Comparative Analysis of Circulating Levels of SARS-CoV-2 Antibodies and Inflammatory Mediators in Healthcare Workers and COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics Statement
2.3. Blood Sample Collection
2.4. SARS-CoV-2 Serology
2.5. The Plaque Reduction Neutralization Test (PRNT)
2.6. Quantification of Cytokines and Chemokines
2.7. Quantification of IFNs
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Anti-SARS-CoV-2 IgA and IgG Antibody Responses in Healthcare Workers and Patients Exposed or Even Infected to SARS-CoV-2 during the Pandemic
3.3. Detection of IFN-λ2/3, IFN-γ and TNF-α in HCWs and COVID-19 Patients in Whom Anti-SARS-CoV-2 Antibodies Had Been Detected
3.4. Detection of CXCL10/IP-10, IL-6, IL-10 and CCL2/MCP-1 in HCWs and COVID-19 Patients in Whom Anti-SARS-CoV-2 Antibodies Had Been Detected
3.5. Principal Component Analysis (PCA) of Cytokines/Chemokines According Asymptomatic and Illness Severity
3.6. ROC Curve Analysis of Cytokines/Chemokines According Asymptomatic and Illness Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Coronavirus Disease 2019 (COVID-19): Situation Report 73. World Health Organization. 2020. Available online: https://apps.who.int/iris/handle/10665/331686 (accessed on 6 October 2021).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.R.; Golding, A.; Sorbello, A.; Ji, P.; Chen, J.; Saluja, B.; Witzmann, K.; Arya, V.; Reynolds, K.S.; Choi, S.Y.; et al. A Comprehensive Updated Review on SARS-CoV-2 and COVID-19. J. Clin. Pharmacol. 2020, 60, 954–975. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 6 October 2021).
- Governo do Brasil. Brasil Confirma Primeiro Caso Do Novo Coronavírus. Available online: https://www.gov.br/pt-br/noticias/saude-e-vigilancia-sanitaria/2020/02/brasil-confirma-primeiro-caso-do-novo-coronavirus (accessed on 6 November 2021).
- Ministerio da Saúde; Secretaria da Vigilância em Saúde. Doença pelo Coronavírus COVID-19: Semana Epidemiológica 6 (6/1 a 12/02/2022). In Boletim Epidemiológico no 100-Boletim COE Coronavirus; Ministério da Saúde: Brasília, Brazil, 2022. [Google Scholar]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2020, 97, 312–320. [Google Scholar] [CrossRef]
- Wenham, C.; Smith, J.; Morgan, R.; Gender and COVID-19 Working Group. COVID-19: The gendered impacts of the outbreak. Lancet 2020, 395, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Haider, N.; Abbasi, A.F.; Jaferi, U.; Prakash, S.; Balendra, V. The emerging SARS-CoV-2 variants of concern. Ther. Adv. Infect. Dis. 2021, 8, 20499361211024372. [Google Scholar] [CrossRef]
- Ahn, D.-G.; Shin, H.-J.; Kim, M.-H.; Lee, S.; Kim, H.-S.; Myoung, J.; Kim, B.-T.; Kim, S.-J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- Gatti, M.; De Ponti, F. Drug Repurposing in the COVID-19 Era: Insights from Case Studies Showing Pharmaceutical Peculiarities. Pharmaceutics 2021, 13, 302. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association of Administration of Interleukin-6 Antagonists with Mortality and Other Outcomes among Hospitalized Patients with COVID-19: A Prospective Meta-Analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Werner, A.P.; Koch, M.; Choi, A.; Narayanan, E.; Stewart-Jones, G.B.; Colpitts, T.; Bennett, H.; Boyoglu-Barnum, S.; Shi, W.; et al. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N. Engl. J. Med. 2021, 384, 1468–1470. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell. Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; da Silva Antunes, R.; Frazier, A.; et al. Negligible impact of SARS-CoV-2 variants on CD4+ and CD8+ T cell reactivity in COVID-19 exposed donors and vaccinees. bioRxiv 2021. [Google Scholar] [CrossRef]
- Chang, D.; Xu, H.; Rebaza, A.; Sharma, L.; Dela Cruz, C.S. Protecting health-care workers from subclinical coronavirus infection. Lancet. Respir. Med. 2020, 8, e13. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Severe acute respiration syndrome Taiwan, 2003. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 461–466. [Google Scholar]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Conselho Federal de Enfermagem (BR). Observatório da Enfermagem 2020. Available online: http://observatoriodaenfermagem.cofen.gov.br/ (accessed on 6 October 2021).
- Bandyopadhyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; Kamath, A.; Parepalli, S.A.; Brown, G.; Iharchane, S.; et al. Infection and mortality of healthcare workers worldwide from COVID-19: A systematic review. BMJ Glob. Health 2020, 5, e003097. [Google Scholar] [CrossRef]
- Meng, L.; Qin, H.; Zhao, J.; He, S.; Wei, Q.; Wang, Z.; Shen, J.; Sooranna, S.; Song, J. Transcriptomic Signatures of Airway Epithelium Infected With SARS-CoV-2: A Balance Between Anti-infection and Virus Load. Front. Cell Dev. Biol. 2021, 9, 735307. [Google Scholar] [CrossRef]
- Canetti, D.; Dell’Acqua, R.; Riccardi, N.; Della Torre, L.; Bigoloni, A.; Muccini, C.; Bruzzesi, E.; Ranzenigo, M.; Chiurlo, M.; Racca, S.; et al. SARS-CoV-2 IgG/IgM Rapid Test as a Diagnostic Tool in Hospitalized Patients and Healthcare Workers, at a large Teaching Hospital in northern Italy, during the 2020 COVID-19 Pandemic. New Microbiol. 2020, 43, 161–165. [Google Scholar] [PubMed]
- Ministério da Saúde. Guia de Vigilância Epidemiológica Emergência de Saúde Pública de Importância Nacional Pela Doença Pelo Coronavirus; Ministerio da Saúde: Brasília, Brasil, 2022.
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.H.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.; et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.K.; Nisalak, A.; Sukhavachana, P.; Vivona, S. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 1967, 99, 285–290. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; The R Foundation. Available online: https://www.R-project.org/ (accessed on 20 April 2021).
