Google Scholar] [CrossRef]
- Borghetti, A.; Lombardi, F.; Gagliardini, R.; Baldin, G.; Ciccullo, A.; Moschese, D.; Emiliozzi, A.; Belmonti, S.; Lamonica, S.; Montagnani, F.; et al. Efficacy and tolerability of lamivudine plus dolutegravir compared with lamivudine plus boosted PIs in HIV-1 positive individuals with virologic suppression: A retrospective study from the clinical practice. BMC Infect. Dis. 2019, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Baldin, G.; Ciccullo, A.; Borghetti, A.; Di Giambenedetto, S. Virological efficacy of dual therapy with lamivudine and dolutegravir in HIV-1-infected virologically suppressed patients: Long-term data from clinical practice. J. Antimicrob. Chemother. 2019, 74, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, J.; Ajana, F.; Bisshop, F.; De Wit, S.; Osiyemi, O.; Portilla Sogorb, J.; Routy, J.P.; Wyen, C.; Ait-Khaled, M.; Nascimento, M.C.; et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose 2-Drug Regimen vs Continuing a Tenofovir Alafenamide–Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living with Human Immunodeficiency Virus Type 1, Phase 3, Randomized, Noninferiority TANGO Study. Clin. Infect. Dis. 2020, 71, 1920–1929. [Google Scholar] [PubMed] [Green Version]
- Llibre, J.M.; Brites, C.A.; Cheng, C.Y.; Osiyemi, O.; Galera, C.; Hocqueloux, L.; Maggiolo, F.; Degen, O.; Blair, L.; Wynne, B.; et al. Switching to the 2-drug regimen of dolutegravir/lamivudine (DTG/3TC) fixed-dose combination (FDC) is non-inferior to continuing a 3-drug regimen through 24 weeks in a randomized clinical trial (SALSA). In Proceedings of the IAS 2021, 11th IAS Conference on HIV Science, Berlin, Germany, 18–21 July 2021. [Google Scholar]
- Cahn, P.; Madero, J.S.; Arribas, J.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.C.; Rockstroh, J.K.; Girard, P.M.; Sievers, J.; et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): Week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019, 393, 143–155. [Google Scholar] [PubMed]
- Cahn, P.; Madero, J.S.; Arribas, J.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.C.; Rockstroh, J.K.; Girard, P.M.; Sievers, J.; et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naive adults with HIV-1 infection. AIDS 2022, 36, 39–48. [Google Scholar] [CrossRef]
- Berger, M.L.; Sox, H.; Willke, R.J.; Brixner, D.L.; Eichler, H.G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPESpecial Task Force on real-world evidence in health care decision making. Pharmacoepidemiol. Drug Saf. 2017, 26, 1033–1039. [Google Scholar] [CrossRef]
- Devlin, N.J.; Brooks, R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl. Health Econ. Health Policy 2017, 15, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Knobel, H.; Alonso, J.; Casado, J.L.; Collazos, J.; González, J.; Ruiz, I.; Kindelan, J.M.; Carmona, A.; Juega, J.; Ocampo, A.; et al. GEEMA Study Group Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: The GEEMA Study. AIDS 2002, 16, 605–613. [Google Scholar] [CrossRef]
- Gadzik, J. How much sholud I weigh? Quetelet´s equation, upper weight limits, and BMI prime. Conn. Med. 2006, 70, 81–88. [Google Scholar]
- Lee, P.K.; Kieffer, T.L.; Siliciano, R.F.; Nettles, R.E. HIV-1 viral load blips are of limited clinical significance. J. Antimicrob. Chemother. 2006, 57, 803–805. [Google Scholar] [CrossRef]
