Distinct Plasma Concentrations of Acyl-CoA-Binding Protein (ACBP) in HIV Progressors and Elite Controllers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Measurements
2.3. Calculation of CD4 T-Cell Count Change
2.4. Statistical Analyses
2.5. Ethical Considerations
3. Results
3.1. Study Population
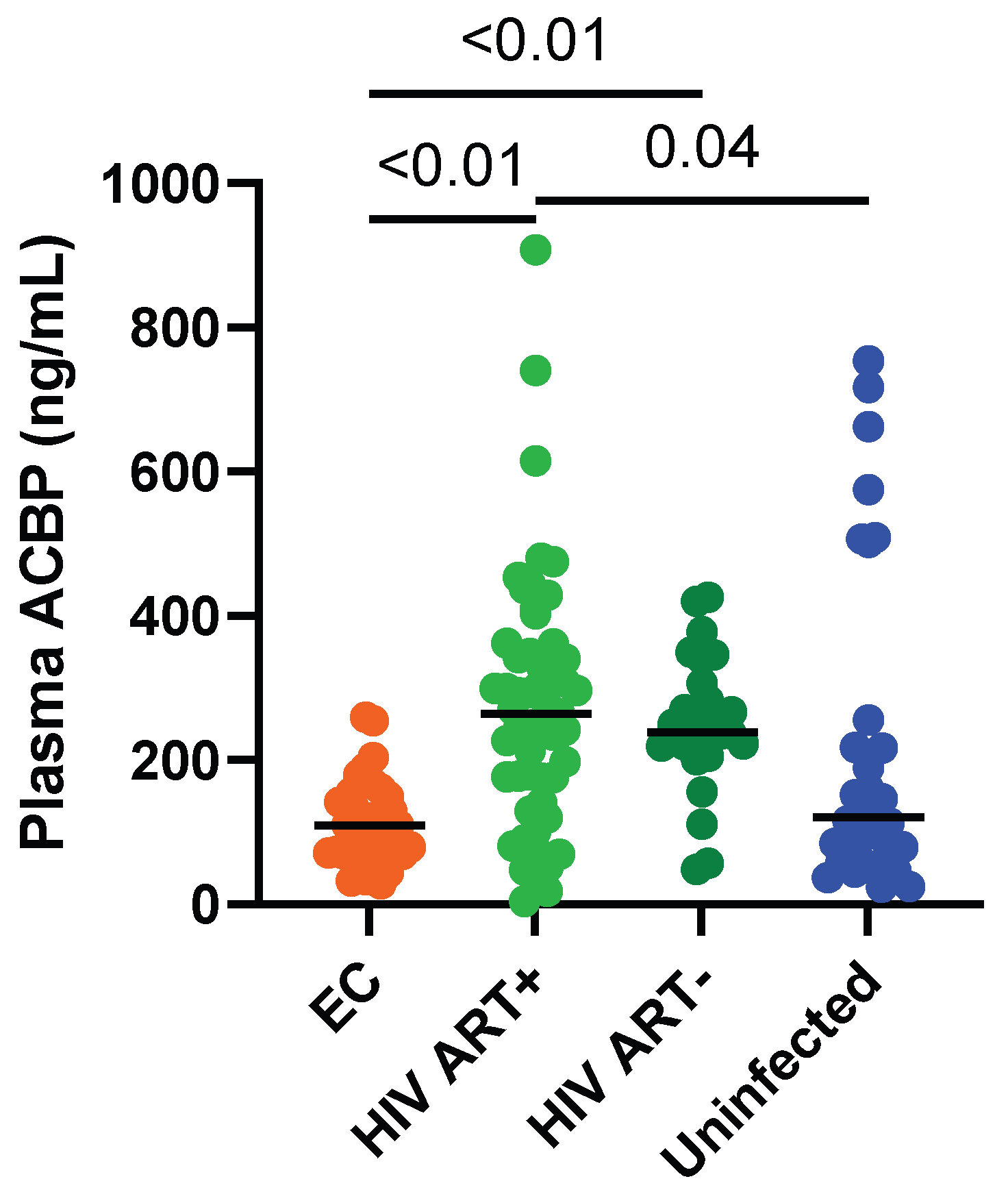
3.2. Circulating ACBP Levels Were Lower in ECs Compared to ART-Treated PLWH
3.3. Markers of HIV Disease Progression Were Not Associated with ACBP Levels in ECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blankson, J.N. The Esperanza Patient: More Hope for a Sterilizing HIV-1 Cure. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Turk, G.; Seiger, K.; Lian, X.; Sun, W.; Parsons, E.M.; Gao, C.; Rassadkina, Y.; Polo, M.L.; Czernikier, A.; Ghiglione, Y.; et al. A Possible Sterilizing Cure of HIV-1 Infection Without Stem Cell Transplantation. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- May, M.E.; Kwaa, A.K.; Blankson, J.N. HIV-1 reservoirs in elite controllers: Clues for developing a functional cure? Future Microbiol. 2017, 12, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, J.; Gianella, S.; Concha-Garcia, S.; Taylor, J.; Kaytes, A.; Christensen, C.; Patel, H.; Ndukwe, S.; Rawlings, S.A.; Hendrickx, S.; et al. Ethical and practical considerations for HIV cure-related research at the end-of-life: A qualitative interview and focus group study in the United States. BMC Med. Ethics 2022, 23, 2. [Google Scholar] [CrossRef]
- Deeks, S.G.; Archin, N.; Cannon, P.; Collins, S.; Jones, R.B.; de Jong, M.; Lambotte, O.; Lamplough, R.; Ndung’u, T.; Sugarman, J.; et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat. Med. 2021, 27, 2085–2098. [Google Scholar] [CrossRef]
- Saag, M.; Deeks, S.G. How do HIV elite controllers do what they do? Clin. Infect. Dis. 2010, 51, 239–241. [Google Scholar] [CrossRef][Green Version]
- Collins, D.R.; Gaiha, G.D.; Walker, B.D. CD8(+) T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 2020, 20, 471–482. [Google Scholar] [CrossRef]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef]
- Miura, T.; Brockman, M.A.; Schneidewind, A.; Lobritz, M.; Pereyra, F.; Rathod, A.; Block, B.L.; Brumme, Z.L.; Brumme, C.J.; Baker, B.; et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J. Virol. 2009, 83, 2743–2755. [Google Scholar] [CrossRef]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 97, 2709–2714. [Google Scholar] [CrossRef]
- Okulicz, J.F.; Lambotte, O. Epidemiology and clinical characteristics of elite controllers. Curr. Opin. HIV AIDS 2011, 6, 163–168. [Google Scholar] [CrossRef]
- Chowdhury, F.Z.; Ouyang, Z.; Buzon, M.; Walker, B.D.; Lichterfeld, M.; Yu, X.G. Metabolic pathway activation distinguishes transcriptional signatures of CD8+ T cells from HIV-1 elite controllers. AIDS 2018, 32, 2669–2677. [Google Scholar] [CrossRef]
- Tarancon-Diez, L.; Rodriguez-Gallego, E.; Rull, A.; Peraire, J.; Vilades, C.; Portilla, I.; Jimenez-Leon, M.R.; Alba, V.; Herrero, P.; Leal, M.; et al. Immunometabolism is a key factor for the persistent spontaneous elite control of HIV-1 infection. EBioMedicine 2019, 42, 86–96. [Google Scholar] [CrossRef]
