Elephant Endotheliotropic Herpesvirus 1, 4 and 5 in China: Occurrence in Multiple Sample Types and Implications for Wild and Captive Population Surveillance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
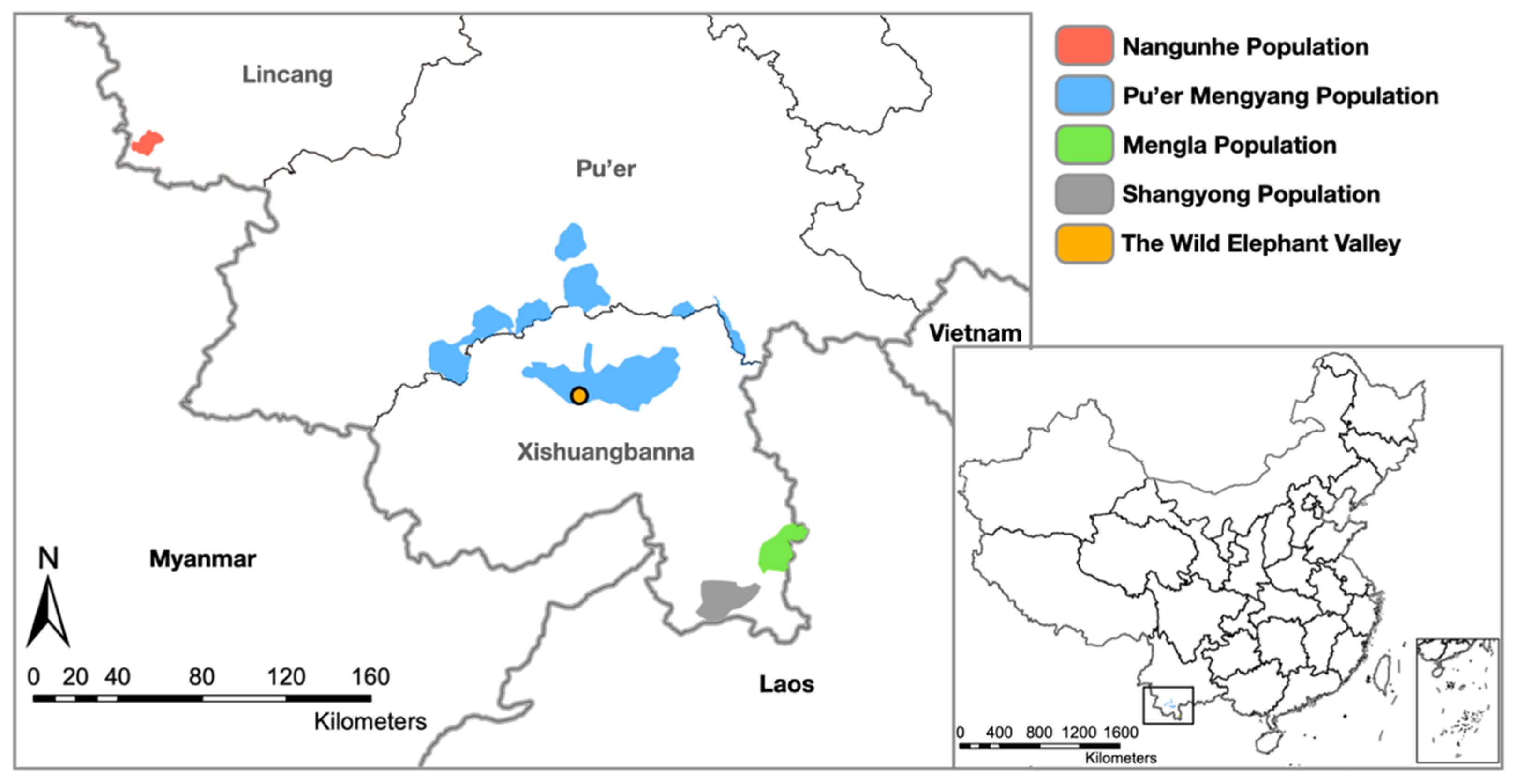
2.2. Investigation of EEHV Presence in China
2.2.1. Sample Collection
- Captive Populations
- Wild Populations
2.2.2. DNA Extraction
2.2.3. PCR Screening for EEHV DNA
- Analytical sensitivity of the PCR assay
- Inhibitor test for the fecal DNA samples
2.2.4. Age and Sex Differences
2.2.5. Phylogenetic Analysis
3. Results
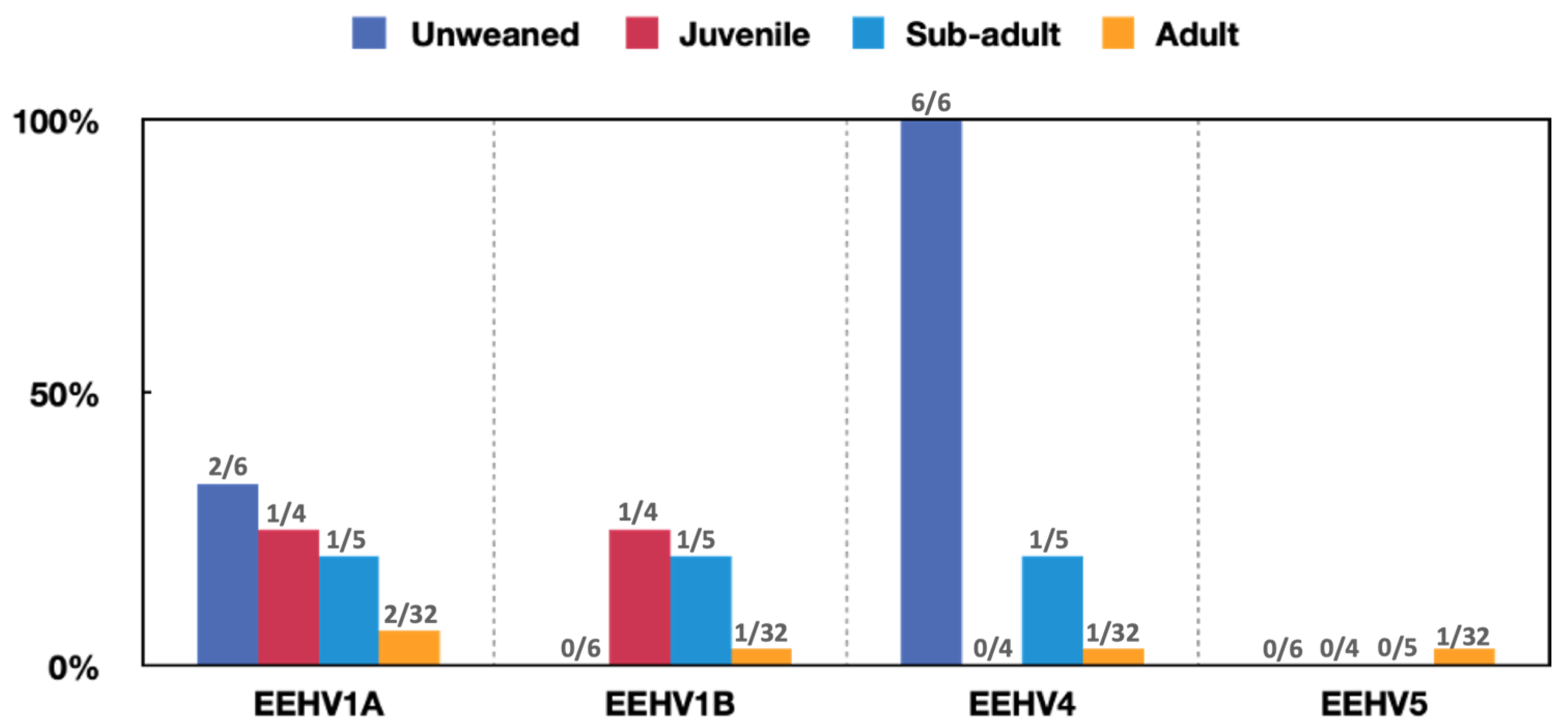
3.1. Number of EEHV-Infected Elephants and EEHVs Detected
3.1.1. Detection of EEHV in Different Sample Types of Wild Elephant Valley Elephants
3.1.2. Detection of EEHV in Fecal Samples from Captive and Wild Elephants
- Wild Populations
- Captive populations
- Analytical sensitivity of the PCR assay and prevalence of inhibitor samples from the Wild Elephant Valley, zoo, and wild elephants
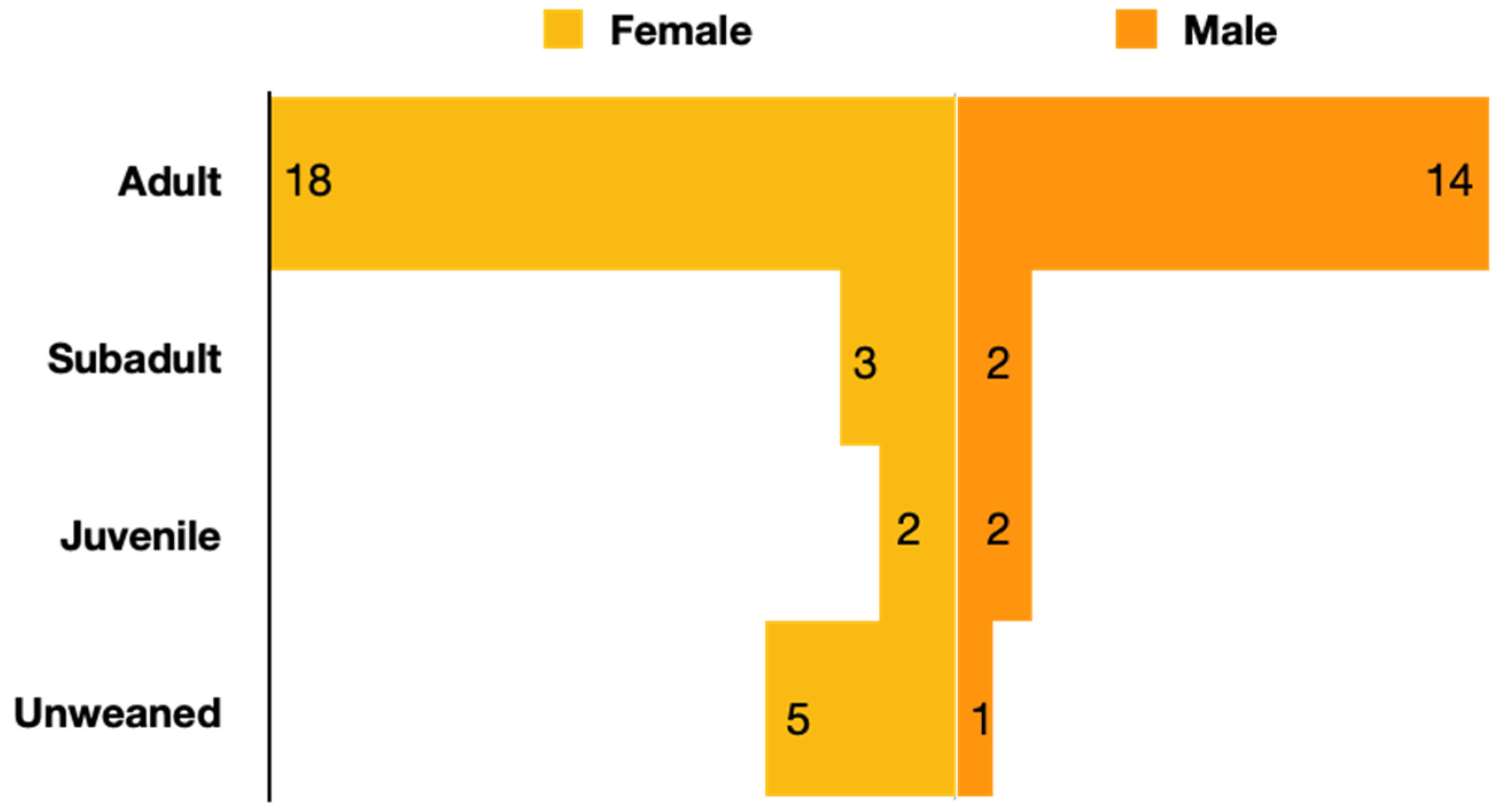
3.2. Age and Sex Differences
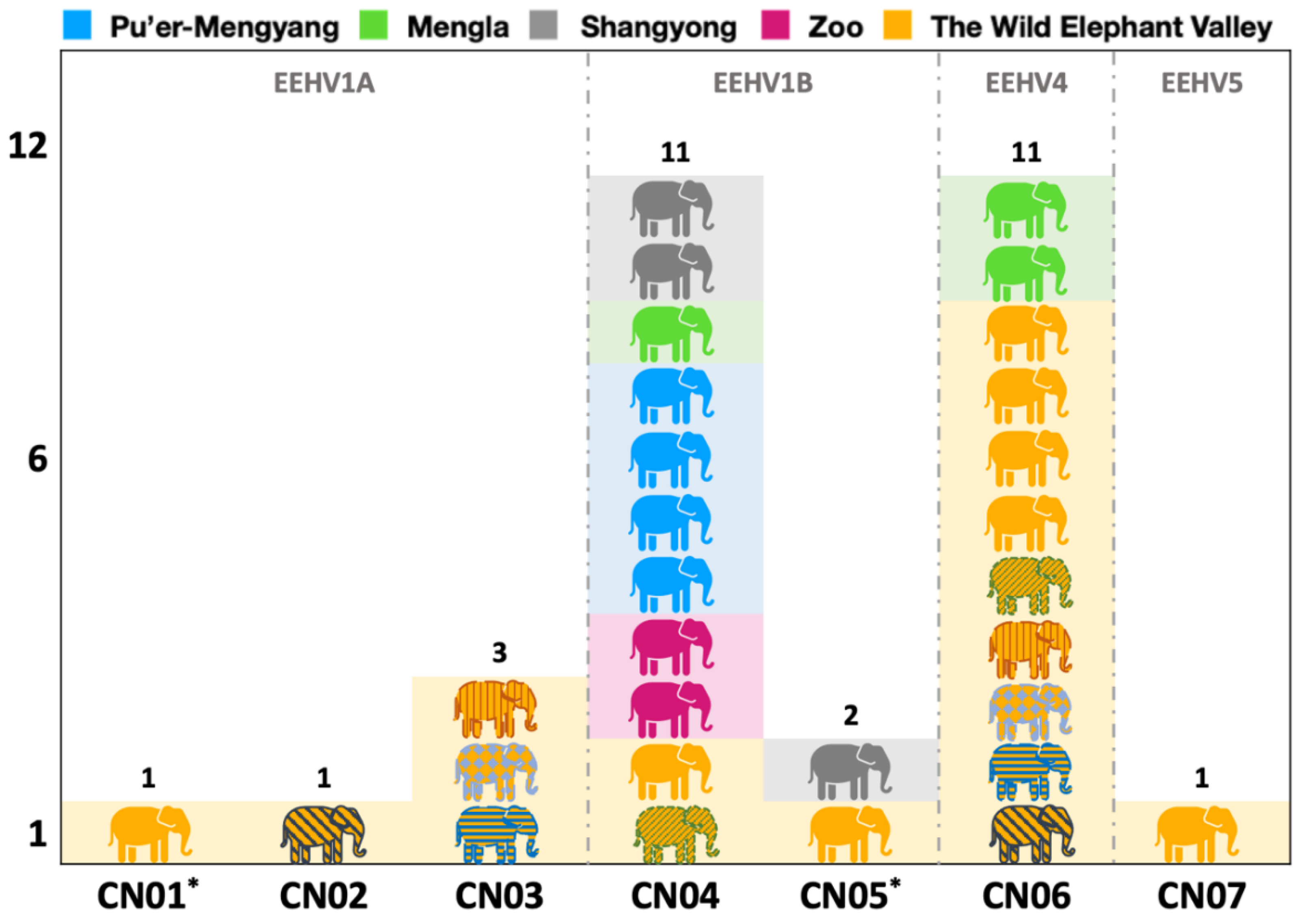
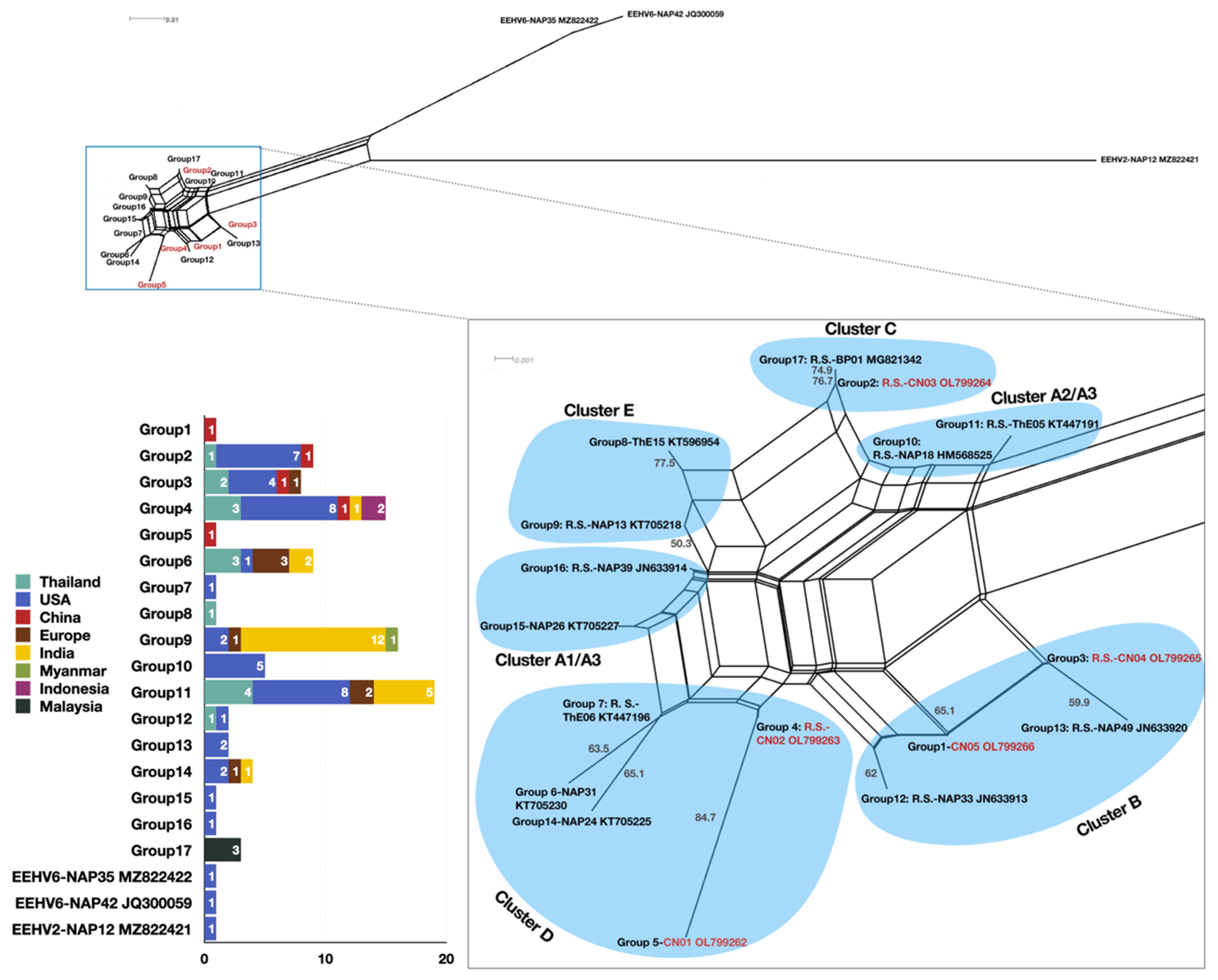
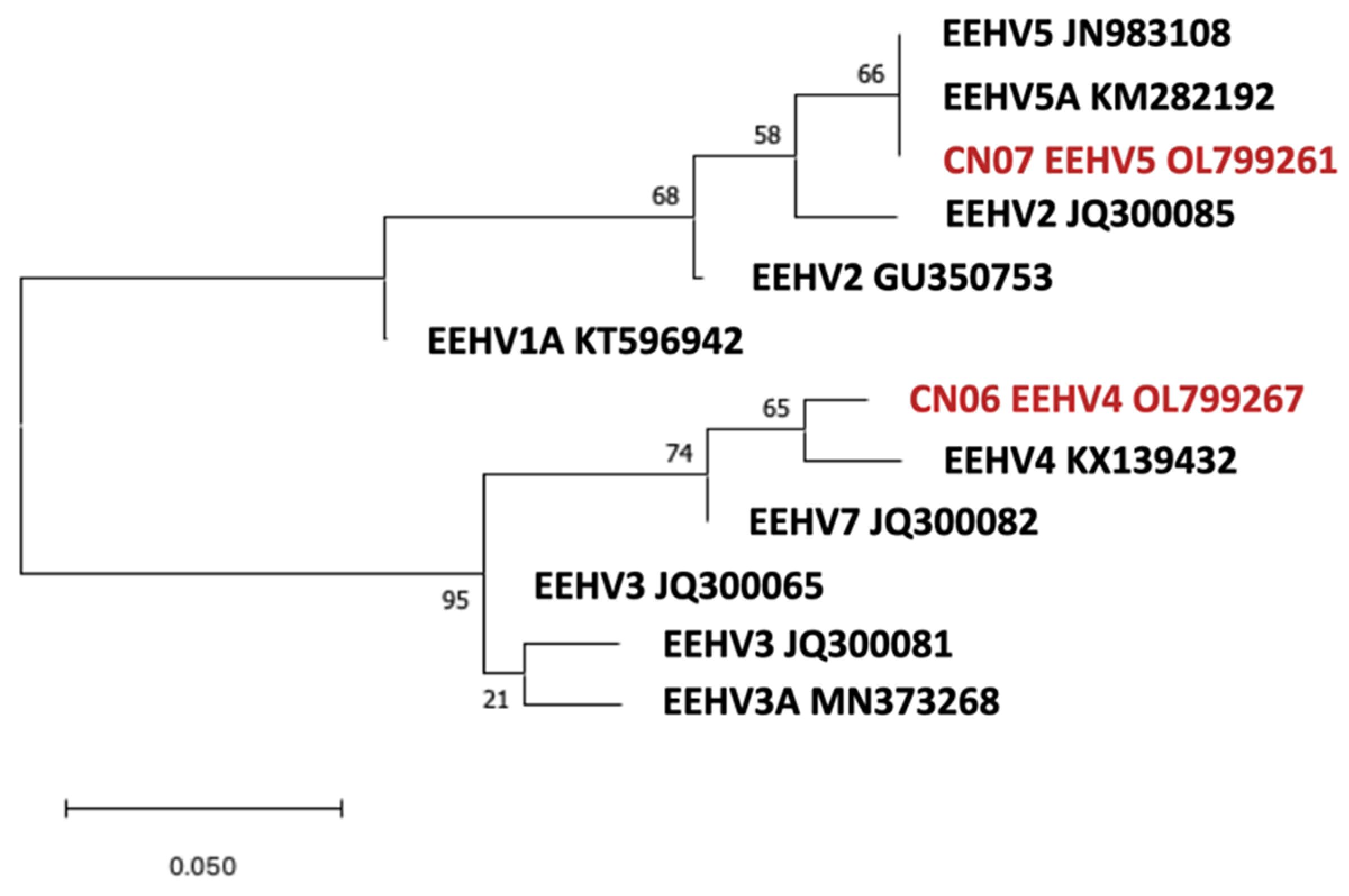
3.3. Phylogenetic Analysis
4. Discussion
4.1. Types, Subtypes, and Strains Identified and the Potential Implications for the Occurrence of EEHV-HD
4.2. Using Multiple Sampling Sites Improved the Detection Rate of EEHV Infections and Types
4.3. Impact of Age on Prevalence of Active EEHV Infections
4.4. Possible Explanations for Variations in Fecal EEHV Prevalence in the Three Elephant Populations Studied
4.5. Molecular versus Serological Screening of Asian Elephants for EEHV
4.6. Implications of These Findings for Asian Elephants in China
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Description | Sequence (5′-3′) | Pairing and Product Size (bp) | ||||
|---|---|---|---|---|---|---|
| Round | ||||||
| 1 | 2A | 2B | 3 | |||
| PANEEHV POL | ||||||
| 1. | 6710 | –ACAAACACGCTGTCRGTRTCYCCRTA– | 500 | 250 | ||
| 2. | 6711 | –GTATTTGATTTYGCNAGYYTGTAYCC– | ||||
| 3. | 6712 | –TGYAAYGCCGTNTAYGGATTYACCGG– | 250 | |||
| EEHV1 POL | ||||||
| 1. | 7445 | –GATTTTGCGAGYCTGTACCY– | 530 | 500 | ||
| 2. | 7446 | –CACGCTGTCAGTATCTCCGTA– | 500 | |||
| 3. | 7447 | –CCCAGTATCATTCAAGCATAC– | 480 | |||
| 4. | 7448 | –CTGTCTACAGGGCARTCAAC– | 500 | |||
| EEHV1 vGPCR | ||||||
| 1. | 7506 | –GATTGTGAACGCTGTATGTC– | 930 | 880 | ||
| 2. | 4963 | –GACTTTCTTCGTCGTAGCCCTCGTCTT– | 700 | |||
| 3. | 7470 | –GGTGGTACTGTATGATGTGC– | 550 | |||
| 4. | 5200 | –CGTGATACGCTTCCAAACATACA– | 880 | |||
| EEHV3/4 POL | ||||||
| 1. | 6719 | –CGTTGAAGGTGTCGCAGAT– | 390 | 250 | ||
| 2. | 7400 | –CAGCATCATCCAGGCCTACAAC– | ||||
| 3. | 6720 | –ATCCTGGCGCAGCTGCTGAC– | 250 | |||
| EEHV5 POL | ||||||
| 1. | 7483 | –CTACATCTATACAGAACTTTCC– | 450 | 200 | ||
