Reversibility of Central Nervous System Adverse Events in Course of Art
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Objectives
2.3. Statistical Analysis
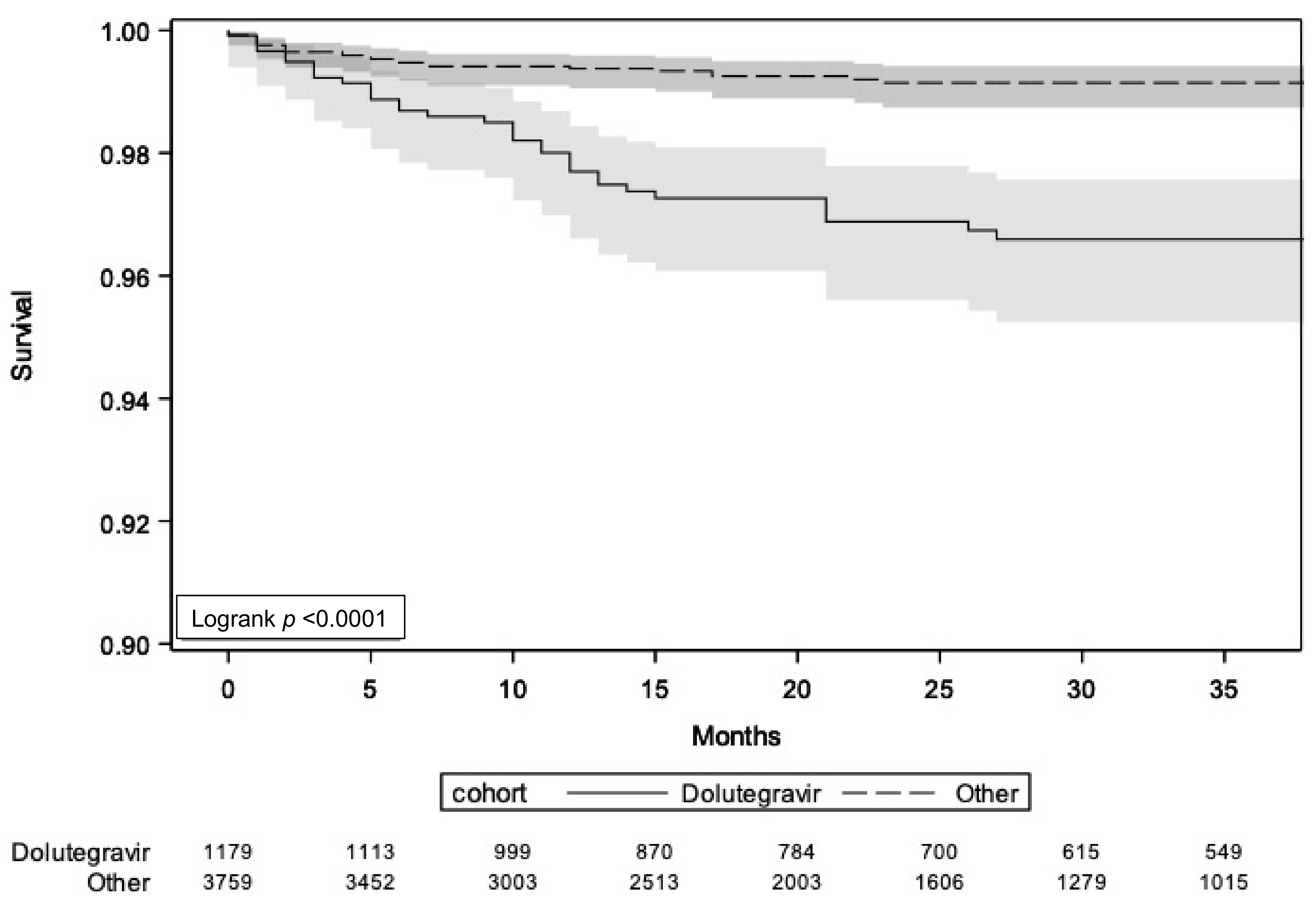
3. Results
3.1. CNS-AEs Leading to ART Discontinuation
3.2. Reversibility after Switching ART
3.3. Factors Associated with CNS-AEs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antela, A.; Rivero, A.; Llibre, J.M.; Moreno, S.; RET Group. Redefining Therapeutic Success in HIV Patients: An Expert View. J. Antimicrob. Chemother. 2021, 76, 2501–2518. [Google Scholar] [CrossRef] [PubMed]
- Lapadula, G.; Bernasconi, D.P.; Bai, F.; Focà, E.; Di Biagio, A.; Bonora, S.; Castelli, F.; Squillace, N.; Bandera, A.; Monforte, A. d’Arminio; et al. Switching from Efavirenz to Rilpivirine Improves Sleep Quality and Self-Perceived Cognition but Has No Impact on Neurocognitive Performances. AIDS 2020, 34, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated Research Nosology for HIV-Associated Neurocognitive Disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffi, F.; Pozniak, A.L.; Wainberg, M.A. Has the Time Come to Abandon Efavirenz for First-Line Antiretroviral Therapy? J. Antimicrob. Chemother. 2014, 69, 1742–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taramasso, L.; Di Biagio, A.; Maggiolo, F.; Tavelli, A.; Lo Caputo, S.; Bonora, S.; Zaccarelli, M.; Caramello, P.; Costantini, A.; Viscoli, C.; et al. First-Line Antiretroviral Therapy with Efavirenz plus Tenofovir Disiproxil Fumarate/Emtricitabine or Rilpivirine plus Tenofovir Disiproxil Fumarate/Emtricitabine: A Durability Comparison. HIV Med. 2018, 19, 475–484. [Google Scholar] [CrossRef]
- Cohen, C.J.; Andrade-Villanueva, J.; Clotet, B.; Fourie, J.; Johnson, M.A.; Ruxrungtham, K.; Wu, H.; Zorrilla, C.; Crauwels, H.; Rimsky, L.T.; et al. Rilpivirine versus Efavirenz with Two Background Nucleoside or Nucleotide Reverse Transcriptase Inhibitors in Treatment-Naive Adults Infected with HIV-1 (THRIVE): A Phase 3, Randomised, Non-Inferiority Trial. Lancet 2011, 378, 229–237. [Google Scholar] [CrossRef]
- Orkin, C.; Squires, K.E.; Molina, J.-M.; Sax, P.E.; Wong, W.-W.; Sussmann, O.; Kaplan, R.; Lupinacci, L.; Rodgers, A.; Xu, X.; et al. Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate Is Non-Inferior to Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate in Treatment-Naive Adults With Human Immunodeficiency Virus-1 Infection: Week 48 Results of the DRIVE-AHEAD Trial. Clin. Infect. Dis. 2019, 68, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Bagella, P.; De Socio, G.V.; Ricci, E.; Menzaghi, B.; Martinelli, C.; Squillace, N.; Maggi, P.; Orofino, G.; Calza, L.; Carenzi, L.; et al. Durability, Safety, and Efficacy of Rilpivirine in Clinical Practice: Results from the SCOLTA Project. Infect. Drug Resist. 2018, 11, 615–623. [Google Scholar] [CrossRef] [Green Version]
- EACS European Aids Clinical Society Guidelines. Version 11.0. October 2021. Available online: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf (accessed on 7 May 2022).
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents.; A Working Group of the Office of AIDS Research; Advisory Council (OARAC). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf (accessed on 7 May 2022).
