Indirect ELISA Using Multi–Antigenic Dominants of p30, p54 and p72 Recombinant Proteins to Detect Antibodies against African Swine Fever Virus in Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Constructs
2.3. Expression in E. coli
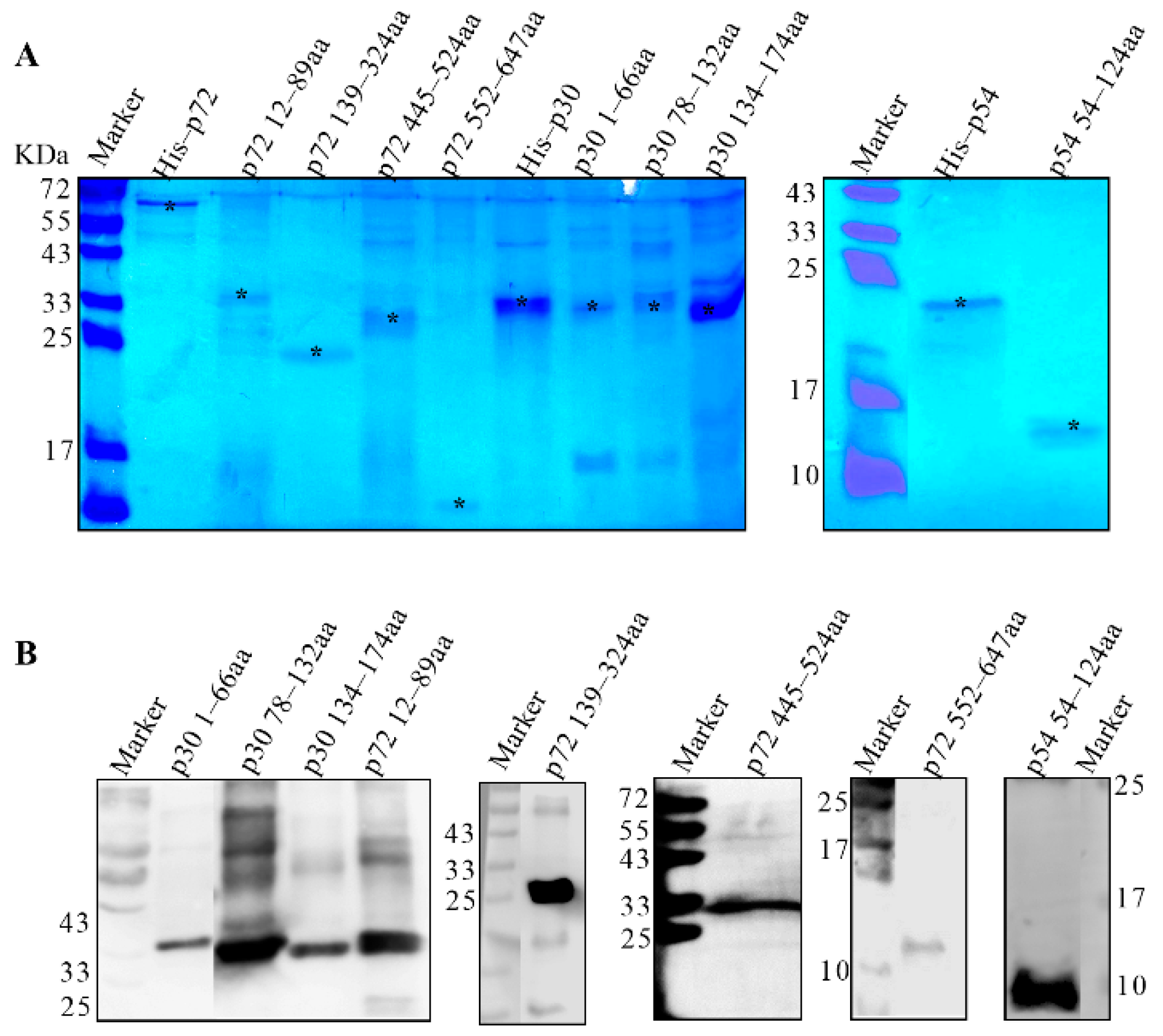
2.4. Purification of Recombinant Proteins
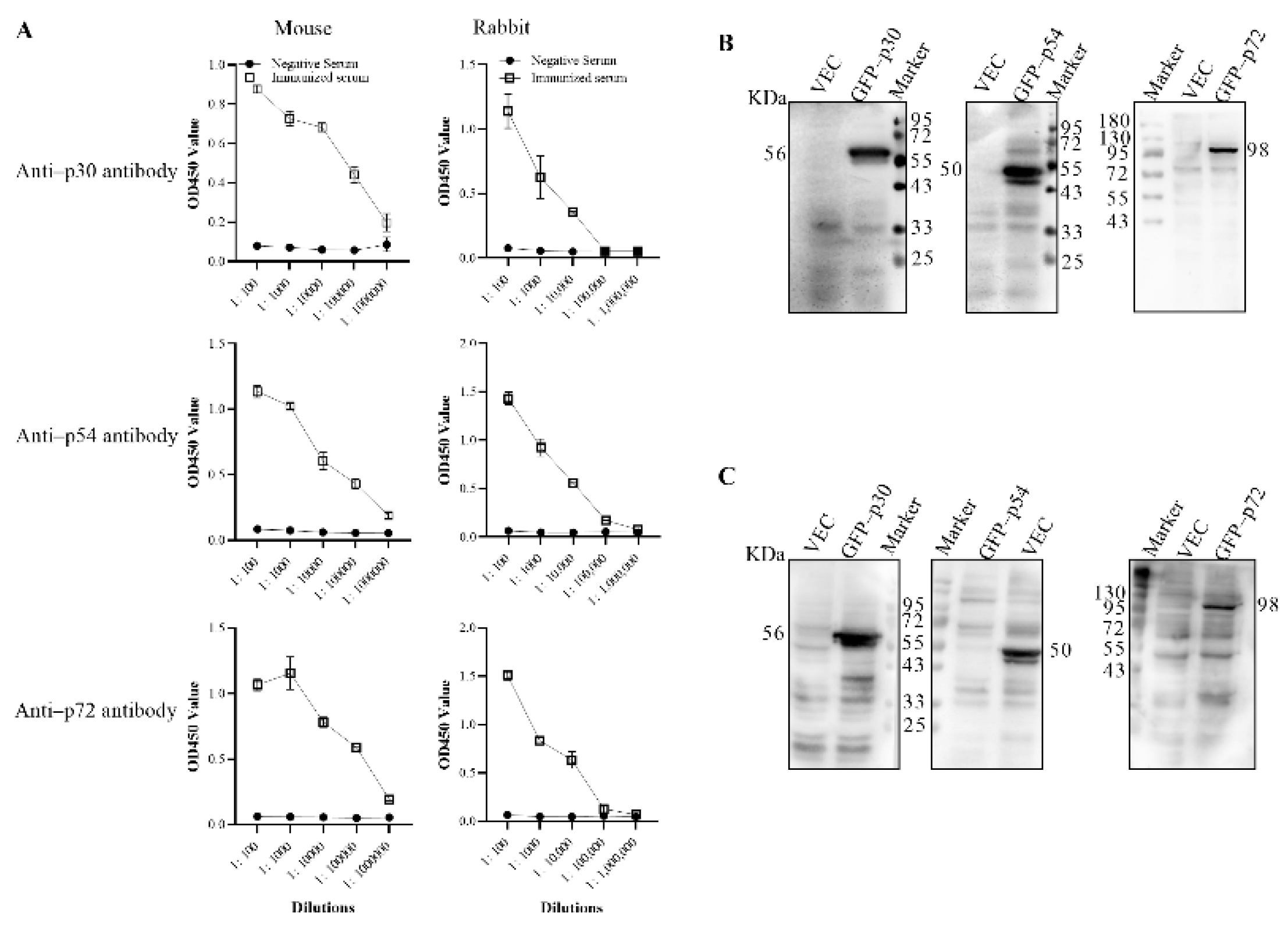
2.5. Preparation of Mouse and Rabbit Polyclonal Antibody
2.6. Western Blot Analysis
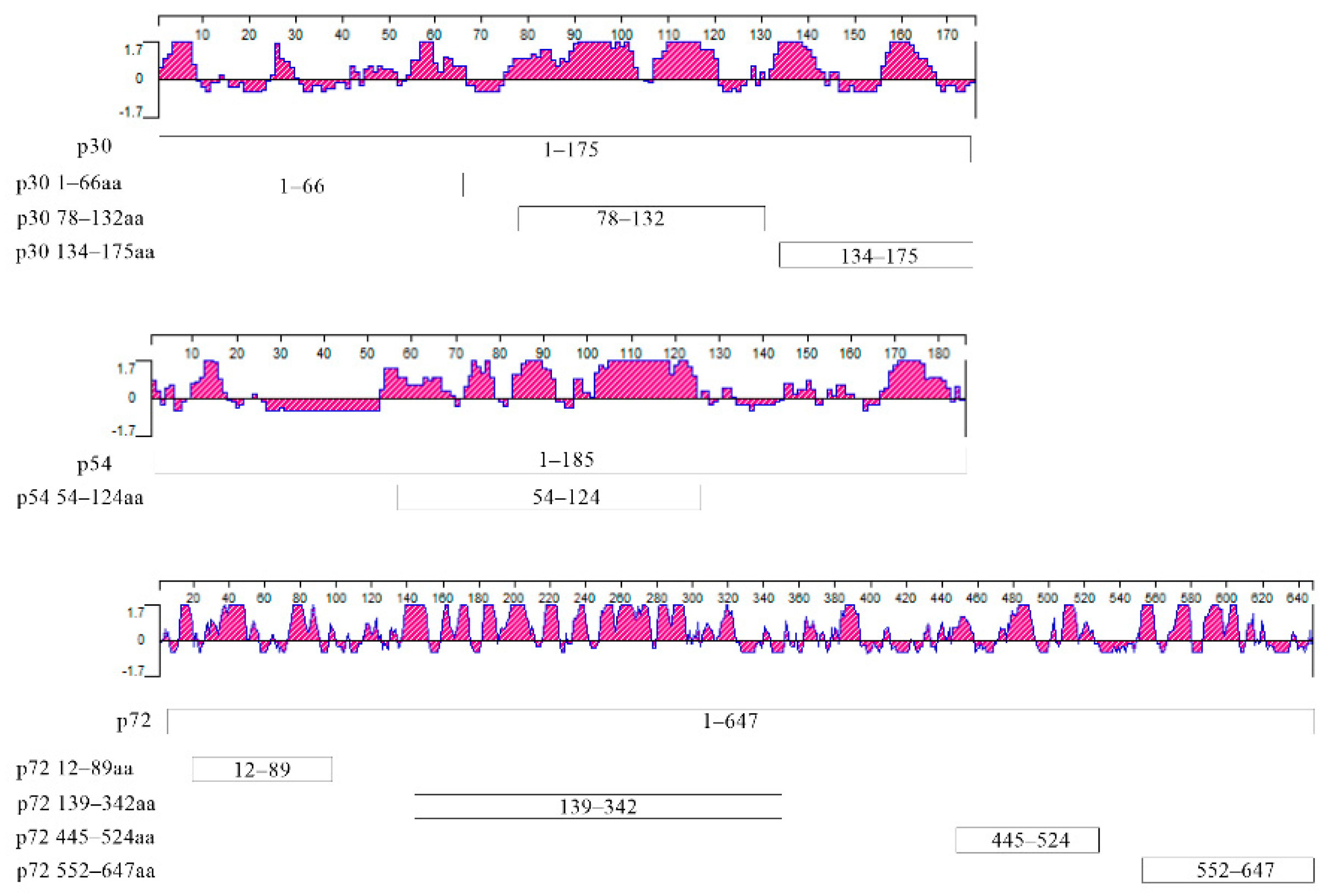
2.7. Screening of Dominant Epitopes
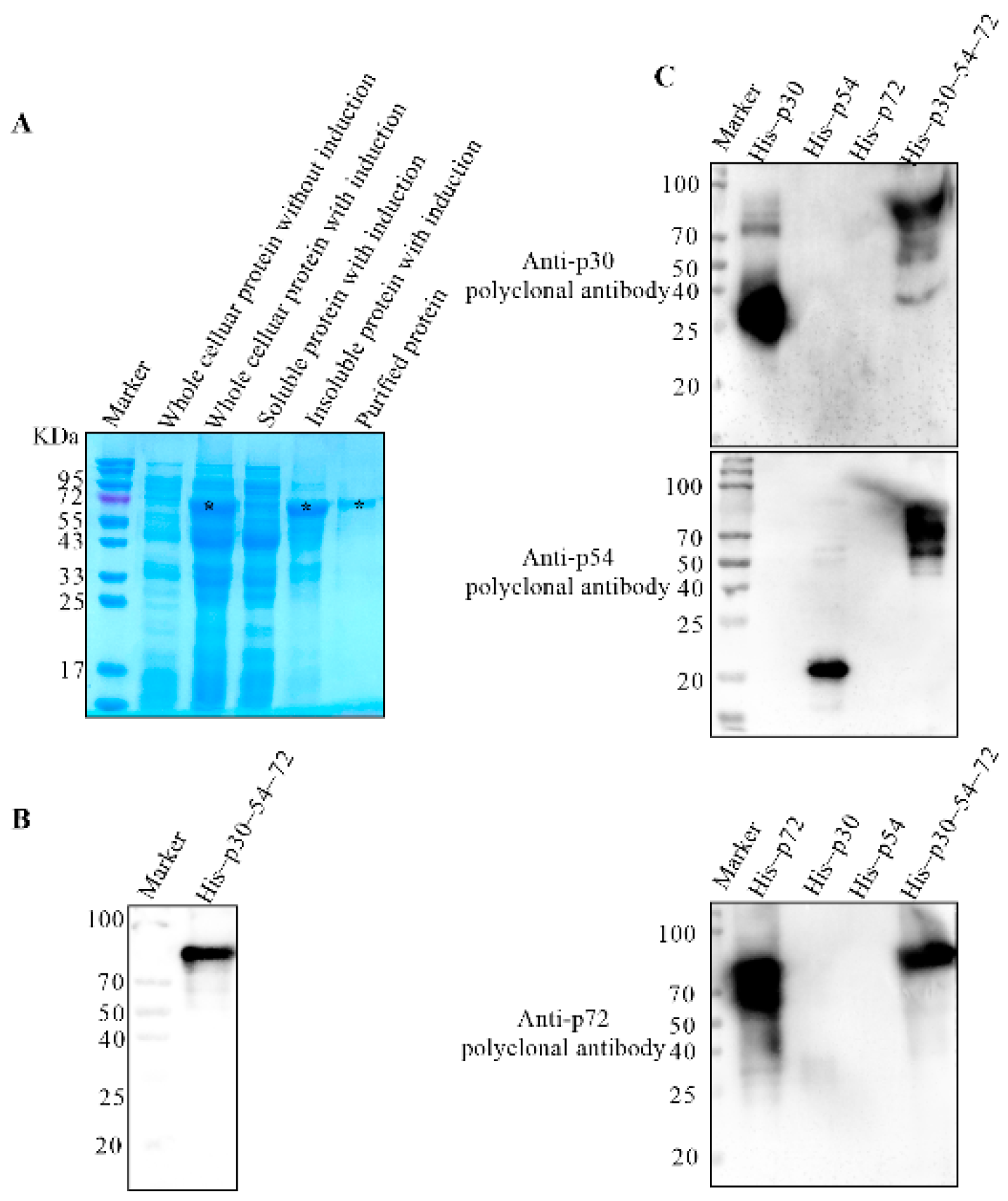
2.8. Expression of the Tandem Antigenic Dominant Protein His–p30–54–72
2.9. Optimization of the p30–54–72 Coated iELISA
2.10. Serum Samples
2.11. Application of the p30–54–72 Coated iELISA
3. Results
3.1. Expression and Purification of His–Fused p30, p54 and p72 Proteins
3.2. Preparation of the Anti–p30, p54 and p72 Polyclonal Antibodies
3.3. Preparation of Truncated p30, p54 and p72 Proteins
3.4. Screening the Dominant Antigenic Domains
3.5. The Tandem Expression of the Dominant Antigenic Domains of p30, p54 and p72 Proteins
3.6. Optimization of the His–p30–54–72 Coated ELISA
3.7. Application of His–p30–54–72–Coated ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Sanchez-Cordon, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, Y.; Qiu, H.J. African swine fever: An unprecedented disaster and challenge to China. Infect. Dis. Poverty 2018, 7, 111. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Escribano, J.M.; Martins, C.; Rock, D.L.; Salas, M.L.; Wilkinson, P.J. Asfarviridae. In Virus Taxonomy; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; VIII Report of the ICTV; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2005; pp. 135–143. [Google Scholar]