| 2. | 7484 | –GTACCTTAGTTACGGACGAGAC– | 430 | |||
| 3. | 7424 | –CGACAGCACACTCCTTTAACAC– | 190 | |||
| 4. | 7485 | –CGCTGTATATGGATTTACCGG– | 200 | |||
Appendix B
References
- Mahato, G.; Sarma, K.K.; Pathak, D.C.; Barman, N.N.; Gogoi, P.; Dutta, M.; Basumatary, P. Endotheliotropic herpesvirus infection in Asian elephants (Elephas maximus) of Assam, India. Vet. World 2019, 12, 1790–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oo, Z.M.; Aung, Y.H.; Aung, T.T.; San, N.; Tun, Z.M.; Hayward, G.S.; Zachariah, A. Elephant Endotheliotropic Herpesvirus Hemorrhagic Disease in Asian Elephant Calves in Logging Camps, Myanmar. Emerg. Infect. Dis. 2020, 26, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-H.; Nathan, S.K.S.S.; Benedict, L.; Nagalingam, P.; Latimer, E.; Hughes, T.; Ramirez, D.; Sukor, J.R.A. The first reported cases of elephant endotheliotropic herpesvirus infectious haemorrhagic disease in Malaysia: Case report. Virol. J. 2021, 18, 231. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.L.; Kristensen, A.T.; Bertelsen, M.F.; Denk, D. Retrospective review of 27 European cases of fatal elephant endotheliotropic herpesvirus-haemorrhagic disease reveals evidence of disseminated intravascular coagulation. Sci. Rep. 2021, 11, 14173. [Google Scholar] [CrossRef] [PubMed]
- Sripiboon, S.; Tankaew, P.; Lungka, G.; Thitaram, C. The occurrence of elephant endotheliotropic herpesvirus in captive Asian elephants (Elephas maximus): First case of EEHV4 in Asia. J. Zoo Wildl. Med. 2013, 44, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Richman, L.K.; Zong, J.-C.; Latimer, E.M.; Lock, J.; Fleischer, R.C.; Heaggans, S.Y.; Hayward, G.S. Elephant Endotheliotropic Herpesviruses EEHV1A, EEHV1B, and EEHV2 from Cases of Hemorrhagic Disease Are Highly Diverged from Other Mammalian Herpesviruses and May Form a New Subfamily. J. Virol. 2014, 88, 13523–13546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, J.-C.; Latimer, E.M.; Long, S.Y.; Richman, L.K.; Heaggans, S.Y.; Hayward, G.S. Comparative Genome Analysis of Four Elephant Endotheliotropic Herpesviruses, EEHV3, EEHV4, EEHV5, and EEHV6, from Cases of Hemorrhagic Disease or Viremia. J. Virol. 2014, 88, 13547–13569. [Google Scholar] [CrossRef] [Green Version]
- Long, S.Y.; Latimer, E.M.; Hayward, G.S. Review of Elephant Endotheliotropic Herpesviruses and Acute Hemorrhagic Disease. ILAR J. 2016, 56, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Richman, L.K.; Montali, R.J.; Garber, R.L.; Kennedy, M.A.; Lehnhardt, J.; Hildebrandt, T.; Schmitt, D.; Hardy, D.; Alcendor, D.J.; Hayward, G.S. Novel Endotheliotropic Herpesviruses Fatal for Asian and African Elephants. Science 1999, 283, 1171–1176. [Google Scholar] [CrossRef]