- Molina, J.-M.; Clotet, B.; van Lunzen, J.; Lazzarin, A.; Cavassini, M.; Henry, K.; Kulagin, V.; Givens, N.; de Oliveira, C.F.; Brennan, C.; et al. Once-Daily Dolutegravir versus Darunavir plus Ritonavir for Treatment-Naive Adults with HIV-1 Infection (FLAMINGO): 96 Week Results from a Randomised, Open-Label, Phase 3b Study. Lancet HIV 2015, 2, e127–e136. [Google Scholar] [CrossRef]
- Eron, J.J.; Orkin, C.; Cunningham, D.; Pulido, F.; Post, F.A.; De Wit, S.; Lathouwers, E.; Hufkens, V.; Jezorwski, J.; Petrovic, R.; et al. Week 96 Efficacy and Safety Results of the Phase 3, Randomized EMERALD Trial to Evaluate Switching from Boosted-Protease Inhibitors plus Emtricitabine/Tenofovir Disoproxil Fumarate Regimens to the Once Daily, Single-Tablet Regimen of Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide (D/C/F/TAF) in Treatment-Experienced, Virologically-Suppressed Adults Living with HIV-1. Antiviral Res. 2019, 170, 104543. [Google Scholar] [CrossRef]
- Orkin, C.; Eron, J.J.; Rockstroh, J.; Podzamczer, D.; Esser, S.; Vandekerckhove, L.; Van Landuyt, E.; Lathouwers, E.; Hufkens, V.; Jezorwski, J.; et al. Week 96 Results of a Phase 3 Trial of Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in Treatment-Naive HIV-1 Patients. AIDS 2020, 34, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Llibre, J.M. Neuropsychiatric Adverse Events with Dolutegravir and Other Integrase Strand Transfer Inhibitors. AIDS Rev. 2019, 21, 4–10. [Google Scholar] [CrossRef]
- Baldin, G.; Ciccullo, A.; Rusconi, S.; Capetti, A.; Sterrantino, G.; Colafigli, M.; d’Ettorre, G.; Giacometti, A.; Cossu, M.V.; Borghetti, A.; et al. Long-Term Data on the Efficacy and Tolerability of Lamivudine plus Dolutegravir as a Switch Strategy in a Multi-Centre Cohort of HIV-1-Infected, Virologically Suppressed Patients. Int. J. Antimicrob. Agents 2019, 54, 728–734. [Google Scholar] [CrossRef]
- De Vito, A.; Caruana, G.; Clark, F.; Nunnari, G.; Pellicanò, G.F.; Angioni, G.; Freedman, A.; Babudieri, S.; Madeddu, G. Efficacy, Safety and Tolerability of Dolutegravir-Based Combination Antiretroviral Therapy in Clinical Practice in HIV-Infected Patients: Results from a Multicenter Study - Infectious Diseases & Tropical Medicine. Infect. Dis. Trop. Med. 2019, 5, e565. [Google Scholar] [CrossRef]
- Nasreddine, R.; Florence, E.; Vandercam, B.; Moutschen, M.; Goffard, J.C.; De Munter, P.; Delforge, M.; Marinus, W.; De Wit, S. Effectiveness of Dolutegravir-Based Antiretroviral Therapy in a Real-World Setting in a Belgian Cohort of 4101 HIV Patients. AIDS 2020, 34, 1151–1159. [Google Scholar] [CrossRef]
- Povar-Echeverría, M.; Comet-Bernad, M.; Gasso-Sánchez, A.; Ger-Buil, A.; Navarro-Aznarez, H.; Martínez-Álvarez, R.; Arazo-Garcés, P. Neuropsychiatric Adverse Effects of Dolutegravir in Real-Life Clinical Practice. Enfermedades Infecciosas y Microbiologia Clinica 2020. [Google Scholar] [CrossRef]
- Bonfanti, P.; Madeddu, G.; Gulminetti, R.; Squillace, N.; Orofino, G.; Vitiello, P.; Rusconi, S.; Celesia, B.M.; Maggi, P.; Ricci, E.; et al. Discontinuation of Treatment and Adverse Events in an Italian Cohort of Patients on Dolutegravir. AIDS 2017, 31, 455–457. [Google Scholar] [CrossRef]