- Andres, G.; Garcia-Escudero, R.; Vinuela, E.; Salas, M.L.; Rodriguez, J.M. African swine fever virus structural protein pE120R is essential for virus transport from assembly sites to plasma membrane but not for infectivity. J. Virol. 2001, 75, 6758–6768. [Google Scholar] [CrossRef]
- Salas, M.L.; Andres, G. African swine fever virus morphogenesis. Virus Res. 2013, 173, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Yanez, R.J.; Rodriguez, J.M.; Nogal, M.L.; Yuste, L.; Enriquez, C.; Rodriguez, J.F.; Vinuela, E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology 1995, 208, 249–278. [Google Scholar] [CrossRef]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Burrage, T.G.; Kutish, G.F.; Rock, D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 2004, 319, 337–342. [Google Scholar] [CrossRef]
- Cao, Y.; Han, D.; Zhang, Y.; Zhang, K.; Du, N.; Tong, W.; Li, G.; Zheng, H.; Liu, C.; Gao, F.; et al. Identification of one novel epitope targeting p54 protein of African swine fever virus using monoclonal antibody and development of a capable ELISA. Res. Vet. Sci. 2021, 141, 19–25. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, X.; Chen, X.; Li, D.; Xu, Q.; Yao, L.; Sun, Q.; Ghonaim, A.H.; Ku, X.; Fan, S.; et al. Establishment of a Blocking ELISA Detection Method for Against African Swine Fever Virus p30 Antibody. Front. Vet. Sci. 2021, 8, 781373. [Google Scholar] [CrossRef]