- Saez-Cirion, A.; Sereti, I. Immunometabolism and HIV-1 pathogenesis: Food for thought. Nat. Rev. Immunol. 2021, 21, 5–19. [Google Scholar] [CrossRef]
- Loucif, H.; Dagenais-Lussier, X.; Avizonis, D.; Choiniere, L.; Beji, C.; Cassin, L.; Routy, J.P.; Fritz, J.H.; Olagnier, D.; van Grevenynghe, J. Autophagy-dependent glutaminolysis drives superior IL21 production in HIV-1-specific CD4 T cells. Autophagy 2021, 1–18. [Google Scholar] [CrossRef]
- Loucif, H.; Dagenais-Lussier, X.; Beji, C.; Cassin, L.; Jrade, H.; Tellitchenko, R.; Routy, J.P.; Olagnier, D.; van Grevenynghe, J. Lipophagy confers a key metabolic advantage that ensures protective CD8A T-cell responses against HIV-1. Autophagy 2021, 17, 3408–3423. [Google Scholar] [CrossRef]
- Angin, M.; Volant, S.; Passaes, C.; Lecuroux, C.; Monceaux, V.; Dillies, M.A.; Valle-Casuso, J.C.; Pancino, G.; Vaslin, B.; Le Grand, R.; et al. Metabolic plasticity of HIV-specific CD8(+) T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nat. Metab. 2019, 1, 704–716. [Google Scholar] [CrossRef]
- Deretic, V.; Kroemer, G. Autophagy in metabolism and quality control: Opposing, complementary or interlinked functions? Autophagy 2021, 1–10. [Google Scholar] [CrossRef]
- Alquier, T.; Christian-Hinman, C.A.; Alfonso, J.; Faergeman, N.J. From benzodiazepines to fatty acids and beyond: Revisiting the role of ACBP/DBI. Trends Endocrinol. Metab. 2021, 32, 890–903. [Google Scholar] [CrossRef]
- Arya, R.; Sundd, M.; Kundu, S. Structural And Functional Aspects Of Acyl-Coenzyme A Binding Proteins (ACBPs): A Comprehensive Review. J. Proteins Proteom. 2012, 3, 61–71. [Google Scholar]
- Lopez-Otin, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Charmpilas, N.; Ruckenstuhl, C.; Sica, V.; Buttner, S.; Habernig, L.; Dichtinger, S.; Madeo, F.; Tavernarakis, N.; Bravo-San Pedro, J.M.; Kroemer, G. Acyl-CoA-binding protein (ACBP): A phylogenetically conserved appetite stimulator. Cell Death Dis. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Pedro, J.M.B.; Sica, V.; Madeo, F.; Kroemer, G. Acyl-CoA-binding protein (ACBP): The elusive ‘hunger factor’ linking autophagy to food intake. Cell Stress 2019, 3, 312–318. [Google Scholar] [CrossRef]
- Bravo-San Pedro, J.M.; Sica, V.; Martins, I.; Pol, J.; Loos, F.; Maiuri, M.C.; Durand, S.; Bossut, N.; Aprahamian, F.; Anagnostopoulos, G.; et al. Acyl-CoA-Binding Protein Is a Lipogenic Factor that Triggers Food Intake and Obesity. Cell Metab. 2019, 30, 754–767.e759. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; Kouassi, P.; Sylla, M.; Zhang, Y.; Fouda, A.; Fabre, T.; Goulet, J.P.; van Grevenynghe, J.; Lee, T.; Singer, J.; et al. Proinflammatory isoforms of IL-32 as novel and robust biomarkers for control failure in HIV-infected slow progressors. Sci. Rep. 2016, 6, 22902. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, V.; Cox, J.; Lebouche, B.; Costiniuk, C.; Cao, W.; Li, T.; Ponte, R.; Thomas, R.; Szabo, J.; Baril, J.G.; et al. Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J. Int. AIDS Soc. 2018, 21, e25034. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Chartrand-Lefebvre, C.; Baril, J.G.; Trottier, S.; Trottier, B.; Harris, M.; Walmsley, S.; Conway, B.; Wong, A.; Routy, J.P.; et al. The Canadian HIV and aging cohort study—Determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: Rationale and study protocol. BMC Infect. Dis. 2017, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Bernard, N.F. Natural killer (NK) cell receptor-HLA ligand genotype combinations associated with protection from HIV infection: Investigation of how protective genotypes influence anti HIV NK cell functions. AIDS Res. Ther. 2017, 14, 38. [Google Scholar] [CrossRef]
- Isnard, S.; Ramendra, R.; Lin, J.; Kant, S.; Fombuena, B.; Ouyang, J.; Peng, X.; El Far, M.; Tremblay, C.; Bernard, N.F.; et al. Anti-CMV IgG is linked to CD4 T-cell count decay in HIV elite controllers. Clin. Infect. Dis. 2020, 1, 144–147. [Google Scholar] [CrossRef]
- Ramendra, R.; Isnard, S.; Lin, J.; Fombuena, B.; Ouyang, J.; Mehraj, V.; Zhang, Y.; Finkelman, M.; Costiniuk, C.; Lebouche, B.; et al. Cytomegalovirus Seropositivity Is Associated With Increased Microbial Translocation in People Living With Human Immunodeficiency Virus and Uninfected Controls. Clin. Infect. Dis. 2020, 71, 1438–1446. [Google Scholar] [CrossRef]
- Caby, F.; Guihot, A.; Lambert-Niclot, S.; Guiguet, M.; Boutolleau, D.; Agher, R.; Valantin, M.A.; Tubiana, R.; Calvez, V.; Marcelin, A.G.; et al. Determinants of a Low CD4/CD8 Ratio in HIV-1-Infected Individuals Despite Long-term Viral Suppression. Clin. Infect. Dis. 2016, 62, 1297–1303. [Google Scholar] [CrossRef]
- Han, W.M.; Apornpong, T.; Kerr, S.J.; Hiransuthikul, A.; Gatechompol, S.; Do, T.; Ruxrungtham, K.; Avihingsanon, A. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res. Ther. 2018, 15, 13. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef]
- Lu, W.; Mehraj, V.; Vyboh, K.; Cao, W.; Li, T.; Routy, J.P. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J. Int. AIDS Soc. 2015, 18, 20052. [Google Scholar] [CrossRef]
- Capeau, J. Weight and antiretrovirals: A new episode in a long series. Lancet HIV 2021, 8, e663–e664. [Google Scholar] [CrossRef]
- Gonzalo-Gil, E.; Ikediobi, U.; Sutton, R.E. Mechanisms of Virologic Control and Clinical Characteristics of HIV+ Elite/Viremic Controllers. Yale J. Biol. Med. 2017, 90, 245–259. [Google Scholar]
- Kamya, P.; Tsoukas, C.M.; Boulet, S.; Routy, J.-P.; Thomas, R.; Côté, P.; Boulassel, M.R.; Lessard, B.; Kaul, R.; Ostrowski, M.; et al. T cell Activation does not drive CD4 decline in longitudinally followed HIV-infected Elite Controllers. AIDS Res. Ther. 2011, 8, 20. [Google Scholar] [CrossRef]
- Noel, N.; Boufassa, F.; Lecuroux, C.; Saez-Cirion, A.; Bourgeois, C.; Dunyach-Remy, C.; Goujard, C.; Rouzioux, C.; Meyer, L.; Pancino, G.; et al. Elevated IP10 levels are associated with immune activation and low CD4(+) T-cell counts in HIV controller patients. AIDS 2014, 28, 467–476. [Google Scholar] [CrossRef]