- Taramasso, L.; De Vito, A.; Ricci, E.D.; Orofino, G.; Squillace, N.; Menzaghi, B.; Molteni, C.; Gulminetti, R.; De Socio, G.V.; Pellicanò, G.F.; et al. Durability of Dolutegravir-Based Regimens: A 5-Year Prospective Observational Study. AIDS Patient Care STDS 2021, 35, 342–353. [Google Scholar] [CrossRef]
- De Boer, M.G.J.; van den Berk, G.E.L.; van Holten, N.; Oryszcyn, J.E.; Dorama, W.; Moha, D.A.; Brinkman, K. Intolerance of Dolutegravir-Containing Combination Antiretroviral Therapy Regimens in Real-Life Clinical Practice. AIDS 2016, 30, 2831–2834. [Google Scholar] [CrossRef]
- Greenberg, L.; Ryom, L.; Wandeler, G.; Grabmeier-Pfistershammer, K.; Öllinger, A.; Neesgaard, B.; Stephan, C.; Calmy, A.; Rauch, A.; Castagna, A.; et al. Uptake and Discontinuation of Integrase Inhibitors (INSTIs) in a Large Cohort Setting. J. Acquir. Immune Defic. Syndr. 2020, 83, 240–250. [Google Scholar] [CrossRef]
- Llibre, J.M.; Montoliu, A.; Miró, J.M.; Domingo, P.; Riera, M.; Tiraboschi, J.; Curran, A.; Homar, F.; Ambrosioni, J.; Abdulghani, N.; et al. Discontinuation of Dolutegravir, Elvitegravir/Cobicistat and Raltegravir Because of Toxicity in a Prospective Cohort. HIV Med. 2019, 20, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Schewe, K.; Fenske, S.; Buhk, T.; Sabranski, M.; Adam, A.; Hansen, S.; Stellbrink, H.-J. Short-Term Neuropsychiatric Tolerability of Bictegravir Combined with Emtricitabine/Tenofovir Alafenamide in Clinical Practice. Antivir. Ther. 2020, 25, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Elzi, L.; Erb, S.; Furrer, H.; Cavassini, M.; Calmy, A.; Vernazza, P.; Günthard, H.; Bernasconi, E.; Battegay, M.; Swiss HIV Cohort Study Group. Adverse Events of Raltegravir and Dolutegravir. AIDS 2017, 31, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeddu, G.; Menzaghi, B.; Ricci, E.; Carenzi, L.; Martinelli, C.; di Biagio, A.; Parruti, G.; Orofino, G.; Mura, M.S.; Bonfanti, P.; et al. Raltegravir Central Nervous System Tolerability in Clinical Practice: Results from a Multicenter Observational Study. AIDS 2012, 26, 2412–2415. [Google Scholar] [CrossRef]
- Hoffmann, C.; Welz, T.; Sabranski, M.; Kolb, M.; Wolf, E.; Stellbrink, H.-J.; Wyen, C. Higher Rates of Neuropsychiatric Adverse Events Leading to Dolutegravir Discontinuation in Women and Older Patients. HIV Med. 2017, 18, 56–63. [Google Scholar] [CrossRef]
- Bonfanti, P.; Martinelli, C.; Ricci, E.; Carradori, S.; Parruti, G.; Armignacco, O.; Magnani, C.; Quirino, T.; CISAI Group (Italian Coordinators for the Study of Allergies HIV Infection). An Italian Approach to Postmarketing Monitoring: Preliminary Results from the SCOLTA (Surveillance Cohort Long-Term Toxicity Antiretrovirals) Project on the Safety of Lopinavir/Ritonavir. J. Acquir. Immune Defic. Syndr. 2005, 39, 317–320. [Google Scholar] [CrossRef]
- Giuntini, R.; Martinelli, C.; Ricci, E.; Vichi, F.; Gianelli, E.; Madeddu, G.; Abeli, C.; Palvarini, L.; Penco, G.; Marconi, P.; et al. Efficacy and Safety of Boosted and Unboosted Atazanavir-Containing Antiretroviral Regimens in Real Life: Results from a Multicentre Cohort Study. HIV Med. 2010, 11, 40–45. [Google Scholar] [CrossRef]
- Menzaghi, B.; Ricci, E.; Carenzi, L.; Parruti, G.; Orofino, G.; Guastavigna, M.; Madeddu, G.; Maggi, P.; Di Biagio, A.; Corsi, P.; et al. Safety and Durability in a Cohort of HIV-1 Positive Patients Treated with Once and Twice Daily Darunavir-Based Therapy (SCOLTA Project). Biomed. Pharmacother. 2013, 67, 293–298. [Google Scholar] [CrossRef]
