High SARS-CoV-2 Prevalence among Healthcare Workers in Cochabamba, Bolivia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethical Approval
2.3. Serological Testing
2.4. Diagnostic Interpretation
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. SARS-CoV-2 Seroprevalence
3.3. Diagnostic Tests
3.4. Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saavedra, V.V.; López, M.A.; Dauby, N. The Heavy Toll of COVID-19 in Bolivia: A Tale of Distrust, Despair, and Health Inequalities. Am. J. Trop. Med. Hyg. 2021, 104, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Daily Update—Region of the Americas—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/covid-19-global-and-regional-daily-update (accessed on 29 November 2021).
- Ministerio de Salud y Deportes de Bolivia—Gobierno Insta a Cumplir la Cuarentena Domiciliaria Para Evitar la Propagación del COVID-19: Bolivia Registra 24 Casos. Available online: https://www.minsalud.gob.bo/3995-gobierno-insta-a-cumplir-la-cuarentena-domiciliaria-para-evitar-la-propagacion-del-covid-19-bolivia-registra-24-casos (accessed on 29 November 2021).
- PAHO COVID-19 Daily Update: 1 January 2021—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/paho-covid-19-daily-update-1-january-2021 (accessed on 29 November 2021).
- Bolivia (Plurinational State of): WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int (accessed on 29 November 2021).
- Ministerio de Salud y Deportes de Bolivia—Inicia El Proceso de Vacunación Al Personal de Salud Que Lucha en Primera Línea Contra la Pandemia. Available online: https://www.minsalud.gob.bo/5230-inicia-proceso-vacunacion (accessed on 29 November 2021).
- Instituto Nacional de Estadística. Available online: https://www.ine.gob.bo/ (accessed on 29 November 2021).
- Carrat, F.; de Lamballerie, X.; Rahib, D.; Blanché, H.; Lapidus, N.; Artaud, F.; Kab, S.; Renuy, A.; de Edelenyi, F.S.; Meyer, L.; et al. Antibody Status and Cumulative Incidence of SARS-CoV-2 Infection among Adults in Three Regions of France Following the First Lockdown and Associated Risk Factors: A Multicohort Study. Int. J. Epidemiol. 2021, 50, 1458–1472. [Google Scholar] [CrossRef] [PubMed]
- Gallian, P.; Pastorino, B.; Morel, P.; Chiaroni, J.; Ninove, L.; de Lamballerie, X. Lower Prevalence of Antibodies Neutralizing SARS-CoV-2 in Group O French Blood Donors. Antivir. Res. 2020, 181, 104880. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Aguirre, M.F.; Ballejo, C.; Marro, M.J.; Gamarnik, A.; Vargas, G.; Pifano, M.; Varela, T.; García, E.; Lawrynowicz, A.; et al. Seroprevalencia de infección por SARS-CoV-2 en personal de salud de la region sanitaria VIII, provincia de Buenos Aires, Argentina. Rev. Argent. Salud Publica 2020, 12, 14. [Google Scholar]
- Insúa, C.; Stedile, G.; Figueroa, V.; Hernández, C.; Svartz, A.; Ferrero, F.; Ossorio, M.F.; Brunetto, O. Seroprevalence of SARS-CoV-2 Antibodies among Physicians from a Children’s Hospital. Arch. Argent. Pediatr. 2020, 118, 381–385. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; Lobo, R.D.; Detta, F.P.; Vieira-Junior, J.M.; de Souza Castro, T.L.; Zambelli, D.B.; Cardoso, L.F.; Borges, I.C.; Tozetto-Mendoza, T.R.; Costa, S.F.; et al. SARS-CoV-2 Seroprevalence and Risk Factors among Health Care Workers: Estimating the Risk of COVID-19 Dedicated Units. Am. J. Infect. Control 2021, 49, 1197–1199. [Google Scholar] [CrossRef]