- Hayward, G.S. Conservation: Clarifying the risk from herpesvirus to captive Asian elephants. Vet. Rec. 2012, 170, 202–203. [Google Scholar] [CrossRef] [Green Version]
- Boonprasert, K.; Punyapornwithaya, V.; Tankaew, P.; Angkawanish, T.; Sriphiboon, S.; Titharam, C.; Brown, J.L.; Somgird, C. Survival analysis of confirmed elephant endotheliotropic herpes virus cases in Thailand from 2006–2018. PLoS ONE 2019, 14, e0219288. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.L.; Saxmose Nielsen, S.; Martinussen, T.; Bertelsen, M.F. Quantification and risk factor analysis of elephant endotheliotropic herpesvirus-haemorrhagic disease fatalities in Asian elephants (Elephas maximus) in Europe (1985–2017). J. Zoo Aquar. Res. 2021, 9, 8–13. [Google Scholar]
- Bouchard, B.; Xaymountry, B.; Thongtip, N.; Lertwatcharasarakul, P.; Wajjwalku, W. First reported case of elephant endotheliotropic herpes virus infection in Laos. J. Zoo Wildl. Med. 2014, 45, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, A.; PK, S.; Santhosh, S.; Bathrachalam, C.; Megha, M.; Pandiyan, J.; Jishnu, M.; Kobragade, R.; Long, S.; Zong, J.-C.; et al. Extended genotypic evaluation and comparison of twenty-two cases of lethal EEHV1 hemorrhagic disease in wild and captive Asian elephants in India. PLoS ONE 2018, 13, e0202438. [Google Scholar] [CrossRef] [Green Version]
- Prompiram, P.; Wiriyarat, W.; Bhusri, B.; Paungpin, W.; Jairak, W.; Sripiboon, S.; Wongtawan, T. The occurrence of elephant endotheliotropic herpesvirus infection in wild and captive Asian elephants in Thailand: Investigation based on viral DNA and host antibody. Vet. World 2021, 14, 545–550. [Google Scholar] [CrossRef]
- Zachariah, A.; Zong, J.-C.; Long, S.Y.; Latimer, E.M.; Heaggans, S.Y.; Richman, L.K.; Hayward, G.S. Fatal herpesvirus hemorrhagic disease in wild and orphan asian elephants in southern India. J. Wildl. Dis. 2013, 49, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Grenus, B.G.; Latimer, E.; Cullinane, A.; Lyons, P.; Creighton, G.; Nutter, F.B. Evaluation of the Efficacy of Two Different Sampling Sites for the Detection of Elephant Endotheliotropic Herpesvirus (EEHV) in Three Asian Elephants (Elephas Maximus) in Ireland. J. Zoo Wildl. Med. 2020, 51, 303–307. [Google Scholar] [CrossRef]
- Bhusri, B.; Suksai, P.; Mongkolphan, C.; Tiyanun, E.; Ratanakorn, P.; Chaichoun, K.; Sariya, L. Detection of elephant endotheliotropic herpesvirus 4 in captive asian elephants (Elephas maximus) in Thailand. Thai J. Vet. Med. 2017, 47, 97–102. [Google Scholar]
- Sripiboon, S.; Ditcham, W.; Vaughan-Higgins, R.; Jackson, B.; Robertson, I.; Thitaram, C.; Angkawanish, T.; Phatthanakunanan, S.; Lertwatcharasarakul, P.; Warren, K. Subclinical infection of captive Asian elephants (Elephas maximus) in Thailand with elephant endotheliotropic herpesvirus. Arch. Virol. 2020, 165, 397–401. [Google Scholar] [CrossRef]