- CISAI Study Group; Taramasso, L.; Ricci, E.; Cascio, A.; Valsecchi, L.; Menzaghi, B.; Squillace, N.; Maggi, P.; De Socio, G.V.; Dentone, C.; et al. Positioning of Darunavir/Cobicistat-Containing Antiretroviral Regimens in Real Life: Results from a Large Multicentre Observational Prospective Cohort (SCOLTA). AIDS Res. Ther. 2019, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Taramasso, L.; Madeddu, G.; Ricci, E.; De Socio, G.V.; Menzaghi, B.; Orofino, G.; Passerini, S.; Franzetti, M.; Maggi, P.; Dentone, C.; et al. Raltegravir-Based Therapy in a Cohort of HIV/HCV Co-Infected Individuals. Biomed. Pharmacother. 2015, 69, 233–236. [Google Scholar] [CrossRef]
- Squillace, N.; Ricci, E.; Quirino, T.; Gori, A.; Bandera, A.; Carenzi, L.; De Socio, G.V.; Orofino, G.; Martinelli, C.; Madeddu, G.; et al. Safety and Tolerability of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil Fumarate in a Real Life Setting: Data from Surveillance Cohort Long-Term Toxicity Antiretrovirals/Antivirals (SCOLTA) Project. PLoS ONE 2017, 12, e0179254. [Google Scholar] [CrossRef]
- Taramasso, L.; Bonfanti, P.; Ricci, E.; Orofino, G.; Squillace, N.; Menzaghi, B.; De Socio, G.V.; Madeddu, G.; Pellicanò, G.F.; Pagnucco, L.; et al. Factors Associated With Weight Gain in People Treated With Dolutegravir. Open Forum Infect. Dis. 2020, 7, ofaa195. [Google Scholar] [CrossRef]
- Madeddu, G.; De Socio, G.V.L.; Ricci, E.; Quirino, T.; Orofino, G.; Carenzi, L.; Franzetti, M.; Parruti, G.; Martinelli, C.; Vichi, F.; et al. Muscle Symptoms and Creatine Phosphokinase Elevations in Patients Receiving Raltegravir in Clinical Practice: Results from the SCOLTA Project Long-Term Surveillance. Int. J. Antimicrob. Agents 2015, 45, 289–294. [Google Scholar] [CrossRef]
- Menard, A.; Montagnac, C.; Solas, C.; Meddeb, L.; Dhiver, C.; Tomei, C.; Ravaux, I.; Tissot-Dupont, H.; Mokhtari, S.; Colson, P.; et al. Neuropsychiatric Adverse Effects on Dolutegravir: An Emerging Concern in Europe. AIDS 2017, 31, 1201–1203. [Google Scholar] [CrossRef]
- Mondi, A.; Cozzi-Lepri, A.; Tavelli, A.; Rusconi, S.; Vichi, F.; Ceccherini-Silberstein, F.; Calcagno, A.; De Luca, A.; Maggiolo, F.; Marchetti, G.; et al. Effectiveness of Dolutegravir-Based Regimens as Either First-Line or Switch Antiretroviral Therapy: Data from the Icona Cohort. J. Int. AIDS Soc. 2019, 22, e25227. [Google Scholar] [CrossRef] [Green Version]
- Clotet, B.; Feinberg, J.; van Lunzen, J.; Khuong-Josses, M.-A.; Antinori, A.; Dumitru, I.; Pokrovskiy, V.; Fehr, J.; Ortiz, R.; Saag, M.; et al. Once-Daily Dolutegravir versus Darunavir plus Ritonavir in Antiretroviral-Naive Adults with HIV-1 Infection (FLAMINGO): 48 Week Results from the Randomised Open-Label Phase 3b Study. Lancet 2014, 383, 2222–2231. [Google Scholar] [CrossRef]
- Raffi, F.; Jaeger, H.; Quiros-Roldan, E.; Albrecht, H.; Belonosova, E.; Gatell, J.M.; Baril, J.-G.; Domingo, P.; Brennan, C.; Almond, S.; et al. Once-Daily Dolutegravir versus Twice-Daily Raltegravir in Antiretroviral-Naive Adults with HIV-1 Infection (SPRING-2 Study): 96 Week Results from a Randomised, Double-Blind, Non-Inferiority Trial. Lancet Infect. Dis. 2013, 13, 927–935. [Google Scholar] [CrossRef]