- Dubiela, A.L.F.; Lana, D.F.D.; Aerts, A.P.K.; de Marques, C.G.; Cassol, R.; Dalarosa, M.G.; Schultz, V.; Ramos, F.; Guasso, I.; Filho, F.F.D.; et al. Prevalence of Coronavirus Disease 2019 (COVID-19) among Healthcare Professionals Working in Hospital Emergencies during the First-Wave Peak in 2020 in Porto Alegre, Brazil. Infect. Control Hosp. Epidemiol. 2021, 29, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.F.; Giavina-Bianchi, P.; Buss, L.; Mesquita Peres, C.H.; Rafael, M.M.; dos Santos, L.G.N.; Bedin, A.A.; Francisco, M.C.P.B.; Satakie, F.M.; Jesus Menezes, M.A.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Seroprevalence and Risk Factors among Oligo/Asymptomatic Healthcare Workers: Estimating the Impact of Community Transmission. Clin. Infect. Dis. 2021, 73, e1214–e1218. [Google Scholar] [CrossRef]
- de Melo, M.S.; Borges, L.P.; de Souza, D.R.V.; Martins, A.F.; Neto, J.M.d.R.; Ribeiro, A.A.; Santos, A.A.; da Invenção, G.B.; Matos, I.L.S.; dos Santos, K.A.; et al. Anti-SARS-CoV-2 IgM and IgG Antibodies in Health Workers in Sergipe, Brazil. medRxiv 2020. [Google Scholar] [CrossRef]
- Zuñiga, M.; O’Ryan, M.; Bertoglia, M.P.; Bravo, P.; Lagomarcino, A.J.; Muñoz, S.; Peña Alonso, A.; Rodriguez, M.A.; Vial, P.A. SARS-CoV-2 Antibody Prevalence Among 85,529 Healthcare Workers Following the First Wave of COVID-19 in Chile; Social Science Research Network: Rochester, NY, USA, 2021. [Google Scholar]
- Iruretagoyena, M.; Vial, M.R.; Spencer-Sandino, M.; Gaete, P.; Peters, A.; Delgado, I.; Perez, I.; Calderon, C.; Porte, L.; Legarraga, P.; et al. Longitudinal Assessment of SARS-CoV-2 IgG Seroconversionamong Front-Line Healthcare Workers during the First Wave of the COVID-19 Pandemic at a Tertiary-Care Hospital in Chile. BMC Infect. Dis. 2021, 21, 478. [Google Scholar] [CrossRef]
- Ariza, B.; Torres, X.; Salgado, D.; Cepeda, M.; Restrepo, C.G.; Castellanos, J.C.; Suárez-Obando, F.; Cuellar, A.; Cardozo, C.; Ángel, J.; et al. Seroprevalence and Seroconversion Rates to SARS-CoV-2 in Interns, Residents, and Medical Doctors in a University Hospital in Bogotá, Colombia. Infectio 2021, 25, 145–152. [Google Scholar] [CrossRef]
- Orozco, M.A.; Aguilera, J.M.R.; Hernández, B.A.B.; Baeza, G.B.G.; del Rosario Sánchez Navarro, M. Seroprevalencia y seguimiento de anticuerpos IgG contra SARS-CoV-2 en personal del Laboratorio Estatal de Salud Pública de Guanajuato LaESaP. Nova Sci. 2021, 13, 1. [Google Scholar] [CrossRef]
- Cruz-Arenas, E.; Cabrera-Ruiz, E.; Laguna-Barcenas, S.; Colin-Castro, C.A.; Chavez, T.; Franco-Cendejas, R.; Ibarra, C.; Perez-Orive, J. Serological Prevalence of SARS-CoV-2 Infection and Associated Factors in Healthcare Workers in a “Non-COVID” Hospital in Mexico City. PLoS ONE 2021, 16, e0255916. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, A.; Rangel, G.; Zhang, X.; Wong, D.; Britton, G.; Fernandez, P.L.; Pérez, A.; Oviedo, D.; Restrepo, C.; Carreirra, M.B.; et al. Performance of a Point of Care Test for Detecting IgM and IgG Antibodies Against SARS-CoV-2 and Seroprevalence in Blood Donors and Health Care Workers in Panama. Front. Med. 2021, 8, 616106. [Google Scholar] [CrossRef]