- Luz, S.; Howard, L. Elephant Endotheliotropic Herpesvirus (EEHV) in Asia—Recommendations from the 1st Asian EEHV Strategy Meeting; Wildlife Reserves Singapore Group: Singapore, 2017. [Google Scholar]
- Fuery, A.; Pursell, T.; Tan, J.; Peng, R.; Burbelo, P.D.; Hayward, G.S.; Ling, P.D. Lethal Hemorrhagic Disease and Clinical Illness Associated with Elephant Endotheliotropic Herpesvirus 1 Are Caused by Primary Infection: Implications for the Detection of Diagnostic Proteins. J. Virol. 2020, 94, e01528-19. [Google Scholar] [CrossRef] [Green Version]
- Bennett, L.; Dunham, S.; Yon, L.; Chapman, S.; Kenaghan, M.; Purdie, L.; Tarlinton, R. Longitudinal study of Asian elephants, Elephas maximus, indicates intermittent shedding of elephant endotheliotropic herpesvirus 1 during pregnancy. Vet. Rec. Open 2015, 2, e000088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Doel, P.B.; Prieto, V.R.; Van Rossum-Fikkert, S.E.; Schaftenaar, W.; Latimer, E.; Howard, L.; Chapman, S.; Masters, N.; Osterhaus, A.D.M.E.; Ling, P.D.; et al. A novel antigen capture ELISA for the specific detection of IgG antibodies to elephant endotheliotropic herpes virus. BMC Vet. Res. 2015, 11, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, K.L.; Latimer, E.; Finnegan, M. Long-term, intermittent, low-level elephant endotheliotropic herpesvirus 1A viremia in a captive Asian elephant calf. J. Vet. Diagn. Investig. 2018, 30, 917–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, M.; Hatt, J.-M.; Schetle, N.; Steinmetz, H. Identification of shedders of elephant endotheliotropic herpesviruses among Asian elephants (Elephas maximus) in Switzerland. PLoS ONE 2017, 12, e0176891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angkawanish, T.; Nielen, M.; Vernooij, H.; Brown, J.L.; Van Kooten, P.J.S.; Van den Doel, P.B.; Schaftenaar, W.; Na Lampang, K.; Rutten, V.P.M.G. Evidence of high EEHV antibody seroprevalence and spatial variation among captive Asian elephants (Elephas maximus) in Thailand. Virol. J. 2019, 16, 33. [Google Scholar] [CrossRef]
- Hoornweg, T.; Schaftenaar, W.; Maurer, G.; Van den Doel, P.; Molenaar, F.; Chamouard-Galante, A.; Vercammen, F.; Rutten, V.P.M.G.; Haan, C. Elephant Endotheliotropic Herpesvirus Is Omnipresent in Elephants in European Zoos and an Asian Elephant Range Country. Viruses 2021, 13, 283. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.; Lin, L.; Feng, L.M.; Yan, F.; Wang, L.X.; Guo, X.M.; Luo, A.D. Asian Elephants in China: Estimating Population Size and Evaluating Habitat Suitability. PLoS ONE 2015, 10, e0124834. [Google Scholar] [CrossRef] [Green Version]