- Walmsley, S.L.; Antela, A.; Clumeck, N.; Duiculescu, D.; Eberhard, A.; Gutiérrez, F.; Hocqueloux, L.; Maggiolo, F.; Sandkovsky, U.; Granier, C.; et al. Dolutegravir plus Abacavir-Lamivudine for the Treatment of HIV-1 Infection. N. Engl. J. Med. 2013, 369, 1807–1818. [Google Scholar] [CrossRef] [Green Version]
- Cahn, P.; Pozniak, A.L.; Mingrone, H.; Shuldyakov, A.; Brites, C.; Andrade-Villanueva, J.F.; Richmond, G.; Buendia, C.B.; Fourie, J.; Ramgopal, M.; et al. Dolutegravir versus Raltegravir in Antiretroviral-Experienced, Integrase-Inhibitor-Naive Adults with HIV: Week 48 Results from the Randomised, Double-Blind, Non-Inferiority SAILING Study. Lancet 2013, 382, 700–708. [Google Scholar] [CrossRef]
- Stellbrink, H.-J.; Reynes, J.; Lazzarin, A.; Voronin, E.; Pulido, F.; Felizarta, F.; Almond, S.; St Clair, M.; Flack, N.; Min, S.; et al. Dolutegravir in Antiretroviral-Naive Adults with HIV-1: 96-Week Results from a Randomized Dose-Ranging Study. AIDS 2013, 27, 1771–1778. [Google Scholar] [CrossRef]
- Cahn, P.; Madero, J.S.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.-C.; Rockstroh, J.K.; Girard, P.-M.; Sievers, J.; et al. Dolutegravir plus Lamivudine versus Dolutegravir plus Tenofovir Disoproxil Fumarate and Emtricitabine in Antiretroviral-Naive Adults with HIV-1 Infection (GEMINI-1 and GEMINI-2): Week 48 Results from Two Multicentre, Double-Blind, Randomised, Non-Inferiority, Phase 3 Trials. Lancet 2019, 393, 143–155. [Google Scholar] [CrossRef]
- Van Bilsen, W.P.H.; Zimmermann, H.M.L.; Boyd, A.; Davidovich, U.; HIV Transmission Elimination Amsterdam Initiative. Burden of Living with HIV among Men Who Have Sex with Men: A Mixed-Methods Study. Lancet HIV 2020, 7, e835–e843. [Google Scholar] [CrossRef]
- Dubé, B.; Benton, T.; Cruess, D.G.; Evans, D.L. Neuropsychiatric Manifestations of HIV Infection and AIDS. J. Psychiatry Neurosci. 2005, 30, 237–246. [Google Scholar]
- Asch, S.M.; Kilbourne, A.M.; Gifford, A.L.; Burnam, M.A.; Turner, B.; Shapiro, M.F.; Bozzette, S.A.; HCSUS Consortium. Underdiagnosis of Depression in HIV: Who Are We Missing? J. Gen. Intern. Med. 2003, 18, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Elliot, E.R.; Wang, X.; Singh, S.; Simmons, B.; Vera, J.H.; Miller, R.F.; Fitzpatrick, C.; Moyle, G.; McClure, M.; Boffito, M. Increased Dolutegravir Peak Concentrations in People Living With Human Immunodeficiency Virus Aged 60 and Over, and Analysis of Sleep Quality and Cognition. Clin. Infect. Dis. 2019, 68, 87–95. [Google Scholar] [CrossRef]
- Calcagno, A.; Moltó, J.; Borghetti, A.; Gervasoni, C.; Milesi, M.; Valle, M.; Avataneo, V.; Alcantarini, C.; Pla-Junca, F.; Trunfio, M.; et al. Older Age Is Associated with Higher Dolutegravir Exposure in Plasma and Cerebrospinal Fluid of People Living with HIV. Clin. Pharmacokinet 2021, 60, 103–109. [Google Scholar] [CrossRef]
- Courlet, P.; Stader, F.; Guidi, M.; Alves Saldanha, S.; Stoeckle, M.; Cavassini, M.; Battegay, M.; Buclin, T.; Decosterd, L.A.; Marzolini, C.; et al. Pharmacokinetic Profiles of Boosted Darunavir, Dolutegravir and Lamivudine in Aging People Living with HIV. AIDS 2020, 34, 103–108. [Google Scholar] [CrossRef]
