Estimation of R0 for the Spread of the First ASF Epidemic in Italy from Fresh Carcasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Estimation of Epidemiological Parameters
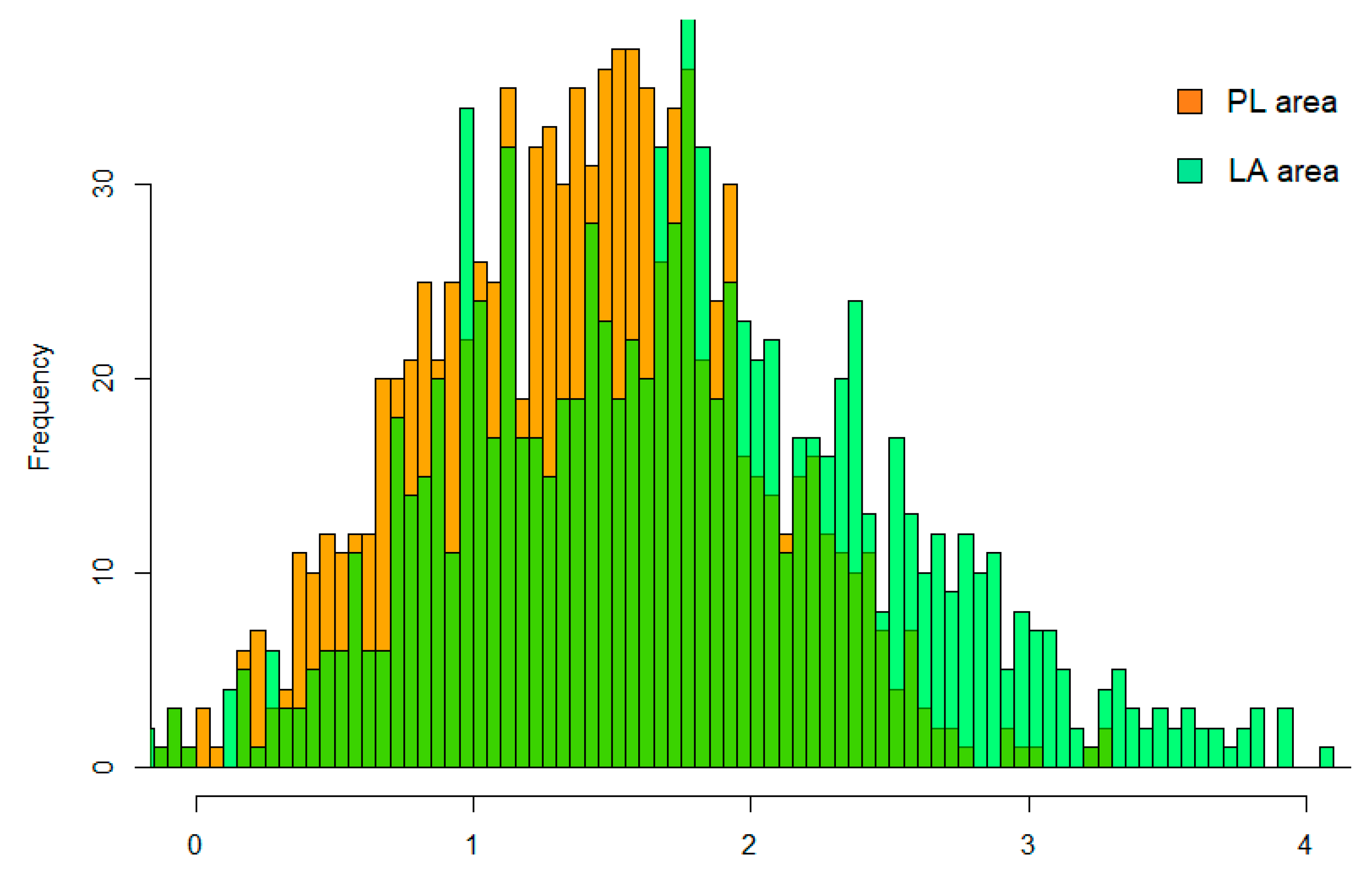
3. Results
Doubling Time and R0 Estimation in PL and LA Infected Areas
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penrith, M.L.; Guberti, V.; Depner, K.; Lubroth, J. Preparation of African swine fever contingency plans. In Animal Production and Health Manual; No. 8; FAO: Rome, Italy, 2009. [Google Scholar]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef]
- OIE (World Organisation for Animal Health). Terrestrial Animal Health Code. 2019. Available online: https://www.oie.int/en/what-we-do/standards/codes-andmanuals/terrestrial-code-online-access/ (accessed on 19 August 2022).