- Stanton, J.J.; Zong, J.-C.; Latimer, E.; Tan, J.; Herron, A.; Hayward, G.S.; Ling, P.D. Detection of pathogenic elephant endotheliotropic herpesvirus in routine trunk washes from healthy adult Asian elephants (Elephas maximus) by use of a real-time quantitative polymerase chain reaction assay. Am. J. Vet. Res. 2010, 71, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, A.; Evans, T.; Molter, C.; Howard, L.; Ling, P.; Goldstein, T.; Gilardi, K. Noninvasive Sampling for Detection of Elephant Endotheliotropic Herpesvirus and Genomic DNA in Asian (Elephas maximus) and African (Loxodonta africana) Elephants. J. Zoo Wildl. Med. 2020, 51, 433. [Google Scholar] [CrossRef]
- Kochagul, V.; Srivorakul, S.; Boonsri, K.; Somgird, C.; Sthitmatee, N.; Thitaram, C.; Pringproa, K. Production of antibody against elephant endotheliotropic herpesvirus (EEHV) unveils tissue tropisms and routes of viral transmission in EEHV-infected Asian elephants. Sci. Rep. 2018, 8, 4675. [Google Scholar] [CrossRef]
- Kochakul, V.; Boonsri, K.; Tiwananthagorn, S.; Somgird, C.; Thitaram, C.; Pringproa, K. Development of in situ hybridization for detection of elephant endotheliotropic herpesvirus in Asian elephants. J. Vet. Diagn. Investig. 2018, 30, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Common, S.; Yun, Y.; Silva-Fletcher, A.; Thitaram, C.; Janyamethakul, T.; Khammesri, S.; Molenaar, F. Developing a non-invasive method of detecting elephant endotheliotropic herpesvirus infections using faecal samples. Vet. Rec. 2021, 190, e833. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, C.; Sukumar, R. Constructing Age Structures of Asian Elephant Populations: A Comparison of Two Field Methods of Age Estimation. Gajah 2008, 29, 11–16. [Google Scholar]
- Zhu, D. Genetic Diversity and Kinship Analysis of Asian Elephant (Elephas maximus) in Xishuangbanan; Beijing Normal University: Beijing, China, 2019. [Google Scholar]
- Von Bertalanffy, L. A Quantitative Theory of Organic Growth (Inquiries on Growth Laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Reilly, J. Growth in the Sumatran elephant (Elephas maximus sumatranus) and age estimation based on dung diameter. J. Zool. 2002, 258, 205–213. [Google Scholar] [CrossRef]
- Latimer, E.; Zong, J.-C.; Heaggans, S.Y.; Richman, L.K.; Hayward, G.S. Detection and evaluation of novel herpesviruses in routine and pathological samples from Asian and African elephants: Identification of two new probosciviruses (EEHV5 and EEHV6) and two new gammaherpesviruses (EGHV3B and EGHV5). Vet. Microbiol. 2011, 147, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, J.I.E. Chapter 13—Hypergeometric Distribution. In Biostatistics for Medical and Biomedical Practitioners; Hoffman, J.I.E., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 179–182. [Google Scholar]
- Microsoft. Microsoft Excel for Mac; Microsoft: Redmond, WA, USA, 2021. [Google Scholar]