- Letendre, S.L.; Mills, A.M.; Tashima, K.T.; Thomas, D.A.; Min, S.S.; Chen, S.; Song, I.H.; Piscitelli, S.C.; Extended ING116070 study team. ING116070: A Study of the Pharmacokinetics and Antiviral Activity of Dolutegravir in Cerebrospinal Fluid in HIV-1-Infected, Antiretroviral Therapy-Naive Subjects. Clin. Infect. Dis 2014, 59, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Borghetti, A.; Calcagno, A.; Lombardi, F.; Cusato, J.; Belmonti, S.; D’Avolio, A.; Ciccarelli, N.; La Monica, S.; Colafigli, M.; Delle Donne, V.; et al. SLC22A2 Variants and Dolutegravir Levels Correlate with Psychiatric Symptoms in Persons with HIV. J. Antimicrob. Chemother. 2019, 74, 1035–1043. [Google Scholar] [CrossRef]
- Yagura, H.; Watanabe, D.; Kushida, H.; Tomishima, K.; Togami, H.; Hirano, A.; Takahashi, M.; Hirota, K.; Ikuma, M.; Kasai, D.; et al. Impact of UGT1A1 Gene Polymorphisms on Plasma Dolutegravir Trough Concentrations and Neuropsychiatric Adverse Events in Japanese Individuals Infected with HIV-1. BMC Infect. Dis. 2017, 17, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parant, F.; Miailhes, P.; Brunel, F.; Gagnieu, M.-C. Dolutegravir-Related Neurological Adverse Events: A Case Report of Successful Management with Therapeutic Drug Monitoring. Curr. Drug Saf. 2018, 13, 69–71. [Google Scholar] [CrossRef] [PubMed]
- George, J.W.; Mattingly, J.E.; Roland, N.J.; Small, C.M.; Lamberty, B.G.; Fox, H.S.; Stauch, K.L. Physiologically Relevant Concentrations of Dolutegravir, Emtricitabine, and Efavirenz Induce Distinct Metabolic Alterations in HeLa Epithelial and BV2 Microglial Cells. Front. Immunol. 2021, 12, 639378. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Gangwani, M.R.; Chaudhari, N.S.; Glazyrin, A.; Bhat, H.K.; Kumar, A. Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotox Res. 2016, 30, 677–697. [Google Scholar] [CrossRef]
- Rivera-Rivera, Y.; Vázquez-Santiago, F.J.; Albino, E.; Sánchez, M.D.C.; Rivera-Amill, V. Impact of Depression and Inflammation on the Progression of HIV Disease. J. Clin. Cell Immunol. 2016, 7, 423. [Google Scholar] [CrossRef] [Green Version]
- Stadtler, H.; Shaw, G.; Neigh, G.N. Mini-Review: Elucidating the Psychological, Physical, and Sex-Based Interactions between HIV Infection and Stress. Neurosci. Lett. 2021, 747, 135698. [Google Scholar] [CrossRef]
- Cohen, R.A.; Seider, T.R.; Navia, B. HIV Effects on Age-Associated Neurocognitive Dysfunction: Premature Cognitive Aging or Neurodegenerative Disease? Alzheimers Res. Ther. 2015, 7, 37. [Google Scholar] [CrossRef] [Green Version]
| DTG | Non-DTG | p | |||
|---|---|---|---|---|---|
| N § = 1179 | % | N § = 3760 | % | ||
| Sex | |||||
| F | 287 | 24.3 | 1047 | 27.8 | |
| M | 892 | 75.7 | 2713 | 72.2 | 0.02 |
| Age (years) | |||||
| mean ± SD | 48.1 ± 12.0 | 44.2 ± 12.7 | <0.0001 | ||
| ≤50 | 643 | 54.5 | 2892 | 76.9 | |
| >50 | 536 | 45.5 | 868 | 23.1 | <0.0001 |
| Ethnicity | |||||
| Caucasian | 1069 | 90.7 | 3469 | 92.3 | |
| Other | 110 | 9.3 | 291 | 7.7 | 0.08 |
| Weight (kg) | |||||
| mean ± SD | 71.0 ± 13.5 | 69.0 ± 13.7 | <0.0001 | ||
| HCV-Ab positive | |||||
| N | 889 | 78.0 | 2385 | 65.8 | |
| Y | 250 | 22.0 | 1238 | 34.2 | <0.0001 |
| Naïve status | |||||
| N | 883 | 74.9 | 3222 | 85.7 | |
| Y | 296 | 25.1 | 538 | 14.3 | <0.0001 |
| CDC stage | |||||
| A | 603 | 51.2 | 1482 | 39.4 | |