- Chafloque-Vásquez, R.A.; Pampa-Espinoza, L.; Celis Salinas, J.C.; Chafloque-Vásquez, R.A.; Pampa-Espinoza, L.; Celis Salinas, J.C. Seroprevalencia de COVID-19 En Trabajadores de Un Hospital de La Amazonía Peruana. Acta Médica Peru. 2020, 37, 390–392. [Google Scholar] [CrossRef]
- Garcia-Basteiro, A.L.; Moncunill, G.; Tortajada, M.; Vidal, M.; Guinovart, C.; Jiménez, A.; Santano, R.; Sanz, S.; Méndez, S.; Llupià, A.; et al. Seroprevalence of Antibodies against SARS-CoV-2 among Health Care Workers in a Large Spanish Reference Hospital. Nat. Commun. 2020, 11, 3500. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-Time Tracking of Self-Reported Symptoms to Predict Potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Kim, G.-U.; Kim, M.-J.; Ra, S.H.; Lee, J.; Bae, S.; Jung, J.; Kim, S.-H. Clinical Characteristics of Asymptomatic and Symptomatic Patients with Mild COVID-19. Clin. Microbiol. Infect. 2020, 26, 948.e1–948.e3. [Google Scholar] [CrossRef]
- Shields, A.; Faustini, S.E.; Perez-Toledo, M.; Jossi, S.; Aldera, E.; Allen, J.D.; Al-Taei, S.; Backhouse, C.; Bosworth, A.; Dunbar, L.A.; et al. SARS-CoV-2 Seroprevalence and Asymptomatic Viral Carriage in Healthcare Workers: A Cross-Sectional Study. Thorax 2020, 75, 1089–1094. [Google Scholar] [CrossRef]
- Lan, F.-Y.; Wei, C.-F.; Hsu, Y.-T.; Christiani, D.C.; Kales, S.N. Work-Related COVID-19 Transmission in Six Asian Countries/Areas: A Follow-up Study. PLoS ONE 2020, 15, e0233588. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic Review of COVID-19 in Children Shows Milder Cases and a Better Prognosis than Adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Fortini, A.; Andreini, R.; Sechi, L.A.; Tascini, C. The Paradox of the Low Prevalence of Current Smokers Among COVID-19 Patients Hospitalized in Nonintensive Care Wards: Results From an Italian Multicenter Case–Control Study. Nicotine Tob. Res. 2021, 23, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.; Bagos, P.G.; Giannouchos, T.; Niaura, R.; Barbouni, A.; Poulas, K. Smoking Prevalence among Hospitalized COVID-19 Patients and Its Association with Disease Severity and Mortality: An Expanded Re-Analysis of a Recent Publication. Harm Reduct. J. 2021, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.; Shahab, L.; Brown, J.; Perski, O. The Association of Smoking Status with SARS-CoV-2 Infection, Hospitalization and Mortality from COVID-19: A Living Rapid Evidence Review with Bayesian Meta-Analyses (Version 7). Addiction 2021, 116, 1319–1368. [Google Scholar] [CrossRef] [PubMed]
- van Westen-Lagerweij, N.A.; Meijer, E.; Meeuwsen, E.G.; Chavannes, N.H.; Willemsen, M.C.; Croes, E.A. Are Smokers Protected against SARS-CoV-2 Infection (COVID-19)? The Origins of the Myth. npj Prim. Care Respir. Med. 2021, 31, 10. [Google Scholar] [CrossRef]
- Smoking and COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19 (accessed on 8 November 2021).
