The Preventive Role of mRNA Vaccines in Reducing Death among Moderate Omicron-Infected Patients: A Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Type of Vaccine for Primary and Booster Vaccination
2.3. Moderate and Severe Omicron-Infected COVID-19
2.4. Statistical Analysis
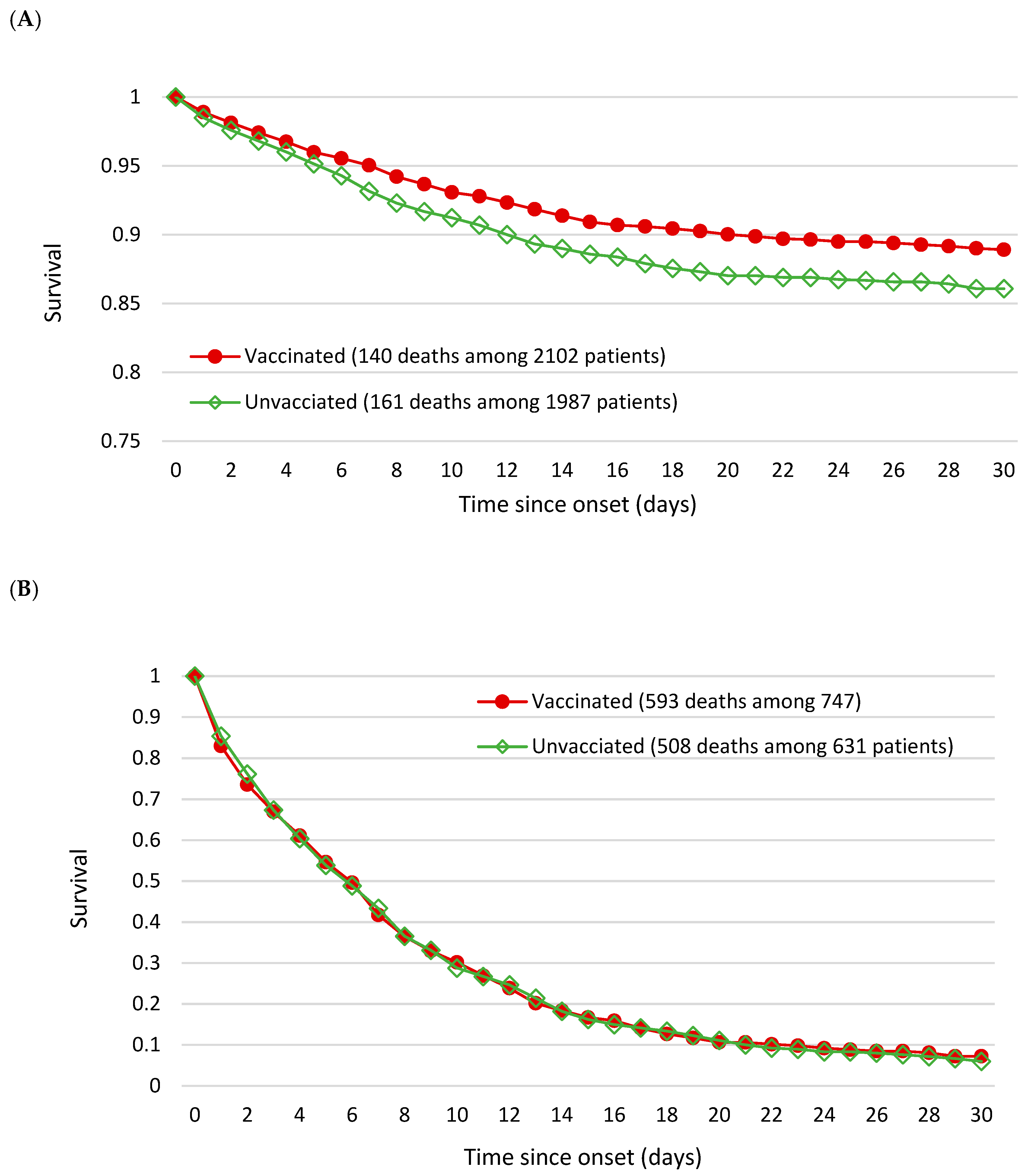
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B. 1.1. 529) and delta (B. 1.617. 2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. Available online: https://covid19.mohw.gov.tw/en/sp-timeline0-206.html (accessed on 2 June 2022).
- World Health Organization. WHO R & D Blueprint Novel Coronavirus: COVID-19 Therapeutic Trial Synopsis. 2020. Available online: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis (accessed on 4 June 2022).
- National Institutes of Health, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 4 June 2022).