- Zhong, K.; Zhu, M.; Yuan, Q.; Deng, Z.; Feng, S.; Liu, D.; Yuan, X. Development of an Indirect ELISA to Detect African Swine Fever Virus pp62 Protein-Specific Antibodies. Front. Vet. Sci. 2021, 8, 798559. [Google Scholar] [CrossRef] [PubMed]
- Heimerman, M.E.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R. Linear epitopes in African swine fever virus p72 recognized by monoclonal antibodies prepared against baculovirus-expressed antigen. J. Vet. Diagn. Invest. 2018, 30, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Carolina, C.; Silvia, G.S.; Noelia, M.; María, C.N.; Leopold, K.M.M.; Carlos, J.Q.; Livio, H.; Eric, M.C.; Ferran, J.; Jose, M.E.; et al. African swine fever virus serodiagnosis: A general review with a focus on the analyses of African serum samples. Virus Res. 2013, 173, 159–167. [Google Scholar] [CrossRef]
- Giménez-Lirola, L.G.; Mur, L.; Rivera, B.; Mogler, M.; Sun, Y.; Lizano, S.; Goodell, C.; Harris, D.L.; Rowland, R.R.; Gallardo, C.; et al. Detection of African Swine Fever Virus Antibodies in Serum and Oral Fluid Specimens Using a Recombinant Protein 30 (p30) Dual Matrix Indirect ELISA. PLoS ONE 2016, 11, e0161230. [Google Scholar] [CrossRef]
- Gallardo, C.; Reis, A.L.; Kalema-Zikusoka, G.; Malta, J.; Soler, A.; Blanco, E.; Parkhouse, R.M.E.; Leitão, A.B. Recombinant Antigen Targets for Serodiagnosis of African Swine Fever. Clin. Vaccine Immun. 2009, 16, 1012–1020. [Google Scholar] [CrossRef]