- Li, J.; Wang, X.; Chen, J.; Cai, Y.; Deng, A.; Yang, M. Association between ABO Blood Groups and Risk of SARS-CoV-2 Pneumonia. Br. J. Haematol. 2020, 190, 24–27. [Google Scholar] [CrossRef]
- Barnkob, M.B.; Pottegård, A.; Støvring, H.; Haunstrup, T.M.; Homburg, K.; Larsen, R.; Hansen, M.B.; Titlestad, K.; Aagaard, B.; Møller, B.K.; et al. Reduced Prevalence of SARS-CoV-2 Infection in ABO Blood Group O. Blood Adv. 2020, 4, 4990–4993. [Google Scholar] [CrossRef]
- Göker, H.; Aladağ Karakulak, E.; Demiroğlu, H.; Ayaz Ceylan, Ç.M.; Büyükaşik, Y.; Inkaya, A.Ç.; Aksu, S.; Sayinalp, N.; Haznedaroğlu, I.C.; Uzun, Ö.; et al. The Effects of Blood Group Types on the Risk of COVID-19 Infection and Its Clinical Outcome. Turk. J. Med. Sci. 2020, 50, 679–683. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Z.; Li, P.; Yu, Q. Relationship between ABO Blood Group Distribution and Clinical Characteristics in Patients with COVID-19. Clin. Chim. Acta 2020, 509, 220–223. [Google Scholar] [CrossRef]
- Ministerio de Salud y Deportes de Bolivia—Ministerio De Salud Recuerda Existen 4 Grupos Sanguíneos “La Sangre Nos Conecta A Todos, Extiende Tu Brazo A La Vida Dona Sangre. Available online: https://www.minsalud.gob.bo/1219-ministerio-de-salud-recuerda-existen-4-grupos-sanguineos-la-sangre-nos-conecta-a-todos-extiende-tu-brazo-a-la-vida-dona-sangre (accessed on 29 November 2021).
- Quispe-Cañari, J.F.; Fidel-Rosales, E.; Manrique, D.; Mascaró-Zan, J.; Huamán-Castillón, K.M.; Chamorro–Espinoza, S.E.; Garayar–Peceros, H.; Ponce–López, V.L.; Sifuentes-Rosales, J.; Alvarez-Risco, A.; et al. Self-Medication Practices during the COVID-19 Pandemic among the Adult Population in Peru: A Cross-Sectional Survey. Saudi Pharm. J. 2021, 29, 1–11. [Google Scholar] [CrossRef]
- Román, B.R.; Moscoso, S.; Chung, S.A.; Terceros, B.L.; Álvarez-Risco, A.; Yáñez, J.A. Tratamiento de la COVID-19 en Perú y Bolivia y los riesgos de la automedicación. Rev. Cuba. Farm. 2020, 53, 1–20. [Google Scholar]
- Heimfarth, L.; Santos, V.S.; Araújo, A.A.d.S.; Quintans-Júnior, L.J.; Martins-Filho, P.R. Ivermectin: Panacea or True Promise for COVID-19. EXCLI J. 2020, 19, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 In Vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Results of an Open-Label Non-Randomized Clinical Trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Mega, E.R. Latin America’s Embrace of an Unproven COVID Treatment Is Hindering Drug Trials. Nature 2020, 586, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Commissioner, O. Of the Coronavirus (COVID-19) Update: FDA Warns Seller Marketing Dangerous Chlorine Dioxide Products that Claim to Treat or Prevent COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-seller-marketing-dangerous-chlorine-dioxide-products-claim (accessed on 29 November 2021).