- National Library of Medicine (US); National Center for Biotechnology Information, GenBank. 2021. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 5 December 2021).
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2005, 23, 254–267. [Google Scholar] [CrossRef]
- Sripiboon, S.; Jackson, B.; Ditcham, W.; Holyoake, C.; Robertson, I.; Thitaram, C.; Tankaew, P.; Letwatcharasarakul, P.; Warren, K. Molecular characterisation and genetic variation of Elephant Endotheliotropic Herpesvirus infection in captive young Asian elephants in Thailand. Infect. Genet. Evol. 2016, 44, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Khammesri, S.; Ampasavate, C.; Hongwiset, D.; Mektrirat, R.; Sangsrijan, S.; Brown, J.; Thitaram, C. Pharmacokinetics and Analytical Determination of Acyclovir in Asian Elephant Calves (Elephas maximus). Vet. Anim. Sci. 2021, 15, 100227. [Google Scholar] [CrossRef]
- Sripiboon, S.; Angkawanish, T.; Boonprasert, K.; Sombutputorn, P.; Langkaphin, W.; Ditcham, W.; Warren, K. Successful treatment of a clinical elephant endotheliotropic herpesvirus infection: The dynamics of viral load, genotype analysis, and treatment with acyclovir. J. Zoo Wildl. Med. 2017, 48, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- He, C.H.; Du, J.J.; Zhu, D.; Zhang, L. Population viability analysis of small population: A case study for Asian elephant in China. Integr. Zool. 2020, 15, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Latimer, E.; Siegal-Willott, J.; Kiso, W.; Padilla, L.; Sanchez, C.; Schmitt, D.; Brown, J. Patterns of serum immune biomarkers during elephant endotheliotropic herpesvirus viremia in Asian and African elephants. PLoS ONE 2021, 16, e0252175. [Google Scholar] [CrossRef] [PubMed]
| Sampling Size Per Sample Type | Total Number of Sampled Individuals | Estimated or Actual Population | |||||
|---|---|---|---|---|---|---|---|
| Feces | Blood | Trunk Swab | Oral Swab | ||||
| Wild (n = 126) | Pu’er-Mengyang | 81 | NA | NA | NA | 81 | ~140 |
| Mengla | 26 | NA | NA | NA | 26 | ~45 | |
| Shangyong | 19 | NA | NA | NA | 19 | ~65 | |
| Captive (n = 202) | Zoo | 155 | NA | NA | NA | 155 | ~270 |
| Wild Elephant Valley | 44 | 40 | 47 | 47 | 47 | 47 | |
| Age a | Sex b | Origin c | Feces d | Blood d | Trunk Swab d | Oral Swab d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EEHV1 | EEHV4 | EEHV5 | EEHV1 | EEHV4 | EEHV5 | EEHV1 | EEHV4 | EEHV5 | EEHV1 | EEHV4 | EEHV5 | |||||
| 1 | V10 | 30 y | M | TL | − | − | − | − | − | − | − | − | + | − | − | + |
| 2 | V15 | 21 y | M | TL | − | − | − | 1A | − | − | 1A | − | − | − | − | − |
| 3 | V31 | 14 y | M | R | − | − | − | 1B | − | − | − | − | − | − | − | − |
| 4 | V33 | 5 y | M | R | − | − | − | 1A | − | − | − | − | − | − | − | − |
| 5 | V34 | 4 y | F | Cb | NA | NA | NA | 1B | − | − | − | − | − | − | − | − |
| 6 | V36 | 2 y | F | Cb | − | + | − | − | − | − | − | + | − | − | + | − |