- Alcaraz, C.; Rodriguez, F.; Oviedo, J.; Eiras, A.; De Diego, M.; Alonso, C.; Escribano, J. Highly specific confirmatory Western blot test for African swine fever virus antibody detection using the recombinant virus protein p54. J. Virol. Methods 1995, 52, 111–119. [Google Scholar] [CrossRef]
- Barderas, M.G.; Rodríguez, F.; Gómez-Puertas, P.; Avilés, M.; Beitia, F.; Alonso, C.; Escribano, J.M. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch. Virol 2001, 146, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernandez-Pinero, J.; Markowska-Daniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R.; Simon, A.; et al. Assessment of African Swine Fever Diagnostic Techniques as a Response to the Epidemic Outbreaks in Eastern European Union Countries: How To Improve Surveillance and Control Programs. J. Clin. Microbiol. 2015, 53, 2555–2565. [Google Scholar] [CrossRef]
- Greiner, M. Two-graph receiver operating characteristic (TG-ROC): A Microsoft-EXCEL template for the selection of cut-off values in diagnostic tests. J. Immunol. Methods 1995, 185, 145–146. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Wang, G.; Xie, M.; Wu, W.; Chen, Z. Structures and Functional Diversities of ASFV Proteins. Viruses 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiu, S.; Xiao, Y.; Yu, H.; Li, H.; Wu, S.; Feng, C.; Lin, X. Development of a Blocking ELISA Kit for Detection of ASFV Antibody Based on a Monoclonal Antibody against Full Length p72. J. AOAC Int. 2022, 105, 1428–1436. [Google Scholar] [CrossRef]