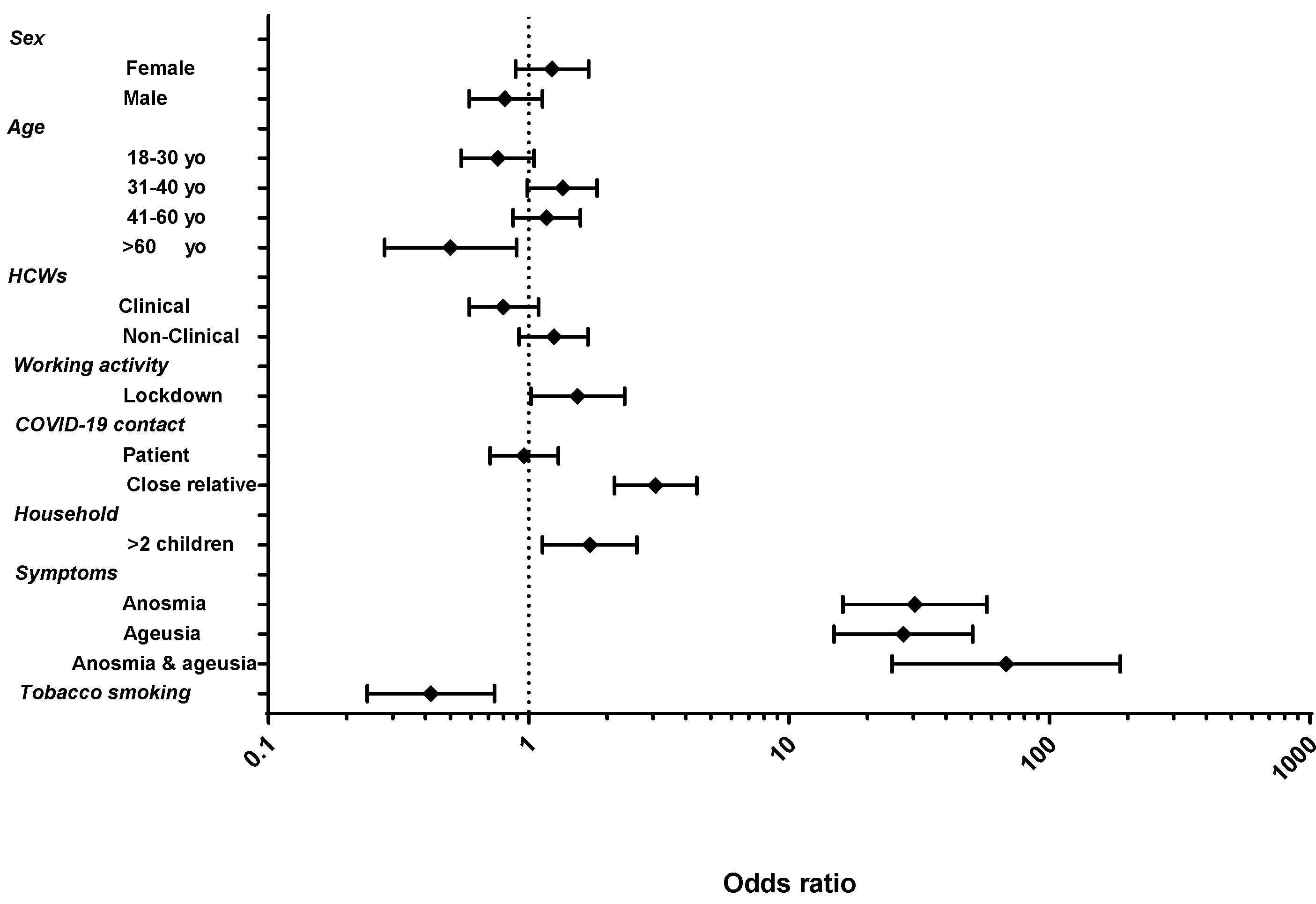
| Variables | Total | Seropositive | p-Value | |
|---|---|---|---|---|
| Age | ||||
| Age (years), mean (SD) | 39.29 (12.3) | 39.18 (11.5) | ||
| 18–30, n (%) | 220 | 85 (38.64) | 0.09 | |
| 31–40, n (%) | 241 | 117 (48.55) | 0.05 | |
| 41–60, n (%) | 263 | 121 (46.01) | 0.30 | |
| >60, n (%) | 59 | 17 (28.81) | 0.02 | |
| Sex | ||||
| Female, n (%) | 579 | 259 (44.73) | 0.21 | |
| Male, n (%) | 204 | 81 (39.71) | ||
| Healthcare facility | ||||
| Viedma Adult Hospital, n (%) | 360 | 150 (41.67) | 0.36 | |
| Manuel Ascencio Villarroel Hospital, n (%) | 206 | 93 (45.15) | 0.32 | |
| Copacabana Clinic, n (%) | 81 | 31 (38.27) | 0.56 | |
| María de los Ángeles Clinic, n (%) | 71 | 36 (50.70) | 0.19 | |
| Others, n (%) | 65 | 30 (46.15) | 0.65 | |
| Occupation | ||||
| Clinical HCWs, n (%) | 532 | 222 (41.73) | 0.15 | |
| Non-clinical HCWs, n (%) | 248 | 117 (47.20) | ||
| Missing, n (%) | 3 | 1 | ||
| Household composition | ||||
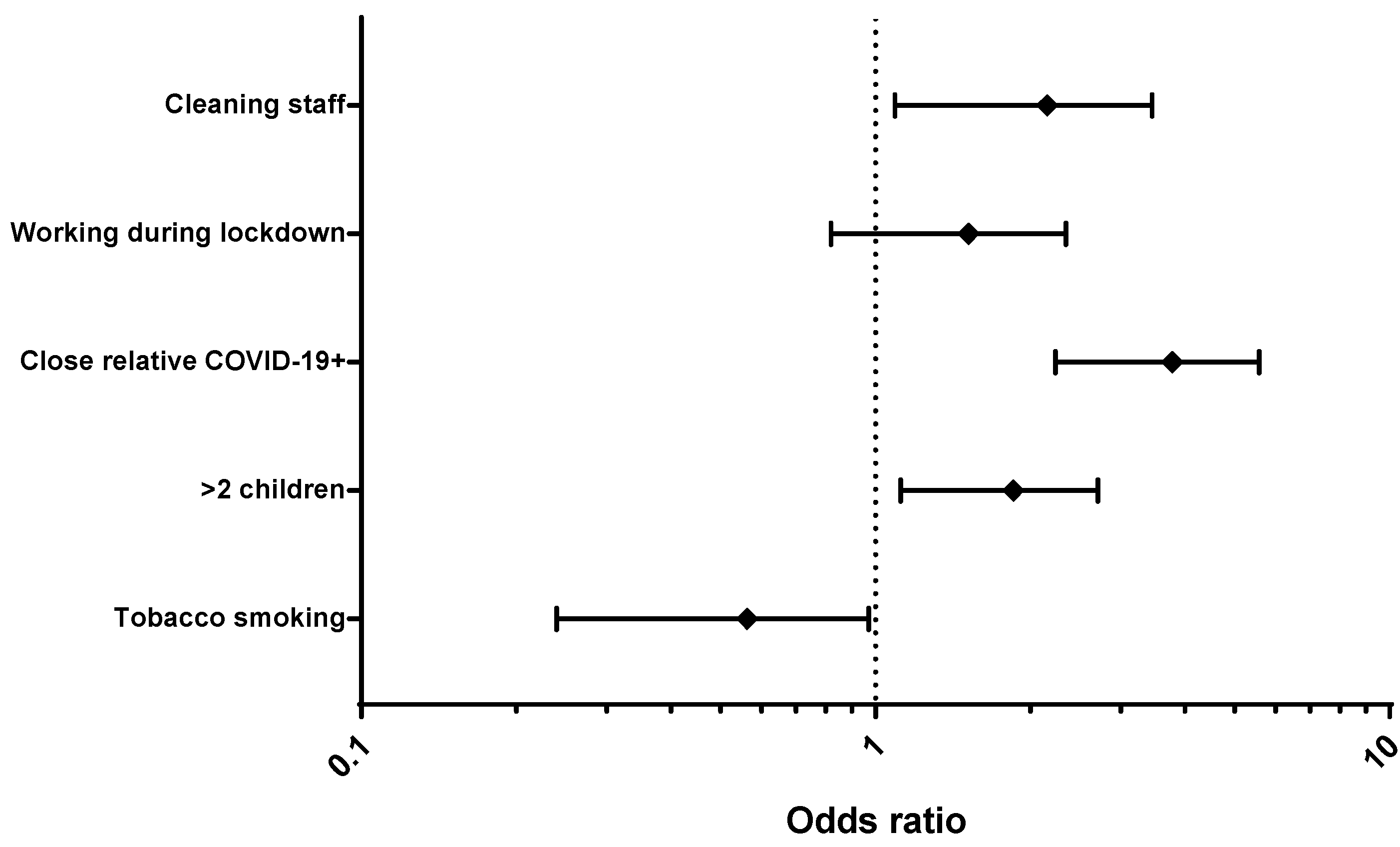
| >two children, n (%) | 116 | 62 (53.45) | 0.01 | |