- Grennan, J.T.; Loutfy, M.R.; Su, D.; Harrigan, P.R.; Cooper, C.; Klein, M.; Machouf, N.; Montaner, J.S.; Rourke, S.; Tsoukas, C.; et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: A recurrent events analysis. J. Infect. Dis. 2012, 205, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.D.; Crofoot, G.; Ramgopal, M.; Gathe, J.; Bolan, R.; Luo, D.; Simonson, R.B.; Nettles, R.E.; Benson, C.; Dunn, K. Darunavir/ Cobicistat/ Emtricitabine/Tenofovir Alafenamide in a Rapid-Initiation Model of Care for Human Immunodeficiency Virus Type 1 Infection: Primary Analysis of the DIAMOND Study. Clin. Infect. Dis. 2020, 71, 3110–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolle, C.P.; Berhe, M.; Singh, T.; Ortiz, R.; Wurapa, A.; Ramgopal, M.; Leone, P.A.; Matthews, J.E.; Dalessandro, M.; Underwood, M.R.; et al. Feasibility, efficacy, and safety of dolutegravir/lamivudine (dtg/3tc) as a first-line regimen in a test-and-treat setting for newly diagnosed people living with hiv (plwh): 48-week results of the stat study. In Proceedings of the 11th IAS Conference on HIV Science, Berlin, Germany, 18–21 July 2021. [Google Scholar]
- Ford, N.; Migone, C.; Calmy, A.; Kerschberger, B.; Kanters, S.; Nsanzimana, S.; Mills, E.J.; Meintjes, G.; Vitoria, M.; Doherty, M.; et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018, 32, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antela, A.; Rivero, A.; Llibre, J.M.; Moreno, S.; RET Group. Redefining therapeutic success in HIV patients: An expert view. J. Antimicrob. Chemother. 2021, 76, 2501–2518. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.; Bacchetti, P.; Sachdev, D.; Bacon, O.; Jones, D.; Ospina-Norvell, C.; Torres, S.; Lynch, E.; Camp, C.; Mercer-Slomoff, R.; et al. RAPID antiretroviral therapy: High virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS 2019, 33, 825–832. [Google Scholar] [CrossRef]
- Labhardt, N.D.; Ringera, I.; Lejone, T.I.; Klimkait, T.; Muhairwe, J.; Amstutz, A.; Glass, T.R. Effect of Offering Same-Day ART vs Usual Health Facility Referral During Home-Based HIV Testing on Linkage to Care and Viral Suppression Among Adults with HIV in Lesotho: The CASCADE Randomized Clinical Trial. JAMA 2018, 319, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Pilcher, C.D.; Ospina-Norvell, C.; Dasgupta, A.; Jones, D.; Hartogensis, W.; Torres, S.; Calderon, F.; Demicco, E.; Geng, E.; Gandhi, M.; et al. The Effect of Same-Day Observed Initiation of Antiretroviral Therapy on HIV Viral Load and Treatment Outcomes in a US Public Health Setting. J. Acquir. Immune Defic. Syndr. 2017, 74, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Cicculo, A.; Baldin, G.; Dusina, A.; Cossu, M.V.; Lombardi, F.; Borghetti, A.; Capetti, A.; Di Giambenedetto, S. Short Communication: Efficacy and Safety of Dolutegravir Plus Lamivudine as a First-Line Regimen in Clinical Practice. AIDS Res. Hum. Retrovir. 2021, 37, 486–488. [Google Scholar] [CrossRef]
- van Wyk, J.; Ait-Khaled, M.; Santos, J.; Scholten, S.; Wohlfeiler, M.; Ajana, F.; Jones, B.; Nascimento, M.C.; Tenorio, A.R.; Smith, D.E.; et al. Brief Report: Improvement in Metabolic Health Parameters at Week 48 After Switching From a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen to the 2-Drug Regimen of Dolutegravir/Lamivudine: The TANGO Study. J. Acquir. Immune. Defic. Syndr. 2021, 87, 794–800. [Google Scholar] [CrossRef]
- Eckard, A.R.; McComsey, G.A. Weight gain and integrase inhibitors. Curr. Opin. Infect. Dis. 2020, 33, 10–19. [Google Scholar] [CrossRef]


| Variable | N = 88 |
|---|---|
| Age (year), mean (±SD) | 35.8 (±12.2) |
| Male, n (%) | 77 (87.5) |
| Rapidly initiated DT, median (IQR) | 0 (0–7) |
| Baseline HIV Viral Load, log10, median (IQR) | 4.49 (4–4.9) |
| Viral Load > 100,000 cop/mL, n (%) | 17 (20) |
| Resistance mutations baseline, n (%) | 3 (3.4) |