| 7 | V37 | 2 y | F | Cb | − | + | − | 1 A | − | − | − | − | − | − | − | − |
| 8 | V38 | 14 m | M | LAO | − | + | − | NA | NA | NA | − | − | − | − | + | − |
| 9 | V39 | 17 m | F | Cb | − | + | − | NA | NA | NA | − | + | − | − | − | − |
| 10 | V41 | 5 m | F | Cb | NA | NA | NA | NA | NA | NA | − | − | − | − | + | − |
| 11 | V42 | 2 m | F | Cb | NA | NA | NA | NA | NA | NA | 1A | − | − | − | + | − |
| 12 | V43 | 36 y | F | MM | − | − | − | − | − | − | 1B | − | − | 1B | + | − |
| 13 | V44 | 23 y | F | MM | − | − | − | − | − | − | − | + | − | 1A | − | − |
| 14 | V45 | 8 y | F | Cb | − | − | − | − | − | − | 1A | − | − | − | + | − |
| Wild/Captive Number of Elephants Sampled | Sample Number | Age | Sex | Origin | Year of Import or Rescue | EEHV1 | EEHV4 | EEHV5 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | ||||||||||
| 1 | Wild (n = 126) | W025 | Sub-adult | NA * | Pu’er-Mengyang | NA | − $ | CN04 | − | − | |
| 2 | W029 | Adult | NA | Pu’er-Mengyang | NA | − | CN04 | − | − | ||
| 3 | W030 | Sub-adult | NA | Pu’er-Mengyang | NA | − | CN04 | − | − | ||
| 4 | W032 | Sub-adult | NA | Pu’er-Mengyang | NA | − | CN04 | − | − | ||
| 5 | W106 | Sub-adult | NA | Shangyong | NA | − | CN04 | − | − | ||
| 6 | W113 | Sub-adult | NA | Shangyong | NA | − | CN04 | − | − | ||
| 7 | W118 | Sub-adult | NA | Shangyong | NA | − | CN04 | − | − | ||
| 8 | W119 | Sub-adult | NA | Mengla | NA | − | − | CN06 | − | ||
| 9 | W120 | Sub-adult | NA | Mengla | NA | − | − | CN06 | − | ||
| 10 | W121 | Sub-adult | NA | Mengla | NA | − | CN05 | − | − | ||
| 11 | Captive (n = 199) | Zoo (n = 155) | Z054 | Adult | F | Myanmar | 1996 | − | CN04 | − | − |
| 12 | Z196 | Sub-adult | M | Laos | 2016 | − | CN04 | − | − | ||
| 13 | Wild Elephant Valley (n = 44) | V36 | Unweaned | F | Captive bred | NA | − | − | CN06 | − | |
| 14 | V37 | Unweaned | F | Captive bred | NA | − | − | CN06 | − | ||
| 15 | V38 | Unweaned | M | Laos | 2019 | − | − | CN06 | − | ||
| 16 | V39 | Unweaned | F | Captive bred | NA | − | − | CN06 | − | ||
| Strain Name | Virus Types | Positive Elephants in This Study | Presence in Other Countries | ||||
|---|---|---|---|---|---|---|---|
| Country | Strain Name | Isolation Date | Origin | GenBank Accession | |||
| CN01 | EEHV1A | V33 | Novel Sequence | ||||
| CN02 | EEHV1A | V37 | Thailand | ThE03 | 2008 | necropsy tissue from 26 month old captive-born male elephant with fatal acute hemorrhagic disease | KT390759 |
| Thailand | ThE07 | 2012 | necropsy tissue from a 2.4 year old captive-born female elephant with fatal acute hemorrhagic disease | KT447201 | |||
| USA | NAP17 | 2000 | necropsy tissue from a 6 year old captive-born female elephant with fatal acute hemorrhagic disease | KT705221 | |||
| USA | NAP79 | 2016 | whole blood from a 2 year old female captive elephant with acute hemorrhagic disease | MK473327 | |||