- Yuan, F.; Petrovan, V.; Gimenez-Lirola, L.G.; Zimmerman, J.J.; Rowland, R.R.R.; Fang, Y. Development of a Blocking Enzyme-Linked Immunosorbent Assay for Detection of Antibodies against African Swine Fever Virus. Pathogens 2021, 10, 760. [Google Scholar] [CrossRef] [PubMed]


| Constructs | Primer (5′–3′) | Vector | Insoluble/Soluble |
|---|---|---|---|
| pET–28a | F: CAAGCTTGCGGCCGCACT | ||
| R: TCGACGGAGCTCGAATTCG | |||
| EGFP–C1 | F: GTCGACGGTACCGCGGGC | ||
| R: TGCAGAATTCGAAGCTTGAGC | |||
| pGEX–6P–1 | F: GTCGACTCGAGCGGCCGCA | ||
| R: CCCGGGAATTCCGGGGATC | |||
| pEGFP–C1–p30 | F: CTCAAGCTTCGAATTCTGCAATGGATTTTATTTTAAATATATCCAT | pEGFP–C1 | Eukaryotic expression |
| R: GGGCCCGCGGTACCGTCGACTTATTTTTTTTTTAAAAGTTTAA | |||
| pEGFP–C1–p54 | F: CTCAAGCTTCGAATTCTGCAATGGATTCTGAATTTTTTCAA | pEGFP–C1 | Eukaryotic expression |
| R: GGGCCCGCGGTACCGTCGACTTACAAGGAGTTTTCTAGGTCT | |||
| pEGFP–C1–p72 | F: CTCAAGCTTCGAATTCTGCAATGGCATCAGGAGGAGCTT | pEGFP–C1 | Eukaryotic expression |
| R: GGGCCCGCGGTACCGTCGACTTAGGTACTGTAACGCAGCACA | |||
| pGEX–6P–1–p30 (1–66aa) | F: GATCCCCGGAATTCCCGGGATGGACCTGCGTAGCAGCAG | pGEX–6P–1 | Insoluble |
| R: TGCGGCCGCTCGAGTCGACTTCCTCTTGCGCCTGGTGC | |||
| pGEX–6P–1–p30 (78–132aa) | F: GATCCCCGGAATTCCCGGGGAGACCGAAAGCAGCGCGAG | pGEX–6P–1 | Insoluble |
| R: TGCGGCCGCTCGAGTCGACGATGTGTTGAACGGTTTTCTGC | |||
| pGEX–6P–1–p30 (134–174aa) | F: GATCCCCGGAATTCCCGGGCAGTATGGCAAGGCGCCGG | pGEX–6P–1 | Insoluble |
| R: TGCGGCCGCTCGAGTCGACCAGTTTGATAACCATCA | |||
| pET–28a–p54 (54–124aa) | F: CCGAATTCGAGCTCCGTCGACGTCCGGCGACCAACCGTC | pET–28a | Soluble |
