Acyl-Coa Thioesterases: A Rheostat That Controls Activated Fatty Acids Modulates Dengue Virus Serotype 2 Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. siRNA Transfection and Knockdown Confirmation
2.3. RNA Extraction and qRT-PCR
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
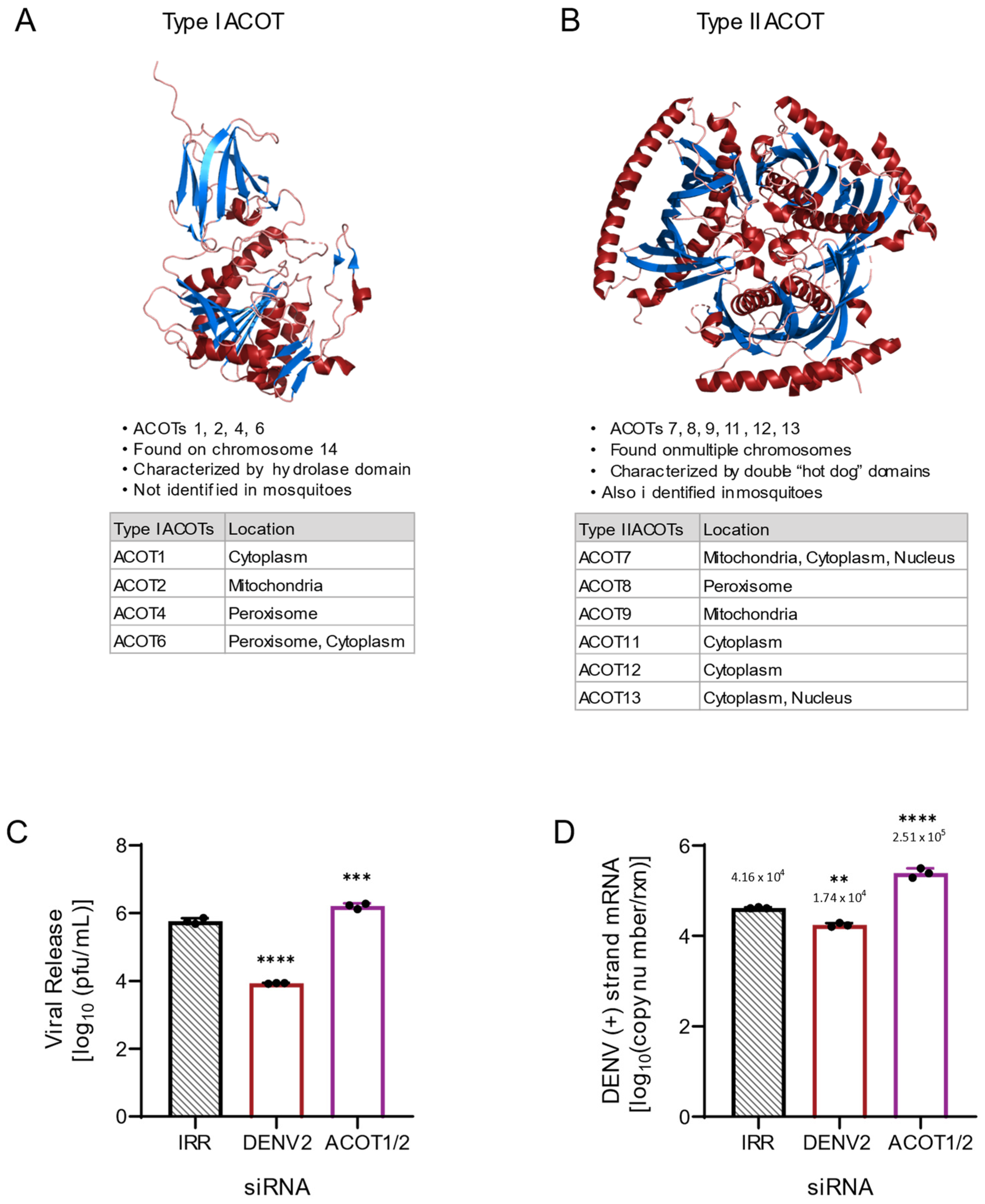
3.1. Combined Loss of ACOT1 and ACOT2 Function Increases DENV2 Genome Replication and Infectious Particle Release
3.2. Loss of ACOT2 Function Reduces DENV2 Replication, Infectious Particle Release and Infectivity
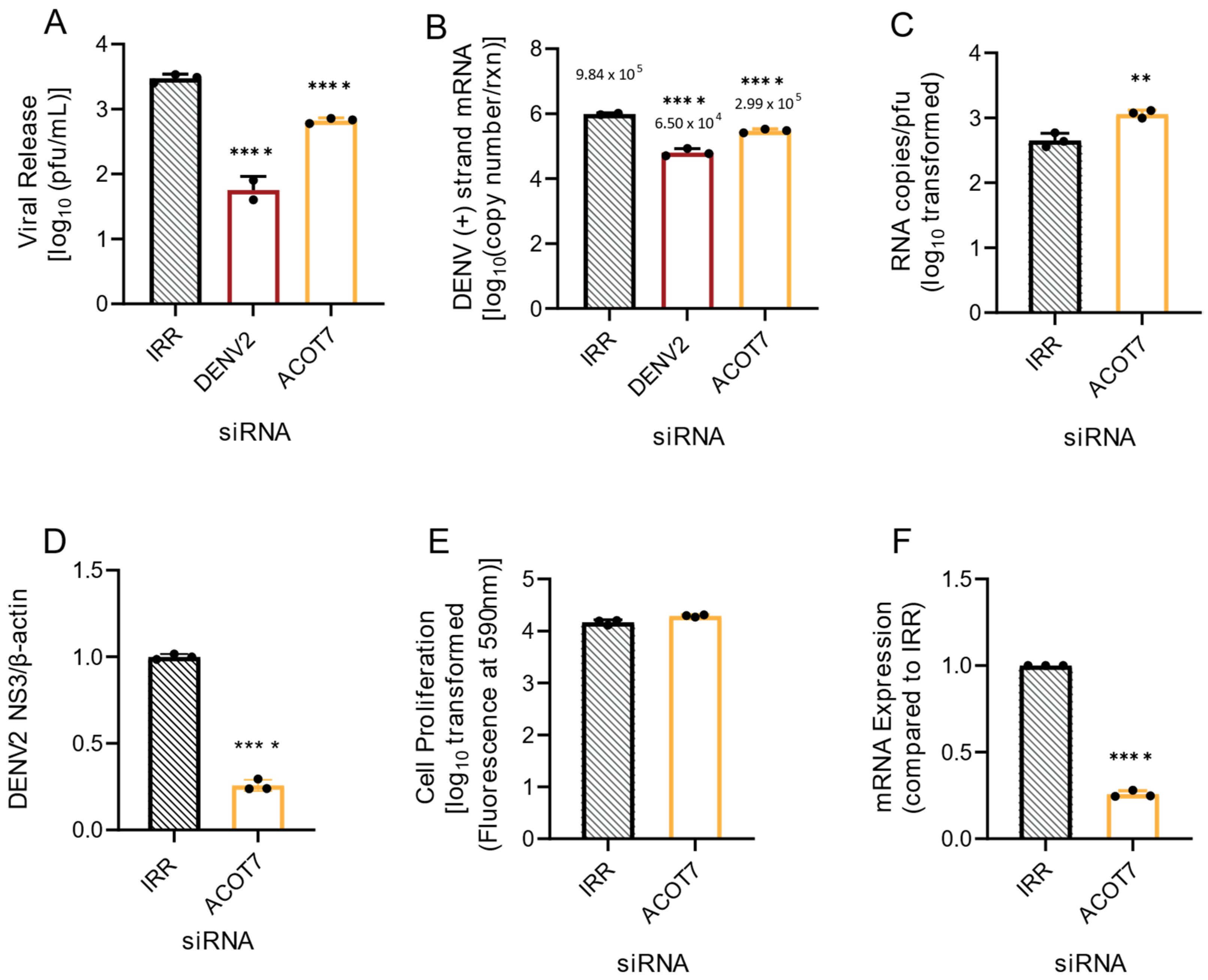
3.3. Mitochondrial ACOTs Are Vital for Productive DENV2 Infection
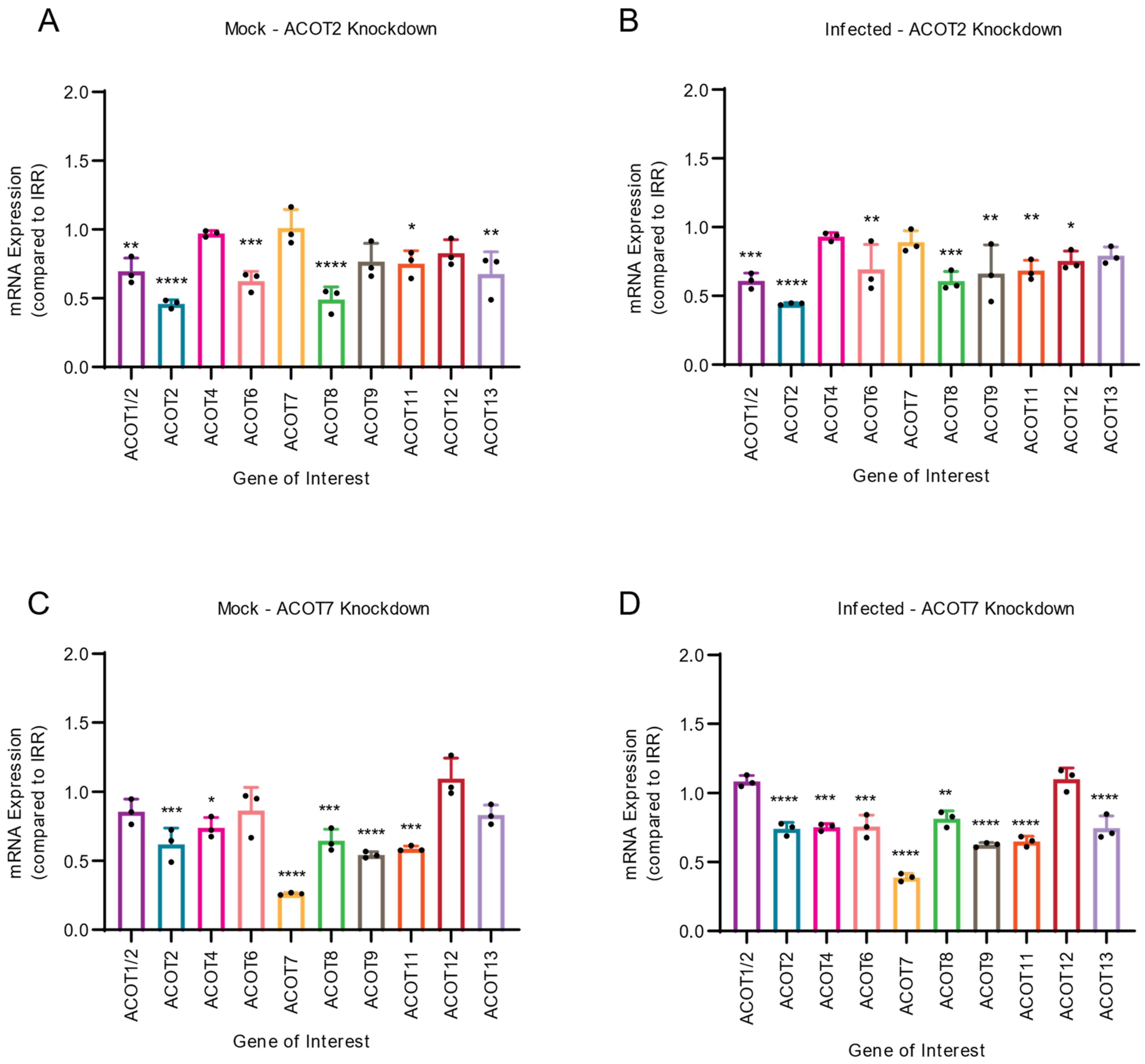
3.4. Both Type I and Type II ACOTs Are Differentially Expressed upon ACOT2 or ACOT7 Knockdown
3.5. Mitochondrial ACOTs Are Upregulated at Early Timepoints of Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Halstead, S.B. Dengue antibody-dependent enhancement: Knowns and unknowns. Microbiol. Spectr. 2014, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Kuhn, R.J. Chapter 4. “Host metabolism and its contribution in flavivirus biogenesis”. In Arboviruses: Molecular Biology, Evolution and Control; Gubler, D., Vasilakis, N., Eds.; Caister Academic Press: Norfolk, UK, 2015; ISBN 978-191-019-021-0. [Google Scholar]
- Zaitseva, E.; Yang, S.T.; Melikov, K.; Pourmal, S.; Chernomordik, L.V. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 2010, 6, e10001131. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 2018, 16, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Chatel-Chaix, L.; Bartenschlager, R. Dengue virus- and hepatitis C virus-induced replication and assembly compartments: The enemy inside—Caught in the web. J. Virol. 2014, 88, 5907–5911. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.K.; Hoenen, A.; Morgan, G.; Mackenzie, J.M. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J. Virol. 2010, 84, 10438–10447. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Riley, C.; Issac, G.; Hopf-Jannasch, A.S.; Moore, R.J.; Weitz, K.W.; Pasa-Tolic, L.; Metz, T.O.; Adamec, J.; Kuhn, R.J. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012, 8, e1002584. [Google Scholar] [CrossRef]
- Chotiwan, N.; Andre, B.G.; Snachez-Vargus, I.; Grabowski, J.M.; Hopf-jannasch, A.; Hedrick, V.; Gough, E.; Nakayasu, E.; Blair, C.D.; Hill, C.A.; et al. Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathog. 2018, 14, e1006853. [Google Scholar] [CrossRef]
- Tillander, V.; Alexson, S.H.E.; Cohen, D.E. Deactivating fatty acids: Acyl-CoA thioesterase-mediated control of lipid metabolism. Trends Endocrinol. Metab. 2017, 28, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.C.; Siponen, M.I.; Alexson, S.E. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim. Biophys. Acta 2012, 1822, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Lee, H.C.; Hwang, H.J.; Park, H.A.; Moon, Y.; Kim, B.C.; Lee, H.M.; Kim, K.P.; Kim, Y.; Lee, B.L.; et al. Acyl-CoA thioesterase 7 is involved in cell cycle progression via regulation of PKCζ-p53-p21 signaling pathway. Cell Death Dis. 2017, 8, e2793. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, J.; Qiu, Z.; Ge, X.; Liu, X.; Zhang, C.; Xu, W.; Wang, F.; Hua, D.; Qi, X.; et al. ACOT1 expression is associated with poor prognosis in gastric adenocarcinoma. Hum. Pathol. 2018, 77, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M.; Wong, G.W.; Wolfgang, M.J. Acyl coenzyme A thioesterase 7 regulates neuronal fatty acid metabolism to prevent neurotoxicity. Mol. Cell Biol. 2013, 33, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, C.; Wang, H.; Rao, X.; Wang, F.; Duan, Q.; Chen, F.; Long, G.; Gong, W.; Zou, M.; et al. Protective effects of acyl-CoA thioesterase 1 on diabetic heart via PPARα/PGC1α signaling. PLoS ONE 2012, 7, e50376. [Google Scholar] [CrossRef] [PubMed]