| ≤two children, n (%) | 407 | 163 (40.05) | ||
| Missing, n (%) | 260 | 115 | ||
| Tobacco smoking | ||||
| Active tobacco smokers, n (%) | 67 | 17 (25.37) | 0.02 | |
| No smoking, n (%) | 693 | 310 (44.73) | ||
| Missing, n (%) | 23 | 13 | ||
| COVID-19 contact | ||||
| Patient, n (%) | 529 | 228 (43.10) | NA | |
| Relative living in the same house, n (%) | 158 | 103 (65.19) | ||
| Relative not living in the same house, n (%) | 109 | 37 (33.94) | ||
| Symptoms | ||||
| No symptoms, n (%) | 348 | 76 (21.84) | <0.001 | |
| Symptoms 1, n (%) | 406 | 256 (63.05) | ||
| Missing, n (%) | 29 | 8 | ||
| Working activity | ||||
| Working during lockdown, n (%) | 656 | 294 (44.82) | 0.041 | |
| Not working during lockdown, n (%) | 113 | 39 (34.51) | ||
| Missing, n (%) | 14 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saba Villarroel, P.M.; Castro Soto, M.d.R.; Undurraga, V.; Sanz, H.; Jaldín, A.M.; Ninove, L.; Nurtop, E.; Pezzi, L.; Mohamed Ali, S.; Amroun, A.; et al. High SARS-CoV-2 Prevalence among Healthcare Workers in Cochabamba, Bolivia. Viruses 2022, 14, 232. https://doi.org/10.3390/v14020232