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Vardhana, S.A.; Wolchok, J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020, 217, e20200678. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Healthcare Workers: A Living Systematic Review and Meta-analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2020, 190, 161–175. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Zhang, Q.; Fang, X.; Li, N.; Lin, Z.; Li, J.; Xiong, N. Mild manifestations of COVID-19 in healthcare workers. PLOS Negl. Trop. Dis. 2020, 14, e0008950. [Google Scholar] [CrossRef]
- Onchonga, D.; Omwoyo, J.; Nyamamba, D. Assessing the prevalence of self-medication among healthcare workers before and during the 2019 SARS-CoV-2 (COVID-19) pandemic in Kenya. Saudi Pharm. J. 2020, 28, 1149–1154. [Google Scholar] [CrossRef]
- Uygun, Ö.; Ertaş, M.; Ekizoğlu, E.; Bolay, H.; Özge, A.; Orhan, E.K.; Çağatay, A.A.; Baykan, B. Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain 2020, 21, 121. [Google Scholar] [CrossRef]
- Canedo-Marroquin, G.; Saavedra, F.; Andrade, C.A.; Berrios, R.V.; Rodriguez-Guilarte, L.; Opazo, M.C.; Riedel, C.A.; Kalergis, A.M. SARS-CoV-2: Immune Response Elicited by Infection and Development of Vaccines and Treatments. Front. Immunol. 2020, 11, 569760. [Google Scholar] [CrossRef]
- Trigo, J.; García-Azorín, D.; Planchuelo-Gómez, Á.; Martínez-Pías, E.; Talavera, B.; Hernández-Pérez, I.; Valle-Peñacoba, G.; Simón-Campo, P.; De Lera, M.; Chavarría-Miranda, A.; et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J. Headache Pain 2020, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, L.; Chen, X.; Deng, Y.; Yang, S.; Xu, H.; Chen, Z.; Gao, X. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir. Med. 2020, 169, 106026. [Google Scholar] [CrossRef]
- Canova, V.; Lederer Schlapfer, H.; Piso, R.J.; Droll, A.; Fenner, L.; Hoffmann, T.; Hoffmann, M. Transmission risk of SARS-CoV-2 to healthcare workers -observational results of a primary care hospital contact tracing. Swiss. Med. Wkly. 2020, 150, w20257. [Google Scholar] [CrossRef] [PubMed]