| CN03 | EEHV1A | V42, V44, V45 | USA | NAP29 | 2007 | necropsy tissue from a 16 month old captive-born female elephant with fatal acute hemorrhagic disease | KT705228 |
| CN04 | EEHV1B | V34, V43, Z054, Z196, W025, W029, W030, W032, W113, W116, W118 | Thailand | ThE04 | 2009 | necropsy tissue from a 2 year old captive-born female elephant with fatal acute hemorrhagic disease | KT447186 |
| USA | NAP38 | 2009 | routine trunk washes from healthy adult Asian elephants | GU350763 | |||
| CN05 | EEHV1B | V31, W121 | Novel Sequence | ||||
| CN06 | EEHV4 | V36, V37, V38, V39, V41, V42, V43, V44, V45, W119, W120 | Thailand | ThE17 | 2014 | necropsy tissue from a 3.7 year old captive-born female elephant with fatal acute hemorrhagic disease | KT596956 |
| USA | NAP69 | 2014.08 | trunk wash from a convalescing 4.5 year old captive-born male elephant | KR781027 | |||
| USA | NAP70 | 2014.10 | trunk wash from a convalescing 4 year old captive-born female elephant | KR781034 | |||
| CN07 | EEHV5 | V10 | USA | NAP50 | 2011 | viremic blood from a 41 year old wild-born female elephant with mild systemic infection | JN983108 |
| USA | NAP51WB | 2011 | whole blood from an asymptomatic 4 month old captive-born female elephant | JX011010 | |||
| USA | NAP52WB | 2011 | whole blood from a 9 month old captive-born male elephant with mild disease symptoms | JX011011 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Bao, M.; Zhu, B.; Shen, Q.; Guo, X.; Li, W.; Tang, R.; Zhu, D.; Tang, Y.; Phalen, D.N.; et al. Elephant Endotheliotropic Herpesvirus 1, 4 and 5 in China: Occurrence in Multiple Sample Types and Implications for Wild and Captive Population Surveillance. Viruses 2022, 14, 411. https://doi.org/10.3390/v14020411
Yang N, Bao M, Zhu B, Shen Q, Guo X, Li W, Tang R, Zhu D, Tang Y, Phalen DN, et al. Elephant Endotheliotropic Herpesvirus 1, 4 and 5 in China: Occurrence in Multiple Sample Types and Implications for Wild and Captive Population Surveillance. Viruses. 2022; 14(2):411. https://doi.org/10.3390/v14020411
Chicago/Turabian StyleYang, Nian, Mingwei Bao, Biru Zhu, Qingzhong Shen, Xianming Guo, Wenwen Li, Ruchun Tang, Di Zhu, Yinpu Tang, David N. Phalen, and et al. 2022. "Elephant Endotheliotropic Herpesvirus 1, 4 and 5 in China: Occurrence in Multiple Sample Types and Implications for Wild and Captive Population Surveillance" Viruses 14, no. 2: 411. https://doi.org/10.3390/v14020411
APA StyleYang, N., Bao, M., Zhu, B., Shen, Q., Guo, X., Li, W., Tang, R., Zhu, D., Tang, Y., Phalen, D. N., & Zhang, L. (2022). Elephant Endotheliotropic Herpesvirus 1, 4 and 5 in China: Occurrence in Multiple Sample Types and Implications for Wild and Captive Population Surveillance. Viruses, 14(2), 411. https://doi.org/10.3390/v14020411