- Blight, K.J.; McKeating, J.A.; Rice, C.M. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 2002, 76, 13001–13014. [Google Scholar] [CrossRef] [PubMed]
- Yoksan, S.; Bhamarapravati, N.; Halstead, S.B. Dengue virus vaccine development: Study on biological markers of uncloned dengue 1–4 viruses serially passaged in primary kidney cells. In Arbovirus Research in Australia, Proceedings of the Fourth Symposium; St George, T.D., Kay, B.H., Blok, J., Eds.; CSIRO/QIMR: Brisbane, Australia, 1986; pp. 35–38. [Google Scholar]
- Kinney, R.M.; Butrapet, S.; Chang, G.J.; Tsuchiya, K.R.; Roehrig, J.T.; Bhamarapravati, N.; Gubler, D.J. Construction of infectious cDNA clones for dengue 2 virus: Strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 1997, 230, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Dulbecco, R.; Vogt, M. Some problems of animal virology as studied by the plaque technique. Cold Spring Harb. Symp. Quant. Biol. 1953, 18, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Perera, R.; Berger, K.L.; Khadka, S.; Lacount, D.J.; Kuhn, R.J.; Randall, G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 17345–17350. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, R.C.; Steel, J.J.; Pujari, V.; Rovnak, J.; Crick, D.C.; Perera, R. Stearoyl-CoA desaturase 1 differentiates early and advanced dengue virus infections and determines virus particle infectivity. PLoS Pathog. 2018, 14, e1007261. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Celta C(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.P.; Sathyanarayan, A.; Mashek, D.G. Acyl-CoA thioesterase 1 (ACOT1) regulates PPARα to couple fatty acid flux with oxidative capacity during fasting. Diabetes 2017, 66, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.C.; Rautanen, A.; Westin, M.A.; Svensson, L.T.; Alexson, S.E. Analysis of the mouse and human acyl-CoA thioesterase (ACOT) gene clusters shows that convergent, functional evolution results in a reduced number of human peroxisomal ACOTs. FASEB J. 2006, 20, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.; Carpenter, C.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human acyl-CoA thioesterase gene (ACOT) family. Hum. Genom. 2010, 4, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.T.; Engberg, S.T.; Aoyama, T.; Usuda, N.; Alexson, S.E.; Hashimoto, T. Molecular cloning and characterization of a mitochondrial peroxisome proliferator-induced acyl-CoA thioesterase from rat liver. Biochem. J. 1998, 329 Pt 3, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Bekeova, C.; Anderson-Pullinger, L.; Boye, K.; Boos, F.; Sharpadskaya, Y.; Herrmann, J.M.; Seifert, E.L. Multiple mitochondrial thioesterases have distinct tissue and substrate specificity and CoA regulation, suggesting unique functional roles. J. Biol. Chem. 2019, 294, 19034–19047. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Randall, G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Samsa, M.M.; Mondotte, J.A.; Iglesias, N.G.; Assunção-Mirando, I.; Barbosa-Lima, G.; Da Poian, A.T.; Bozza, P.T.; Gamarnik, A.V. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009, 5, e1000632. [Google Scholar] [CrossRef]
- Moffat, C.; Bhatia, L.; Nguyen, T.; Lynch, P.; Wang, M.; Wang, D.; Ilkayeva, O.R.; Han, X.; Hirschey, M.D.; Claypool, S.M.; et al. Acyl-CoA thioesterase-2 facilitates mitochondrial fatty acid oxidation in the liver. J. Lipid Res. 2014, 55, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Momose, A.; Ohtomo, T.; Nishinosono, A.; Tanonaka, K.; Toyoda, H.; Morikawa, M.; Yamada, J. Upregulation of fatty acyl-CoA thioesterases in the heart and skeletal muscles of rats fed a high-fat diet. Bio. Pharm. Bull. 2011, 34, 87–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cook, K.C.; Moreno, J.A.; Jean Beltran, P.M.; Cristea, I.M. Peroxisome plasticity at the virus-host interface. Trends Microbiol. 