- EU Regulation EU 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). 2016. Available online: https://eur-lex.europa.eu/eli/reg/2016/429/2021-04-21 (accessed on 19 August 2022).
- Pepin, K.M.; Golnar, A.J.; Abdo, Z.; Podgórski, T. Ecological drivers of African swine fever virus persistence in wild boar populations: Insight for control. Ecol. Evol. 2020, 10, 2846–2859. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, V.; Guberti, V. African swine fever endemic persistence in wild boar populations: Key mechanisms explored through modelling. Transbound. Emerg. Dis. 2021, 68, 2812–2825. [Google Scholar] [CrossRef]
- Gervasi, V.; Marcon, A.; Guberti, V. Estimating the risk of environmental contamination by forest users in African Swine Fever endemic areas. Acta Vet. Scand. 2022, 64, 16. [Google Scholar] [CrossRef]
- Gavier-Widén, D.; Ståhl, K.; Dixon, L. No hasty solutions for African swine fever. Science 2020, 367, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Rohani, P. Modeling Infectious Diseases in Humans and Animals; Princeton University Press: Princeton, NJ, USA, 2008; p. 35. ISBN 9781400841035. [Google Scholar]
- Council Directive of the European Union 2002/60/EC. Specific Provisions for the Control of African Swine Fever and Amending Directive 92/119/EEC as Regards Teschen Disease and African Swine Fever. Off. J. Eur. Union L. 2002, 192, 27–46. [Google Scholar]
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar—Ecology and Biosecurity, 2nd ed.; FAO Animal Production and Health Manual No. 28; World Organisation for Animal Health; European Commission; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Gervasi, V.; Guberti, V. Combining hunting and intensive carcass removal to eradicate African swine fever from wild boar populations. Prev. Vet. Med. 2022, 203, 105633. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Reichold, A.; Thulke, H.-H. Modelling advanced knowledge of African swine fever, resulting surveillance patterns on the population level and impact to reliable exit strategy definition. EFSA Support. Publ. 2021, 6429, 33. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; et al. ASF exit strategy: Providing cumulative evidence of the absence of African swine fever virus circulation in wild boar populations using standard surveillance measures. EFSA J. 2021, 19, e06419. [Google Scholar]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African swine fever: An epidemiological update. Transbound. Emerg. Dis. 2012, 59, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, A.; Sotgia, S.; Patta, C.; Oggiano, A.; Carboni, A.; Cossu, P.; Laddomada, A. Temporal and spatial patterns of African swine fever in Sardinia. Prev. Vet. Med. 1998, 35, 297–306. [Google Scholar] [CrossRef]
- Cappai, S.; Rolesu, S.; Coccollone, A.; Laddomada, A.; Loi, F. Evaluation of biological and socio-economic factors related to persistence of African swine fever in Sardinia. Prev. Vet. Med. 2018, 152, 1–11. [Google Scholar] [CrossRef]
- Gulenkin, V.M.; Korennoy, F.I.; Karaulov, A.K.; Dudnikov, S.A. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Prev. Vet. Med. 2011, 102, 167–174. [Google Scholar] [CrossRef]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African swine fever virus isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef]
- Bosch, J.; Rodríguez, A.; Iglesias, I.; Muñoz, M.J.; Jurado, C.; Sánchez-Vizcaíno, J.M.; de la Torre, A. Update on the risk of introduction of African swine fever by wild boar into disease-free European Union countries. Transbound. Emerg. Dis. 2017, 64, 1424–1432. [Google Scholar] [CrossRef]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African swine fever status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Göldner, M.; Schatz, J.; et al. Joining the club: First detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 2020, 68, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Boklund, A.; Bøtner, A.; Chesnoiu, V.T.; Depner, K.; Desmecht, D.; Guberti, V.; Helyes, G.; Korytarova, D.; Linden, A.; et al. Epidemiological analyses of African swine fever in the European union (November 2018 to October 2019). EFSA J. 2020, 18, 5996. [Google Scholar]
- Iscaro, C.; Dondo, A.; Ruoco, L.; Masoero, L.; Giammarioli, M.; Zoppi, S.; Guberti, V.; Feliziani, F. January 2022: Index case of new African Swine Fever incursion in main land Italy. Transbound. Emerg. Dis. 2022, 69, 1707–1711. [Google Scholar] [CrossRef]
- Loi, F.; Cappai, S.; Coccollone, A.; Rolesu, S. Standardized Risk Analysis Approach Aimed to Evaluate the Last African Swine Fever Eradication Program Performance, in Sardinia. Front. Vet. Sci. 2019, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- OIE (World Organisation for Animal Health). African Swine Fever (ASF)—Situation Report 6. 2022. Available online: https://www.woah.org/app/uploads/2022/02/asf-report6.pdf (accessed on 19 August 2022).