Saba Villarroel PM, Castro Soto MdR, Undurraga V, Sanz H, Jaldín AM, Ninove L, Nurtop E, Pezzi L, Mohamed Ali S, Amroun A, et al. High SARS-CoV-2 Prevalence among Healthcare Workers in Cochabamba, Bolivia. Viruses. 2022; 14(2):232. https://doi.org/10.3390/v14020232
Chicago/Turabian StyleSaba Villarroel, Paola Mariela, María del Rosario Castro Soto, Verónica Undurraga, Heydi Sanz, Ana María Jaldín, Laetitia Ninove, Elif Nurtop, Laura Pezzi, Souand Mohamed Ali, Abdennour Amroun, and et al. 2022. "High SARS-CoV-2 Prevalence among Healthcare Workers in Cochabamba, Bolivia" Viruses 14, no. 2: 232. https://doi.org/10.3390/v14020232
APA StyleSaba Villarroel, P. M., Castro Soto, M. d. R., Undurraga, V., Sanz, H., Jaldín, A. M., Ninove, L., Nurtop, E., Pezzi, L., Mohamed Ali, S., Amroun, A., Seston, M., & de Lamballerie, X. (2022). High SARS-CoV-2 Prevalence among Healthcare Workers in Cochabamba, Bolivia. Viruses, 14(2), 232. https://doi.org/10.3390/v14020232