| K103 N | 1 (33.3) |
| V106I, E138A | 1 (33.3) |
| V108I | 1 (33.3) |
| Baseline CD4, (Cell/uL), mean (±SD) | 512.2 ± 250.3 |
| CD4 < 200 cell/uL, n (%) | 7 (8) |
| Baseline CD4/CD8 ratio, mean (±SD) | 0.6 (±0.37) |
| CDC AIDS stage (A3, B3, C), n (%) | 10 (11.4) |
| A1 | 42 (47.7) |
| A2 | 33 (37.5) |
| A3 | 8 (9.1) |
| B1 | 1 (1.1) |
| B2 | 2 (2.3) |
| C2 | 1 (1.1) |
| C3 | 1 (1.1) |
| Chronic hepatitis C cured, n (%) | 3 (3.4) |
| Positive HVB surface and core antibodies, n (%) | 1 (1.1) |
| Risk factor for HIV infection, n (%) | |
| Heterosexual | 18 (20.5) |
| MSM | 67 (76.1) |
| IVDU | 3 (3.4) |
| Smoker, n (%) | 47 (53.4) |
| Non-alcohol consumer, n (%) | 62 (70.5) |
| Social drinker, n (%) | 26 (29.5) |
| Weight, mean (±SD) (Kg) | 72.3 (11.8) |
| BMI, mean (±SD), Kg/m2 | 23.8 (3.9) |
| Waist, mean (±SD) (cm) | 84.7 (12.7) |
| Underweight, n (%) | 3 (3.5) |
| Normal weight, n (%) | 53 (61.6) |
| Overweight, n (%) | 25 (29.1) |
| Obese | 5 (5.8) |
| Patient | Age | Sex | Baseline VL | Baseline CD4 | VL in VF | Baseline RS ** | RS of VF | Week of * VF | Observations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | 370,000 cop/uL | 235 cell/uL | 1st VL: 423 cop/uL 2nd VL: 763 cop/uL | No mutations | No mutations | 24 | Poor adherence After starting ART 1° BIC/FTC/TAF, with VL 215 and 183 cop/mL RS: negative 2° DRV/cob/FTC/TAF with VL 311 and 2211 cop/mL RS: Negative |
| Baseline | Week 4 | Week 24 | Week 48 | p-Value (Baseline vs. Week 48) | |
|---|---|---|---|---|---|
| Creatinine (mg/dL), mean ± SD Creatinine Clearance (CKD-EPI), mL/min, mean ± SD | 0.82 ± 0.1 109 ± 16 | 0.94 ± 0.14 99.5 ± 14.2 | 0.94 ± 0.15 99.3 ± 16.1 | 0.9 ± 1.04 100.8 ± 15.7 | 0.0001 0.0001 |
| Total cholesterol (mg/dL), mean ± SD | 158.4 ± 39.8 | 177.1 ± 39.5 | 174.8 ± 35.7 | 177.2 ± 38.7 | 0.0001 |
| HDL cholesterol (mg/dL), mean ± SD | 48.3 ± 43 | 45.3 ± 11.1 | 46.2 ± 11.5 | 46.3 ± 12.3 | 0.005 |
| LDL cholesterol (mg/dL), mean ± SD | 102.7 ± 34.8 | 112 ± 35.2 | 110.5 ± 35.2 | 112.1 ± 32.3 | 0.012 |
| TC/HDL ratio, mean ± SD | 3.9 ± 1.3 | 4.2 ± 1.26 | 4.1 ± 1.1 | 4 ± 1.3 | 0.579 |
| Triglycerides (mg/dL), mean ± SD | 112.2 ± 66.4 | 121.8 ± 85.9 | 126.3 ± 76.7 | 122.8 ± 81 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Tenorio, C.; Pasquau, J.; Vinuesa, D.; Ferra, S.; Terrón, A.; SanJoaquín, I.; Payeras, A.; Martínez, O.J.; López-Ruz, M.Á.; Omar, M.; et al. DOLAVI Real-Life Study of Dolutegravir Plus Lamivudine in Naive HIV-1 Patients (48 Weeks). Viruses 2022, 14, 524. https://doi.org/10.3390/v14030524
Hidalgo-Tenorio C, Pasquau J, Vinuesa D, Ferra S, Terrón A, SanJoaquín I, Payeras A, Martínez OJ, López-Ruz MÁ, Omar M, et al. DOLAVI Real-Life Study of Dolutegravir Plus Lamivudine in Naive HIV-1 Patients (48 Weeks). Viruses. 2022; 14(3):524. https://doi.org/10.3390/v14030524
Chicago/Turabian StyleHidalgo-Tenorio, Carmen, Juan Pasquau, David Vinuesa, Sergio Ferra, Alberto Terrón, Isabel SanJoaquín, Antoni Payeras, Onofre Juan Martínez, Miguel Ángel López-Ruz, Mohamed Omar, and et al. 2022. "DOLAVI Real-Life Study of Dolutegravir Plus Lamivudine in Naive HIV-1 Patients (48 Weeks)" Viruses 14, no. 3: 524. https://doi.org/10.3390/v14030524
APA StyleHidalgo-Tenorio, C., Pasquau, J., Vinuesa, D., Ferra, S., Terrón, A., SanJoaquín, I., Payeras, A., Martínez, O. J., López-Ruz, M. Á., Omar, M., de la Torre-Lima, J., López-Lirola, A., Palomares, J., Blanco, J. R., Montero, M., & García-Vallecillos, C. (2022). DOLAVI Real-Life Study of Dolutegravir Plus Lamivudine in Naive HIV-1 Patients (48 Weeks). Viruses, 14(3), 524. https://doi.org/10.3390/v14030524