- Yombi, J.C.; De Greef, J.; Marsin, A.S.; Simon, A.; Rodriguez-Villalobos, H.; Penaloza, A.; Belkhir, L. Symptom-based screening for COVID-19 in health care workers: The importance of fever. J. Hosp. Infect. 2020, 105, 428. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Cruz, C.S.D.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef]
- Chinazzi, M.; Davis, J.T.; Ajelli, M.; Gioannini, C.; Litvinova, M.; Merler, S.; Piontti, Y.; Pastore, A.; Mu, K.; Rossi, L.; et al. The Effect of Travel Restrictions on the Spread of the 2019 Novel Coronavirus (COVID-19) Outbreak. Science 2020, 368, 395–400. [Google Scholar] [CrossRef] [Green Version]
- da Silva, S.J.R.; do Nascimento, J.C.F.; dos Santos Reis, W.P.M.; da Silva, C.T.A.; da Silva, P.G.; Mendes, R.P.G.; Mendonça, A.A.; Santos, B.N.R.; de Magalhães, J.J.F.; Kohl, A.; et al. Widespread Contamination of SARS-CoV-2 on Highly Touched Surfaces in Brazil During the Second Wave of the COVID-19 Pandemic. Environ. Microbiol. 2021, 23, 7382–7395. [Google Scholar] [CrossRef]
- Peirlinck, M.; Linka, K.; Costabal, F.S.; Bhattacharya, J.; Bendavid, E.; Ioannidis, J.P.; Kuhl, E. Visualizing the invisible: The effect of asymptomatic transmission on the outbreak dynamics of COVID-19. Comput. Methods Appl. Mech. Eng. 2020, 372, 113410. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Cornforth, D.M.; Dushoff, J.; Weitz, J.S. The time scale of asymptomatic transmission affects estimates of epidemic potential in the COVID-19 outbreak. Epidemics 2020, 31, 100392. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, M.; Bertuzzo, E.; Mari, L.; Miccoli, S.; Carraro, L.; Casagrandi, R.; Rinaldo, A. Spread and dynamics of the COVID-19 epidemic in Italy: Effects of emergency containment measures. Proc. Natl. Acad. Sci. USA 2020, 117, 10484–10491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Chen, D.; Xia, Y.; Wu, X.; Li, T.; Ou, X.; Zhou, L.; Liu, J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect. Dis. 2020, 20, 410–411. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [Green Version]