- Clavier, T.; Tonon, M.C.; Foutel, A.; Besnier, E.; Lefevre-Scelles, A.; Morin, F.; Gandolfo, P.; Tuech, J.J.; Quillard, M.; Veber, B.; et al. Increased plasma levels of endozepines, endogenous ligands of benzodiazepine receptors, during systemic inflammation: A prospective observational study. Crit. Care 2014, 18, 633. [Google Scholar] [CrossRef]
- Ferrarese, C.; Appollonio, I.; Frigo, M.; Meregalli, S.; Piolti, R.; Tamma, F.; Frattola, L. Cerebrospinal fluid levels of diazepam-binding inhibitor in neurodegenerative disorders with dementia. Neurology 1990, 40, 632–635. [Google Scholar] [CrossRef]
- Conti, E.; Andreoni, S.; Tomaselli, D.; Storti, B.; Brovelli, F.; Acampora, R.; Da Re, F.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Serum DBI and biomarkers of neuroinflammation in Alzheimer’s disease and delirium. Neurol. Sci. 2021, 42, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, C.; Gorwood, J.; Olivo, A.; Le Pelletier, L.; Capeau, J.; Lambotte, O.; Bereziat, V.; Lagathu, C. Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment. Front. Immunol. 2021, 12, 670566. [Google Scholar] [CrossRef] [PubMed]
| ACBP vs. | EC | HIV ART+ | HIV ART- | Uninfected Controls | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| CD4 count | −0.08 | 0.62 | −0.06 | 0.64 | −0.48 | 0.01 | 0.33 | 0.22 |
| CD8 count | −0.14 | 0.41 | −0.06 | 0.68 | −0.38 | 0.048 | −0.53 | 0.04 |
| CD4/CD8 ratio | 0.21 | 0.21 | 0.03 | 0.80 | −0.12 | 0.56 | 0.68 | <0.01 |
| CD4 decay | 0.32 | 0.07 | ND | ND | ND | |||
| Weight | −0.25 | 0.24 | −0.002 | 0.99 | 0.14 | 0.69 | ND | |
| BMI | −0.03 | 0.90 | 0.11 | 0.44 | 0.23 | 0.50 | ND | |
| CMV IgG titers | −0.19 | 0.31 | 0.05 | 0.79 | −0.06 | 0.8 | 0.12 | 0.66 |
| Presence of protective HLA | 0.2 * | ND | ND | ND | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isnard, S.; Royston, L.; Lin, J.; Fombuena, B.; Bu, S.; Kant, S.; Mabanga, T.; Berini, C.; El-Far, M.; Durand, M.; et al. Distinct Plasma Concentrations of Acyl-CoA-Binding Protein (ACBP) in HIV Progressors and Elite Controllers. Viruses 2022, 14, 453. https://doi.org/10.3390/v14030453
Isnard S, Royston L, Lin J, Fombuena B, Bu S, Kant S, Mabanga T, Berini C, El-Far M, Durand M, et al. Distinct Plasma Concentrations of Acyl-CoA-Binding Protein (ACBP) in HIV Progressors and Elite Controllers. Viruses. 2022; 14(3):453. https://doi.org/10.3390/v14030453
Chicago/Turabian StyleIsnard, Stéphane, Léna Royston, John Lin, Brandon Fombuena, Simeng Bu, Sanket Kant, Tsoarello Mabanga, Carolina Berini, Mohamed El-Far, Madeleine Durand, and et al. 2022. "Distinct Plasma Concentrations of Acyl-CoA-Binding Protein (ACBP) in HIV Progressors and Elite Controllers" Viruses 14, no. 3: 453. https://doi.org/10.3390/v14030453
APA StyleIsnard, S., Royston, L., Lin, J., Fombuena, B., Bu, S., Kant, S., Mabanga, T., Berini, C., El-Far, M., Durand, M., Tremblay, C. L., Bernard, N. F., Kroemer, G., & Routy, J.-P. (2022). Distinct Plasma Concentrations of Acyl-CoA-Binding Protein (ACBP) in HIV Progressors and Elite Controllers. Viruses, 14(3), 453. https://doi.org/10.3390/v14030453