- Iscaro, C.; Cambiotti, V.; Bessi, O.; Pacelli, F.; Ruoco, L.; Feliziani, F. Analysis of surveillance and prevention plan for African Swine Fever in Italy in 2020. Vet. Med. Sci. 2022, 8, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alcrudo, D.; Falco, J.R.; Raizman, E.; Dietze, K. Transboundary spread of pig diseases: The role of international trade and travel. BMC Vet. Res. 2019, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Dellicour, S.; Desmecht, D.; Paternostre, J.; Malengreaux, C.; Licoppe, A.; Gilbert, M.; Linden, A. Unravelling the dispersal dynamics and ecological drivers of the African swine fever outbreak in Belgium. J. Appl. Ecol. 2020, 57, 1619–1629. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Cortinas Abrahantes, J.; Gogin, A.; Richardson, J.; Gervelmeyer, A. Scientific report on epidemiological analyses on African swine fever in the Baltic countries and Poland. EFSA J. 2017, 15, 4732–4773. [Google Scholar]
- EFSA (European Food Safety Authority); Alvarez, J.; Bicout, D.; Boklund, A.; Bøtner, A.; Depner, K.; More, S.J.; Roberts, H.; Stahl, K.; Thulke, H.H.; et al. Research gap analysis on African swine fever. EFSA J. 2019, 17, e05811. [Google Scholar]
- Linden, A.; Licoppe, A.; Volpe, R.; Paternostre, J.; Lesenfants, C.; Cassart, D.; Garigliany, M.; Tignon, M.; Berg, T.V.D.; Desmecht, D.; et al. Summer 2018: African swine fever virus hits north-western Europe. Transbound. Emerg. Dis. 2019, 66, 54–55. [Google Scholar] [CrossRef]
- Saegerman, C. Découverte Inattendue De La Peste Porcine Africaine En Belgique. Épidémiol. St. Anim. 2018, 73, 147–164. [Google Scholar]
- Food and Agriculture Organization of the United Nations; World Organisation for Animal Health and European Commission. African Swine Fever in Wild Boar Ecology and Biosecurity; FAO: Rome, Italy, 2019. [Google Scholar]
- World Organisation for Animal Health. African Swine Fever. 2020. Available online: https://www.oie.int/en/animal-health-in-the-world/animal-diseases/african-swine-fever (accessed on 4 October 2022).