| R: CGAGTGCGGCCGCAAGCTTGATAGGTGTTACGTTGACGC | |||
| pGEX–6P–1–p72 (12–89aa) | F: GATCCCCGGAATTCCCGGGGATGGGAAGGCCGACAAG | pGEX–6P–1 | Insoluble |
| R: TGCGGCCGCTCGAGTCGACAAGCTTGTTTCCCAAGGTG | |||
| pET–28a–p72 (139–324aa) | F: CCGAATTCGAGCTCCGTCGACGCAACGGATATGACTGGG | pET–28a | Insoluble |
| R: CGAGTGCGGCCGCAAGCTTGCTGATAGTATTTAGGGGTTTG | |||
| pGEX–6P–1–p72 (445–524aa) | F: GATCCCCGGAATTCCCGGGCACACCAACAATAACCACCAC | pGEX–6P–1 | Insoluble |
| R: TGCGGCCGCTCGAGTCGACATCCGATATAGATGAACATG | |||
| pET–28a–p72 (552–647aa) | F: CCGAATTCGAGCTCCGTCGAAAGTTTCCATCAAAGTTCTG | pET–28a | Insoluble |
| R: CGAGTGCGGCCGCAAGCTGAAGTTTCCATCAAAGTTC TG | |||
| Linker–p30 (78–132aa) | F: CCGAATTCGAGCTCCGTCGAGAGACCGAAAGCAGCGCG | ||
| R: GATCCTCCACCTCCTGATCCACCTCCACCGATGTGTTGAACGGTTT | |||
| Linker–p30 (134–174aa) | F: GATCAGGAGGTGGAGGATCACAGTATGGCAAGGCGCCG | ||
| R: AGAACCACCGCCACCCGAGCCGCCACCGCCCAGTTTGATAACCA | |||
| Linker–p54 (54–124aa) | F: GGCGGTGGCGGCTCGGGTGGCGGTGGTTCTCGTCCGGCGACCAA | ||
| R: CGATCCGCCTCCACCGGAACCTCCGCCTCCATAGGTGTTACGTTGA | |||
| Linker–p72 (12–89aa) | F: GGAGGCGGAGGTTCCGGTGGAGGCGGATCGGATGGGAAGGCCG | ||
| R: GGAGCCTCCGCCGCCAGATCCGCCTCCCCCAAGCTTGTTTCCCAA | |||
| Linker––p72 (139––324aa) | F: CTGGCGGCGGAGGCTCCCGCAACGGATATGACTGGGA | ||
| R: AGAACCACCGCCACCCGAGCCGCCACCGCCCTGATAGTATTTAG | |||
| Linker––p72 (445––524aa) | F: CGGGTGGCGGTGGTTCTCACACCAACAATAACCACCAC | ||
| R: CGAGTGCGGCCGCAAGCTTGATCCGATATAGATGAACATGCGT |
| Target Proteins | a/b/p30–54–72 (+) | a/b (+)/p30–54–72(+) | a/b (−)/p30–54–72 (−) | a or b (−)/p30–54–72(+) |
|---|---|---|---|---|