- Young, B.E.; Ong, S.W.X.; Ng, L.F.P.; Anderson, D.E.; Chia, W.N.; Chia, P.Y.; Ang, L.W.; Mak, T.M. Viral dynamics and immune correlates of COVID-19 disease severity. Clin. Infect. Dis. 2021, 73, e2932–e2942. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J.; Luo, W.; Chen, T.; Qin, Q.; Deng, P. Characterization of Cytokine/Chemokine Profiles of Severe Acute Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhan, Y.; Wu, L.; Yu, X.; Zhang, W.; Ye, L.; Xu, S.; Sun, R.; Wang, Y.; et al. Analysis of Serum Cytokines in Patients with Severe Acute Respiratory Syndrome. Infect. Immun. 2004, 72, 4410–4415. [Google Scholar] [CrossRef] [Green Version]
- Mahallawi, W.H.; Khabour, O.F.; Zhang, Q.; Makhdoum, H.M.; Suliman, B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018, 104, 8–13. [Google Scholar] [CrossRef]
- Mubarak, A.; Alturaiki, W.; Hemida, M.G. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development. J. Immunol. Res. 2019, 2019, 6491738. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Poissy, J.; Goffard, A.; Fournier, C.; Kipnis, E.; Titecat, M.; Bortolotti, P.; Martinez, L.; Dubucquoi, S.; Dessein, R.; et al. Distinct immune response in two MERS-CoV-infected patients: Can we go from bench to bedside? PLoS ONE 2014, 9, e88716. [Google Scholar] [CrossRef]
- Walsh, K.A.; Jordan, K.; Clyne, B.; Rohde, D.; Drummond, L.; Byrne, P.; Ahern, S.; Carty, P.G.; O’Brien, K.K.; O’Murchu, E.; et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 2020, 81, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Shin, N.Y.; Jang, J.H.; Lee, W.J.; Lee, D.; Choi, Y.; Choi, S.H.; Kang, D.H. Relationships among stress, emotional intelligence, cognitive intelligence, and cytokines. Medicine 2019, 98, e15345. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-W.; Zhou, Z.; Wang, J.-L.; Deng, Y.-F.; Jing, H.; Qiu, Y. Viral loads, lymphocyte subsets and cytokines in asymptomatic, mildly and critical symptomatic patients with SARS-CoV-2 infection: A retrospective study. Virol. J. 2021, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Caricchio, R.; Gallucci, M.; Dass, C.; Zhang, X.; Gallucci, S.; Fleece, D.; Bromberg, M.; Criner, G.J. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021, 80, 88–95. [Google Scholar] [CrossRef]
- Awasthi, S.; Wagner, T.; Venkatakrishnan, A.J.; Puranik, A.; Hurchik, M.; Agarwal, V.; Conrad, I.; Kirkup, C.; Arunachalam, R.; O’Horo, J.; et al. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov. 2021, 7, 55. [Google Scholar] [CrossRef]
- Wu, J.; Shen, J.; Han, Y.; Qiao, Q.; Dai, W.; He, B.; Pang, R.; Zhao, J.; Luo, T.; Guo, Y.; et al. Upregulated IL-6 Indicates a Poor COVID-19 Prognosis: A Call for Tocilizumab and Convalescent Plasma Treatment. Front. Immunol. 2021, 12, 598799. [Google Scholar] [CrossRef]
- Dorgham, K.; Quentric, P.; Gökkaya, M.; Marot, S.; Parizot, C.; Sauce, D.; Guihot, A.; Luyt, C.-E.; Schmidt, M.; Mayaux, J.; et al. Distinct cytokine profiles associated with COVID-19 severity and mortality. J. Allergy Clin. Immunol. 2021, 147, 2098–2107. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.L.; Feng, Y.; Deng, Y.Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977.e3. [Google Scholar] [CrossRef] [PubMed]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.-Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef]
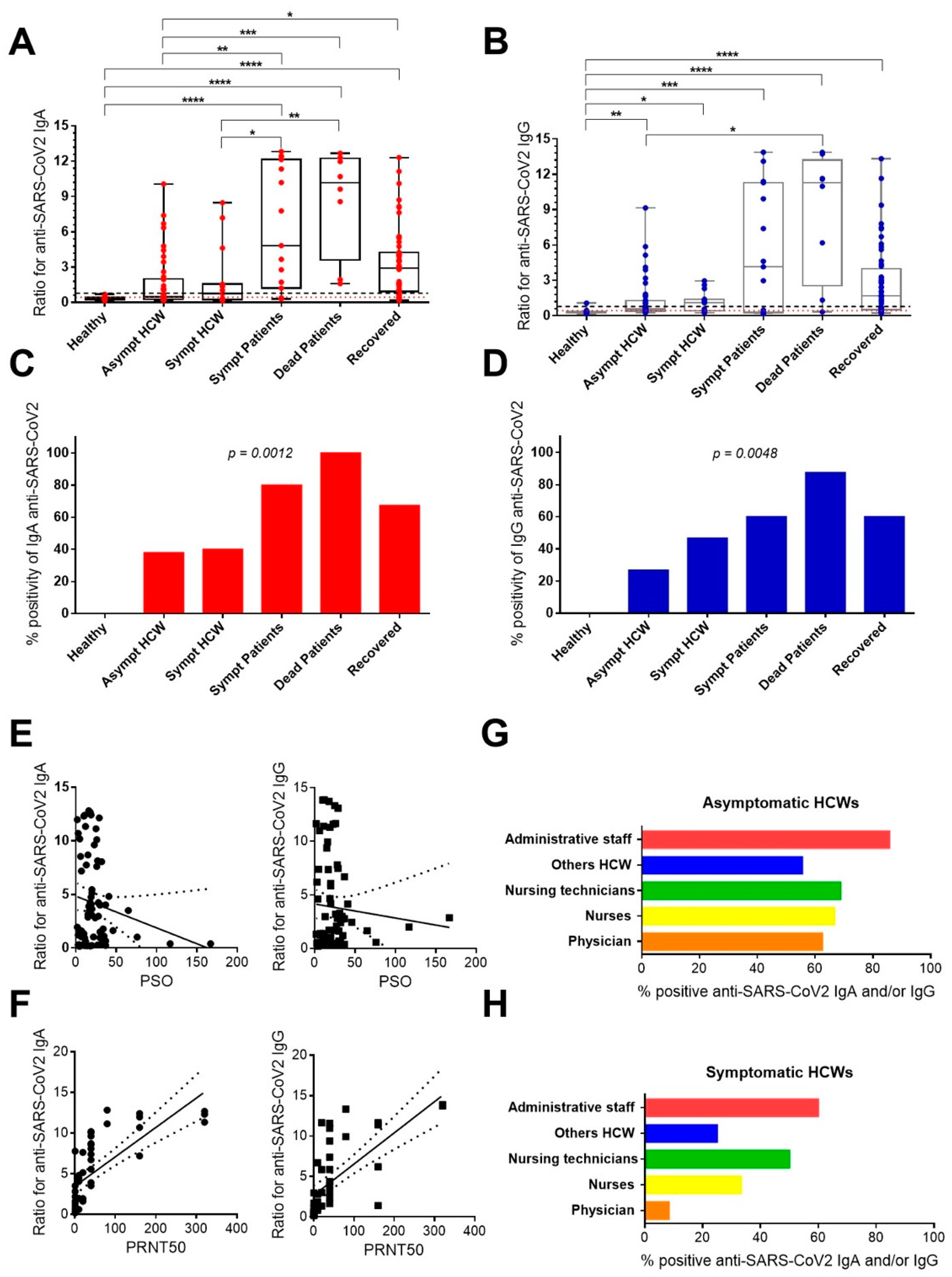
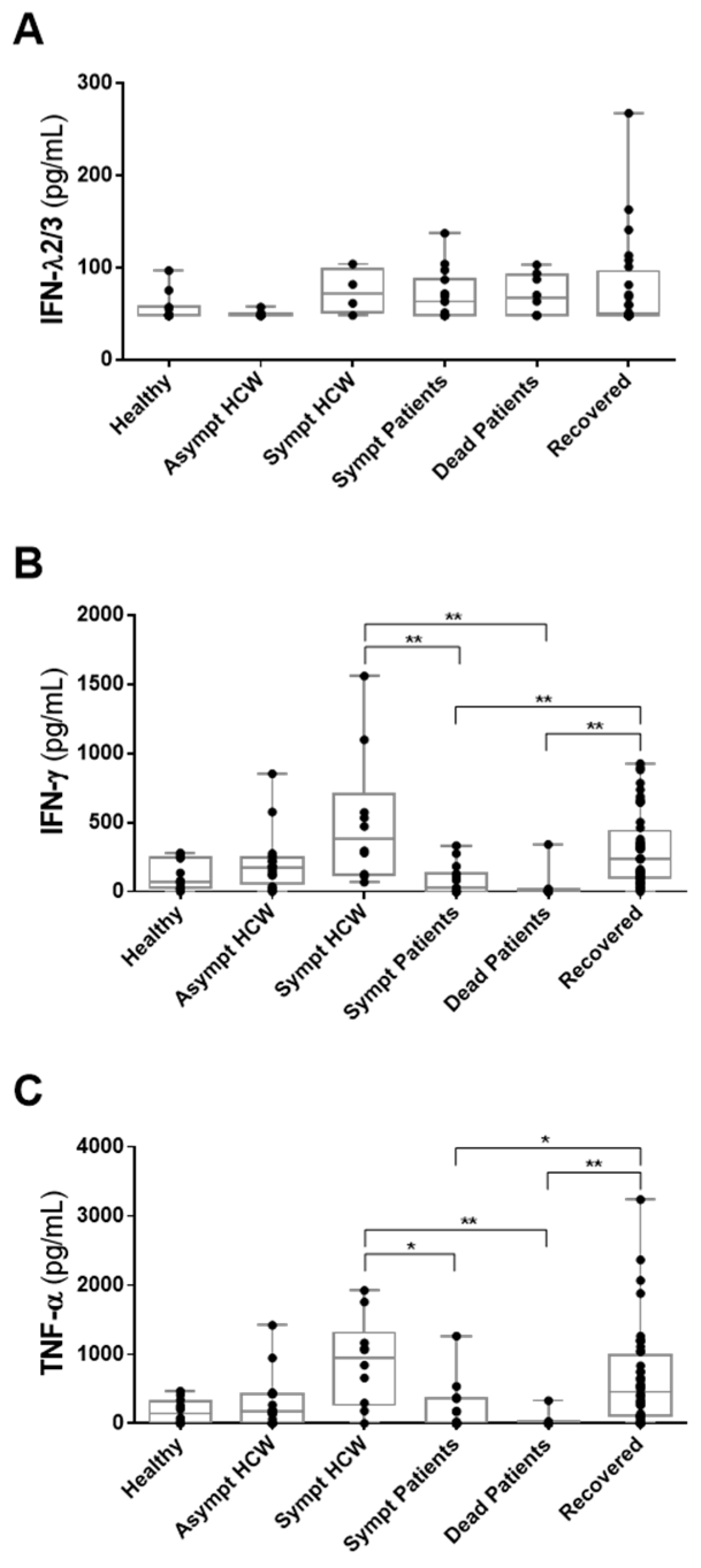

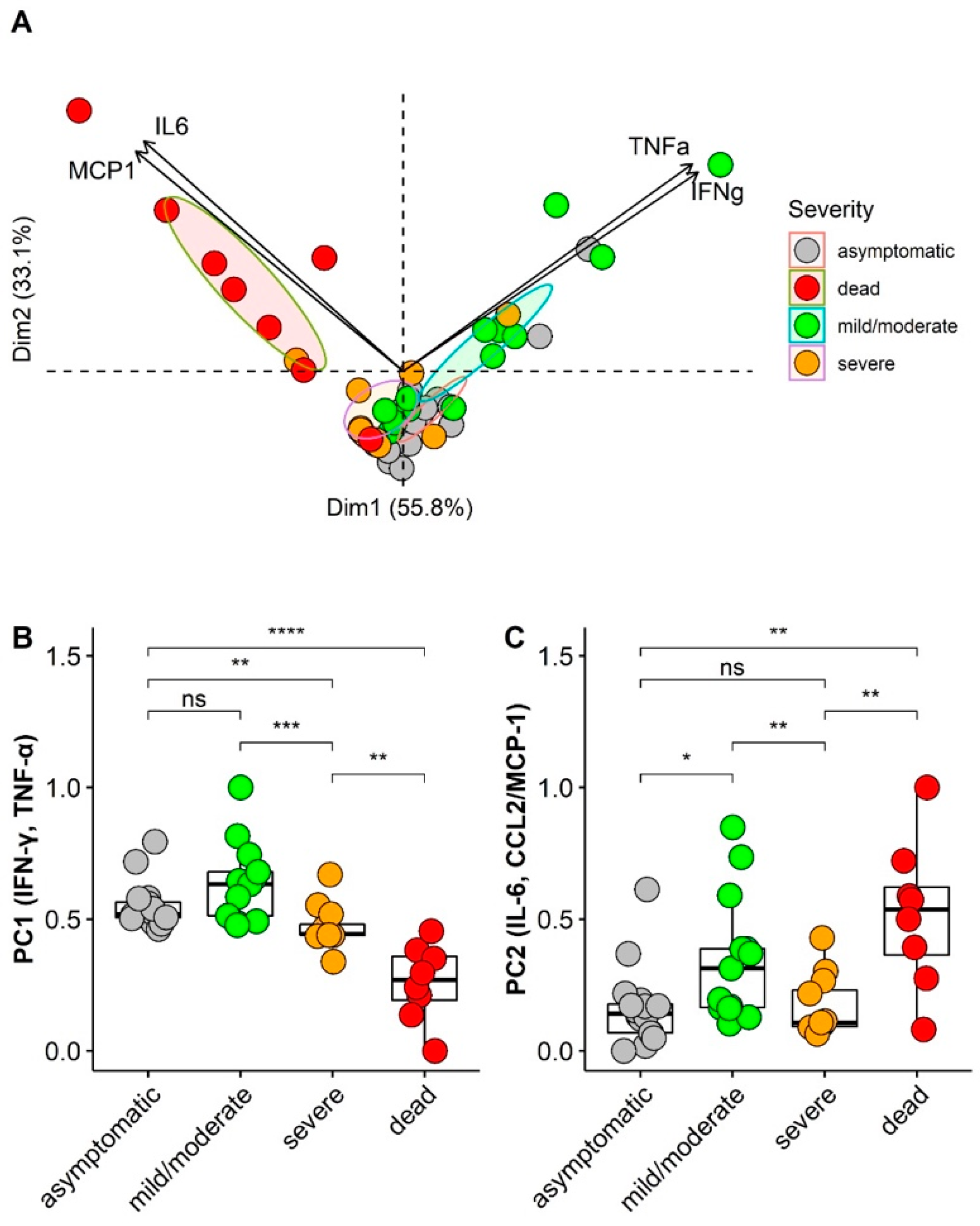