| B | 310 | 26.3 | 1104 | 29.4 | |
| C | 266 | 22.6 | 1174 | 31.2 | <0.0001 |
| Baseline HIVRNA (experienced) | |||||
| Detectable | 146 | 16.5 | 1670 | 51.8 | |
| Undetectable | 737 | 83.5 | 1552 | 48.2 | <0.0001 |
| CD4 (cells/mm3) | |||||
| median (IQR) | 566 (328–798) | 371 (197–604) | <0.0001 | ||
| <250 | 212 | 18.0 | 1233 | 32.8 | |
| 250–499 | 287 | 24.3 | 1233 | 32.8 | |
| 500–749 | 310 | 26.3 | 711 | 18.9 | |
| ≥750 | 347 | 29.4 | 574 | 15.3 | <0.0001 |
| Baseline psychiatric disorder * | 84 | 7.1 | 235 | 6.2 | 0.29 |
| AE Resolved N (%) | AE Not Resolved N (%) | p-Value | |
|---|---|---|---|
| All (n = 63) | 58 (92.1) | 5 (7.9) | |
| DTG (n = 39) | 35 (89.7) | 4 (10.3) | 0.64 |
| Non-DTG (n = 24) | 23 (95.8) | 1 (4.2) | |
| INSTI (n = 53) | 49 (92.9) | 4 (7.6) | 0.79 |
| Non-INSTI (n = 10) | 9 (90.0) | 1 (10.0) | |
| Female (n = 19) | 17 (89.5) | 2 (10.5) | |
| Male (n = 44) | 41 (93.2) | 3 (6.8) | 0.63 |
| Age ≤ 50 years (n = 34) | 34 (100) | 0 | |
| Age > 50 years (n = 29) | 24 (82.8) | 5 (17.2) | 0.017 |
| Caucasian (n = 57) | 52 (91.2) | 5 (8.8) | |
| Other (n = 6) | 6 (100) | 0 | 1.0 |
| HCV-Ab negative (n = 40) | 35 (87.5) | 5 (12.5) | |
| HCV-Ab positive (n = 20) | 20 (100) | 0 (0.0) | 0.16 |
| ART-experienced (n = 44) | 40 (90.9) | 4 (9.1) | |
| ART-naïve (n = 19) | 18 (94.7) | 1 (5.3) | 1.0 |
| CDC stage A (n = 30) | 28 (93.3) | 2 (6.7) | 0.95 |
| B (n = 17) | 15 (88.2) | 2 (11.8) | |
| C (n = 16) | 15 (93.8) | 1 (6.2) | |
| CD4 < 250 cells/mmc (n = 13) | 12 (92.3) | 1 (7.7) | 0.43 |
| 250–499 cells/mmc (n = 18) | 18 (100) | 0 (0.0) | |
| 500–749 cells/mmc (n = 12) | 10 (83.3) | 2 (16.7) | |
| ≥750 cells/mmc (n = 20) | 18 (90.0) | 2 (10.0) | |
| Baseline psychiatric disorder no (n = 54) | 51 (94.4) | 3 (5.6) | |
| yes (n = 9) | 7 (77.8) | 2 (22.2) | 0.14 |
| Switch to INSTI (n = 26) | 22 (84.6) | 4 (15.4) | 0.15 |
| Switch to NNRTI (n = 14) | 13 (92.9) | 1 (7.1) | 1.0 |
| Switch to PI (n = 24) | 23 (95.8) | 1 (4.2) | 0.64 |
| Inter-class switch (n = 37) * | 36 (97.3) | 1 (2.7) | 0.15 |
| Intra-class switch (n = 26) | 22 (88.5) | 4 (15.4) |
| Variable | Crude HR | 95% CI | p | Adjusted HR ** | 95% CI | p |
|---|---|---|---|---|---|---|
| Sex F (ref. M) | 1.29 | 0.77–2.17 | 0.33 | |||
| Age (by 1 year) | 1.02 | 1.00–1.04 | 0.10 | |||
| Age (ref. < 50 years) | 1.97 | 1.21–3.21 | 0.006 | 1.52 | 0.91–2.54 | 0.10 |
| Weight (by 5 Kg) | 0.99 | 0.91–1.08 | 0.89 | |||
| Ethnicity (ref. Caucasian) | 1.23 | 0.53–2.85 | 0.62 | |||
| HCV-Ab+ (ref. HCV-Ab negative) | 1.14 | 0.67–1.92 | 0.63 | |||
| Naïve (ref. experienced) | 2.06 | 1.21–3.51 | 0.008 | 2.23 | 1.24–3.99 | 0.007 |
| CDC stage (ref. A) | ||||||
| B | 0.88 | 0.50–1.56 | 0.66 | |||
| C | 0.75 | 0.41–1.36 | 0.34 | |||
| CDC stage: chi-square for trend | 0.92 | 0.34 | ||||
| CD4 (ref. < 250) | ||||||
| 250–499 | 1.32 | 0.65–2.69 | 0.44 | 1.32 | 0.65–2.70 | 0.44 |
| 500–749 | 1.30 | 0.59–2.84 | 0.51 | 1.11 | 0.50–2.47 | 0.80 |
| ≥750 | 2.65 | 1.34–5.25 | 0.005 | 2.22 | 1.07–4.62 | 0.03 |
| CD4 class: chi-square for trend | 6.89 | 0.009 | 3.86 | 0.049 | ||
| Baseline psychiatric disorder (ref. no) * | 2.35 | 1.16–4.75 | 0.017 | 2.13 | 1.04–4.34 | 0.038 |
| Non-DTG Cohort (ref. DTG) | 0.25 | 0.15–0.41 | <0.0001 | 0.33 | 0.19–0.55 | <0.0001 |