- Taiwan Centers for Disease Control. Treatment Guidelines for SARS-CoV-2 Infection. Available online: https://www.cdc.gov.tw/File/Get/3Uk4nSt0rUljIOh7czgiWw (accessed on 4 June 2022).
- TCDC, Taiwan Centers for Disease Control. Available online: https://www.cdc.gov.tw/En (accessed on 31 May 2022).
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, P.J.M. Using correlates to accelerate vaccinology. Science 2022, 375, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Schelde, A.B.; Moustsen-Helm, I.R.; Emborg, H.-D.; Krause, T.G.; Mølbak, K.; Valentiner-Branth, P.; on behalf of the Infectious Disease Preparedness Group at Statens Serum Institut. Vaccine effectiveness against SARS-CoV-2 infection with the omicron or delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, CoVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Olson, S.M.; Patel, M.M. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 2345–2346. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef] [PubMed]
| Moderate | Severe | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients | (%) | Death | Fatality (%) | Patients | (%) | Death | Fatality (%) | |
| Total | 4090 | - | 302 | 7.4 | 1378 | 1100 | 79.9 | |
| Age | ||||||||
| <10 | 61 | 1.5% | 2 | 3.3 | 14 | 1.0% | 6 | 42.9 |
| 10–29 | 74 | 1.8% | 2 | 2.7 | 13 | 0.9% | 7 | 53.8 |
| 30–49 | 204 | 5.0% | 9 | 4.4 | 53 | 3.8% | 35 | 67.3 |
| 50–69 | 844 | 20.6% | 47 | 5.6 | 251 | 18.2% | 185 | 73.7 |
| 70–79 | 911 | 22.3% | 62 | 6.8 | 299 | 21.7% | 246 | 82.3 |
| 80–89 | 1266 | 31.0% | 103 | 8.1 | 434 | 31.5% | 357 | 82.3 |
| 90+ | 730 | 17.8% | 77 | 10.5 | 314 | 22.8% | 264 | 84.1 |
| Vaccination | ||||||||
| Unvaccinated (0 or 1 dose) | 1988 | 48.6% | 160 | 8.0 | 747 | 54.2% | 592 | 79.3 |
| Two-dose | 590 | 14.4% | 45 | 7.6 | 211 | 15.3% | 160 | 75.8 |
| Booster | 1512 | 37.0% | 95 | 6.3 | 420 | 30.5% | 348 | 82.9 |
| Variable | aHR | 95% CI |
|---|---|---|
| Patients admitted in a moderate status | ||
| Unvaccinated (0 or 1 dose) | (Reference) | |
| Vaccinated (2 or more doses) | 0.75 | 0.60–0.94 |
| Patients admitted in a severe status | ||
| Unvaccinated (0 or 1 dose) | (Reference) | |
| Vaccinated (2 or more doses) | 1.00 | 0.89–1.12 |
| Patients admitted in a moderate status | ||
| Unvaccinated (0 or 1 dose) | (Reference) | |
| Two-dose group | 0.87 | 0.63–1.22 |
| Booster group | 0.71 | 0.55–0.91 |
| Patients admitted in a severe status | ||
| Unvaccinated (0 or 1 dose) | (Reference) | |
| Two-dose group | 0.90 | 0.76–1.08 |
| Booster group | 1.05 | 0.92–1.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, A.M.-F.; Chen, S.L.-S.; Hsu, C.-Y.; Chen, T.H.-H. The Preventive Role of mRNA Vaccines in Reducing Death among Moderate Omicron-Infected Patients: A Follow-Up Study. Viruses 2022, 14, 2622. https://doi.org/10.3390/v14122622
Yen AM-F, Chen SL-S, Hsu C-Y, Chen TH-H. The Preventive Role of mRNA Vaccines in Reducing Death among Moderate Omicron-Infected Patients: A Follow-Up Study. Viruses. 2022; 14(12):2622. https://doi.org/10.3390/v14122622
Chicago/Turabian StyleYen, Amy Ming-Fang, Sam Li-Sheng Chen, Chen-Yang Hsu, and Tony Hsiu-Hsi Chen. 2022. "The Preventive Role of mRNA Vaccines in Reducing Death among Moderate Omicron-Infected Patients: A Follow-Up Study" Viruses 14, no. 12: 2622. https://doi.org/10.3390/v14122622
APA StyleYen, A. M.-F., Chen, S. L.-S., Hsu, C.-Y., & Chen, T. H.-H. (2022). The Preventive Role of mRNA Vaccines in Reducing Death among Moderate Omicron-Infected Patients: A Follow-Up Study. Viruses, 14(12), 2622. https://doi.org/10.3390/v14122622