| Asymptomatic HCWs | Symptomatic HCWs | Symptomatic Patients | Dead Patients | Recovered HCWs/Patients | p * | |
|---|---|---|---|---|---|---|
| Total | 44 | 15 | 15 | 8 | 52 | |
| Gender, n (%) | ||||||
| Female | 32 (73) | 7 (47) | 9 (60) | 5 (62.5) | 38 (73) | 0.303 |
| Age (years) a | 37 (29–42) | 34 (27–41) | 61 (48–69) | 76 (71–85) | 36 (29–47) | <0.001 |
| Post- symptoms onset | NA | 7 (4–12) | 12 (4–19) | 7 (3–14) c | 27 (19–33) | <0.001 |
| Signs/Symptoms, n (%) | ||||||
| cough b | 0 | 8 (53) | 9 (60) | 5 (63) | 33 (63) c | 0.956 |
| headache | 0 | 7 (47) | 3 (20) | 1 (13) | 33 (63) | 0.003 |
| coryza | 0 | 4 (27) | 2 (13) | 1 (13) | 33 (63) | <0.001 |
| fatigue | 0 | 4 (27) | 5 (33) | 3 (38) | 27 (52) | 0.286 |
| myalgia | 0 | 4 (27) | 4 (27) | 3 (38) | 26 (50) | 0.234 |
| fever | 0 | 4 (27) | 7 (47) | 5 (63) | 21 (40) | 0.420 |
| dyspnea | 0 | 2 (13) | 6 (40) | 8 (100) | 20 (38) | 0.001 |
| diarrhea | 0 | 6 (40) | 3 (20) | 0 | 15 (29) | 0.208 |
| Hospitalization, n (%) | 0 | 0 | 12 (80) | 8 (100) | 2 (4) | <0.001 |
| Comorbidity, n (%) | ||||||
| hypertension | 0 | 1 (7) | 7 (47) | 4 (50) | 7 (47) | <0.001 |
| diabetes | 0 | 0 | 5 (33) | 2 (25) | 1 (2) | <0.001 |
| obesity | 0 | 0 | 5 (33) | 0 | 3 (6) | 0.001 |
| dementia | 0 | 0 | 3 (20) | 2 (25) | 0 | <0.001 |
| stroke | 0 | 0 | 2 (13) | 2 (25) | 0 | 0.001 |
| other cardiovascular disease | 0 | 0 | 1 (7) | 2 (25) | 1 (13) | 0.177 |
| chronic kidney disease | 0 | 0 | 1 (7) | 0 | 0 | 0.284 |
| cancer | 0 | 0 | 1 (7) | 0 | 0 | 0.284 |
| HIV | 0 | 0 | 0 | 0 | 3 (6) | 0.551 |
| Charlson Comorbidity Index * | 0 | 0 | 2 (1–2) | 4 (3–5) | 0 | <0.001 |
| Cytokine/Chemokine | ROC Area | 95% CI |
|---|---|---|
| IFN-γ | 0.164 | 0.041–0.287 |
| TNF-α | 0.200 | 0.071–0.329 |
| IL-6 | 0.805 | 0.652–0.958 |
| IL-10 | 0.678 | 0.526–0.831 |