| Variable | Crude HR | 95% CI | p | Adjusted HR ** | 95% CI | p |
|---|---|---|---|---|---|---|
| DTG cohort | ||||||
| Sex F (ref. M) | 1.10 | 0.54–2.25 | 0.79 | |||
| Age (by 1 year) | 1.00 | 0.97–1.02 | 0.89 | |||
| Age (ref. < 50 years) | 1.32 | 0.71–2.46 | 0.38 | |||
| Weight (by 5 Kg) | 0.98 | 0.88–1.10 | 0.78 | |||
| Ethnicity (ref. Caucasian) | 1.28 | 0.46–3.60 | 0.64 | |||
| HCV–Ab+ (ref. HCV–Ab negative) | 1.36 | 0.66–2.84 | 0.40 | |||
| Naïve (ref. experienced) | 2.53 | 1.35–4.73 | 0.004 | |||
| CDC stage (ref. A) | ||||||
| B | 1.29 | 0.62–2.71 | 0.50 | |||
| C | 1.29 | 0.59–2.82 | 0.52 | |||
| CDC stage: chi–square for trend | 0.53 | 0.96 | ||||
| CD4 (ref. < 250) | ||||||
| 250–499 | 0.72 | 0.29–1.80 | 0.48 | |||
| 500–749 | 0.53 | 0.20–1.42 | 0.21 | |||
| ≥750 | 0.97 | 0.42–2.23 | 0.94 | |||
| CD4 class: chi–square for trend | 0.001 | 0.98 | ||||
| Baseline psychiatric disorder (ref. no) * | 1.05 | 0.32–3.42 | 0.93 | |||
| Non–DTG cohort | ||||||
| Sex F (ref. M) | 1.81 | 0.84–3.90 | 0.13 | |||
| Age (by 1 year) | 1.02 | 0.89–1.06 | 0.21 | |||
| Age (ref. < 50 years) | 1.68 | 0.75–3.75 | 0.20 | |||
| Weight (by 5 Kg) | 0.96 | 0.84–1.10 | 0.59 | |||
| Ethnicity (ref. Caucasian) | 0.99 | 0.24–4.18 | 0.99 | |||
| HCV–Ab+ (ref. HCV–Ab negative) | 1.34 | 0.62–2.39 | 0.45 | |||
| Naïve (ref. experienced) | 0.48 | 0.11–2.03 | 0.32 | |||
| CDC stage (ref. A) | ||||||
| B | 0.66 | 0.29–1.63 | 0.37 | |||
| C | 0.54 | 0.21–1.40 | 0.21 | |||
| CDC stage: chi–square for trend | 1.72 | 0.19 | ||||
| CD4 (ref. < 250) | ||||||
| 250–499 | 2.42 | 0.76–7.74 | 0.14 | 2.41 | 0.76–7.68 | 0.14 |
| 500–749 | 2.17 | 0.59–8.03 | 0.25 | 2.01 | 0.54–7.51 | 0.30 |
| ≥750 | 4.33 | 1.32–14.28 | 0.016 | 4.04 | 1.21–13.44 | 0.02 |
| CD4 class: chi–square for trend | 5.58 | 0.018 | 4.46 | 0.035 | ||
| Baseline psychiatric disorder (ref. no) * | 4.42 | 1.78–10.93 | 0.001 | 4.15 | 1.67–10.33 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taramasso, L.; Orofino, G.; Ricci, E.; Menzaghi, B.; De Socio, G.V.; Squillace, N.; Madeddu, G.; Vichi, F.; Celesia, B.M.; Molteni, C.; et al. Reversibility of Central Nervous System Adverse Events in Course of Art. Viruses 2022, 14, 1028. https://doi.org/10.3390/v14051028
Taramasso L, Orofino G, Ricci E, Menzaghi B, De Socio GV, Squillace N, Madeddu G, Vichi F, Celesia BM, Molteni C, et al. Reversibility of Central Nervous System Adverse Events in Course of Art. Viruses. 2022; 14(5):1028. https://doi.org/10.3390/v14051028
Chicago/Turabian StyleTaramasso, Lucia, Giancarlo Orofino, Elena Ricci, Barbara Menzaghi, Giuseppe Vittorio De Socio, Nicola Squillace, Giordano Madeddu, Francesca Vichi, Benedetto Maurizio Celesia, Chiara Molteni, and et al. 2022. "Reversibility of Central Nervous System Adverse Events in Course of Art" Viruses 14, no. 5: 1028. https://doi.org/10.3390/v14051028
APA StyleTaramasso, L., Orofino, G., Ricci, E., Menzaghi, B., De Socio, G. V., Squillace, N., Madeddu, G., Vichi, F., Celesia, B. M., Molteni, C., Conti, F., Del Puente, F., Sarchi, E., Angioni, G., Cascio, A., Grosso, C., Parruti, G., Di Biagio, A., & Bonfanti, P., on behalf of CISAI Study Group. (2022). Reversibility of Central Nervous System Adverse Events in Course of Art. Viruses, 14(5), 1028. https://doi.org/10.3390/v14051028