| p30 a | 140 (72.9%) | 137 (97.9%) | 40 (76.9%) | 9 (4.7%) |
| p72 b | 121 (63%) | 121 (100%) | 43 (60.6%) | 28 (14.6%) |
| His–p30–p54–p72 | 149 (77.6%) | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhang, Q.; Liu, Y.; Wang, M.; Zhang, L.; Han, L.; Chu, X.; Ding, G.; Li, Y.; Hou, Y.; et al. Indirect ELISA Using Multi–Antigenic Dominants of p30, p54 and p72 Recombinant Proteins to Detect Antibodies against African Swine Fever Virus in Pigs. Viruses 2022, 14, 2660. https://doi.org/10.3390/v14122660
Li D, Zhang Q, Liu Y, Wang M, Zhang L, Han L, Chu X, Ding G, Li Y, Hou Y, et al. Indirect ELISA Using Multi–Antigenic Dominants of p30, p54 and p72 Recombinant Proteins to Detect Antibodies against African Swine Fever Virus in Pigs. Viruses. 2022; 14(12):2660. https://doi.org/10.3390/v14122660
Chicago/Turabian StyleLi, Dexin, Qin Zhang, Yutian Liu, Miaoli Wang, Lei Zhang, Liyuan Han, Xuefei Chu, Guofei Ding, Yingchao Li, Yanmeng Hou, and et al. 2022. "Indirect ELISA Using Multi–Antigenic Dominants of p30, p54 and p72 Recombinant Proteins to Detect Antibodies against African Swine Fever Virus in Pigs" Viruses 14, no. 12: 2660. https://doi.org/10.3390/v14122660
APA StyleLi, D., Zhang, Q., Liu, Y., Wang, M., Zhang, L., Han, L., Chu, X., Ding, G., Li, Y., Hou, Y., Liu, S., Wang, Z., & Xiao, Y. (2022). Indirect ELISA Using Multi–Antigenic Dominants of p30, p54 and p72 Recombinant Proteins to Detect Antibodies against African Swine Fever Virus in Pigs. Viruses, 14(12), 2660. https://doi.org/10.3390/v14122660