| CCL2/MCP-1 | 0.837 | 0.716–0.958 |
| CXCL10/IP-10 | 0.752 | 0.574–0.876 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de-Oliveira-Pinto, L.M.; Fiestas Solórzano, V.E.; de Lourdes Martins, M.; Fernandes-Santos, C.; Damasco, P.H.; de Siqueira, M.A.M.T.; Dias, H.G.; Pauvolid-Corrêa, A.; Damasco, P.V.; de Azeredo, E.L. Comparative Analysis of Circulating Levels of SARS-CoV-2 Antibodies and Inflammatory Mediators in Healthcare Workers and COVID-19 Patients. Viruses 2022, 14, 455. https://doi.org/10.3390/v14030455
de-Oliveira-Pinto LM, Fiestas Solórzano VE, de Lourdes Martins M, Fernandes-Santos C, Damasco PH, de Siqueira MAMT, Dias HG, Pauvolid-Corrêa A, Damasco PV, de Azeredo EL. Comparative Analysis of Circulating Levels of SARS-CoV-2 Antibodies and Inflammatory Mediators in Healthcare Workers and COVID-19 Patients. Viruses. 2022; 14(3):455. https://doi.org/10.3390/v14030455
Chicago/Turabian Stylede-Oliveira-Pinto, Luzia Maria, Victor Edgar Fiestas Solórzano, Maria de Lourdes Martins, Caroline Fernandes-Santos, Paula Hesselberg Damasco, Marilda Agudo Mendonça Teixeira de Siqueira, Helver Gonçalves Dias, Alex Pauvolid-Corrêa, Paulo Vieira Damasco, and Elzinandes Leal de Azeredo. 2022. "Comparative Analysis of Circulating Levels of SARS-CoV-2 Antibodies and Inflammatory Mediators in Healthcare Workers and COVID-19 Patients" Viruses 14, no. 3: 455. https://doi.org/10.3390/v14030455
APA Stylede-Oliveira-Pinto, L. M., Fiestas Solórzano, V. E., de Lourdes Martins, M., Fernandes-Santos, C., Damasco, P. H., de Siqueira, M. A. M. T., Dias, H. G., Pauvolid-Corrêa, A., Damasco, P. V., & de Azeredo, E. L. (2022). Comparative Analysis of Circulating Levels of SARS-CoV-2 Antibodies and Inflammatory Mediators in Healthcare Workers and COVID-19 Patients. Viruses, 14(3), 455. https://doi.org/10.3390/v14030455