2019, 27, 906–914. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Hou, S.; Malik-Soni, N.; Xu, Z.; Kumar, A.; Rachubinski, R.A.; Frappier, L.; Hobman, T.C. Flavivirus infection impairs peroxisome biogenesis and early antiviral signaling. J. Virol. 2015, 89, 12349–12361. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
St Clair, L.A.; Mills, S.A.; Lian, E.; Soma, P.S.; Nag, A.; Montgomery, C.; Ramirez, G.; Chotiwan, N.; Gullberg, R.C.; Perera, R. Acyl-Coa Thioesterases: A Rheostat That Controls Activated Fatty Acids Modulates Dengue Virus Serotype 2 Replication. Viruses 2022, 14, 240. https://doi.org/10.3390/v14020240
St Clair LA, Mills SA, Lian E, Soma PS, Nag A, Montgomery C, Ramirez G, Chotiwan N, Gullberg RC, Perera R. Acyl-Coa Thioesterases: A Rheostat That Controls Activated Fatty Acids Modulates Dengue Virus Serotype 2 Replication. Viruses. 2022; 14(2):240. https://doi.org/10.3390/v14020240
Chicago/Turabian StyleSt Clair, Laura A., Stephanie A. Mills, Elena Lian, Paul S. Soma, Aritra Nag, Caroline Montgomery, Gabriela Ramirez, Nunya Chotiwan, Rebekah C. Gullberg, and Rushika Perera. 2022. "Acyl-Coa Thioesterases: A Rheostat That Controls Activated Fatty Acids Modulates Dengue Virus Serotype 2 Replication" Viruses 14, no. 2: 240. https://doi.org/10.3390/v14020240
APA StyleSt Clair, L. A., Mills, S. A., Lian, E., Soma, P. S., Nag, A., Montgomery, C., Ramirez, G., Chotiwan, N., Gullberg, R. C., & Perera, R. (2022). Acyl-Coa Thioesterases: A Rheostat That Controls Activated Fatty Acids Modulates Dengue Virus Serotype 2 Replication. Viruses, 14(2), 240. https://doi.org/10.3390/v14020240