- Bailey, N.T.J. The Mathematical Theory of Infectious Diseases and Its Applications, 2nd ed.; Hafner: New York, NY, USA, 1975. [Google Scholar]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control, 2nd ed.; Oxford University Press: Oxford, UK, 1991; Chapter 6. [Google Scholar]
- Antia, R.; Regoes, R.R.; Koella, J.C.; Bergstrom, C.T. The role of evolution in the emergence of infectious diseases. Nature 2003, 426, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Arinaminpathy, N.; McLean, A.R. Evolution and emergence of novel human infections. Proc. R. Soc. B Biol. Sci. 2009, 276, 3937–3944. [Google Scholar] [CrossRef]
- Marcon, A.; Linden, A.; Satran, P.; Gervasi, V.; Licoppe, A.; Guberti, V. R0 Estimation for the African Swine Fever Epidemics in Wild Boar of Czech Republic and Belgium. Vet. Sci. 2020, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Muñoz, M.J.; Montes, F.; Perez, A.; Gogin, A.; Kolbasov, D.; de la Torre, A. Reproductive Ratio for the Local Spread of African Swine Fever in Wild Boars in the Russian Federation. Transbound. Emerg. Dis. 2016, 63, e237–e245. [Google Scholar] [CrossRef]
- Barongo, M.B.; Ståhl, K.; Bett, B.; Bishop, R.P.; Fèvre, E.M.; Aliro, T.; Okoth, E.; Masembe, C.; Knobel, D.; Ssematimba, A. Estimating the Basic Reproductive Number (R0) for African Swine Fever Virus (ASFV) Transmission between Pig Herds in Uganda. PLoS ONE 2015, 10, e0125842. [Google Scholar] [CrossRef] [PubMed]
- Korennoy, F.I.; Gulenkin, V.M.; Gogin, A.E.; Vergne, T.; Karaulov, A.K. Estimating the Basic Reproductive Number for African Swine Fever Using the Ukrainian Historical Epidemic of 1977. Transbound. Emerg. Dis. 2017, 64, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Nunez, A.; Neimanis, A.; Wikström-Lassa, E.; Montoya, M.; Crooke, H.; Gavier-Widén, D. African Swine Fever: Disease Dynamics in Wild Boar Experimentally Infected with ASFV Isolates Belonging to Genotype I and II. Viruses 2019, 11, 852. [Google Scholar] [CrossRef]
- Huang, M.; Xu, Z. Solving Systems of Quadratic Equations via Exponential-type Gradient Descent Algorithm. arXiv 2018, arXiv:1806.00904. [Google Scholar]
- Matschke, G.H. Aging European wild hogs by dentition. J. Wildl. Manag. 1967, 31, 109–113. [Google Scholar] [CrossRef]
- ENETWILD Consortium; Acevedo, P.; Croft, S.; Smith, G.; Blanco-Aguiar, J.A.; Fernandez-Lopez, J.; Scandura, M.; Apollonio, M.; Ferroglio, E.; Keuling, O.; et al. Update of occurrence and hunting yield-based data models for wild boar at European scale: New approach to handle the bioregion effect. EFSA J. 2020, 17, EN-1871. [Google Scholar]
- Hayes, B.H.; Andraud, M.; Salazar, L.G.; Rose, N.; Vergne, T. Mechanistic modelling of African swine fever: A systematic review. Prev. Vet. Med. 2021, 191, 105358. [Google Scholar] [CrossRef]
- Vynnycky, E.; White, R. An Introduction to Infectious Disease Modelling; OUP Oxford: New York, NY, USA, 2010; ISBN 978-0-19-151136-3. [Google Scholar]
- Desvaux, S.; Urbaniak, C.; Petit, T.; Chaigneau, P.; Gerbier, G.; Decors, A.; Reveillaud, E.; Chollet, J.-Y.; Petit, G.; Faure, E.; et al. How to Strengthen Wildlife Surveillance to Support Freedom from Disease: Example of ASF Surveillance in France, at the Border with an Infected Area. Front. Vet. Sci. 2021, 8, 647439. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.T.T. and Alexander, L.E. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998, 29, 207–231. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Sperling, D.; Bissonette, J.A.; Clevenger, A.P.; Cutshall, C.D.; Dale, V.H.; Fahrig, L.; France, R.; Goldman, C.R.; Heanue, K.; et al. Road Ecology: Science and Solutions; Island Press: Washington, DC, USA, 2003; 481p. [Google Scholar]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Forman, R.T.T. Land Mosaics. In The Ecology of Landscapes and Regions; Cambridge University Press: Cambridge, UK, 1995; 632p. [Google Scholar]
- Harris, L.D. The Fragmented Forest: Island Biogeography Theory and the Preservation Ofbiotic Diversity; University of Chicago Press: Chicago, IL, USA, 1984; 211p. [Google Scholar]
- Hanski, I. Metapopulation Ecology; Oxford University Press: Oxford, UK, 1999; 313p. [Google Scholar]
- Rossi, S.; Artois, M.; Pontier, D.; Hars, J.; Barrat, J.; Pacholek, X.; Fromont, E. Long-term monitoring of classical swine fever in wild boar (Sus scrofa sp.) using serological data. Vet. Res. 2005, 36, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.G.; Rose, N.; Hayes, B.; Hammami, P.; Baubet, E.; Desvaux, S.; Andraud, M. Effects of habitat fragmentation and hunting activities on African swine fever dynamics among wild boar populations. Prev. Vet. Med. 2022, 208, 105750. [Google Scholar] [CrossRef] [PubMed]
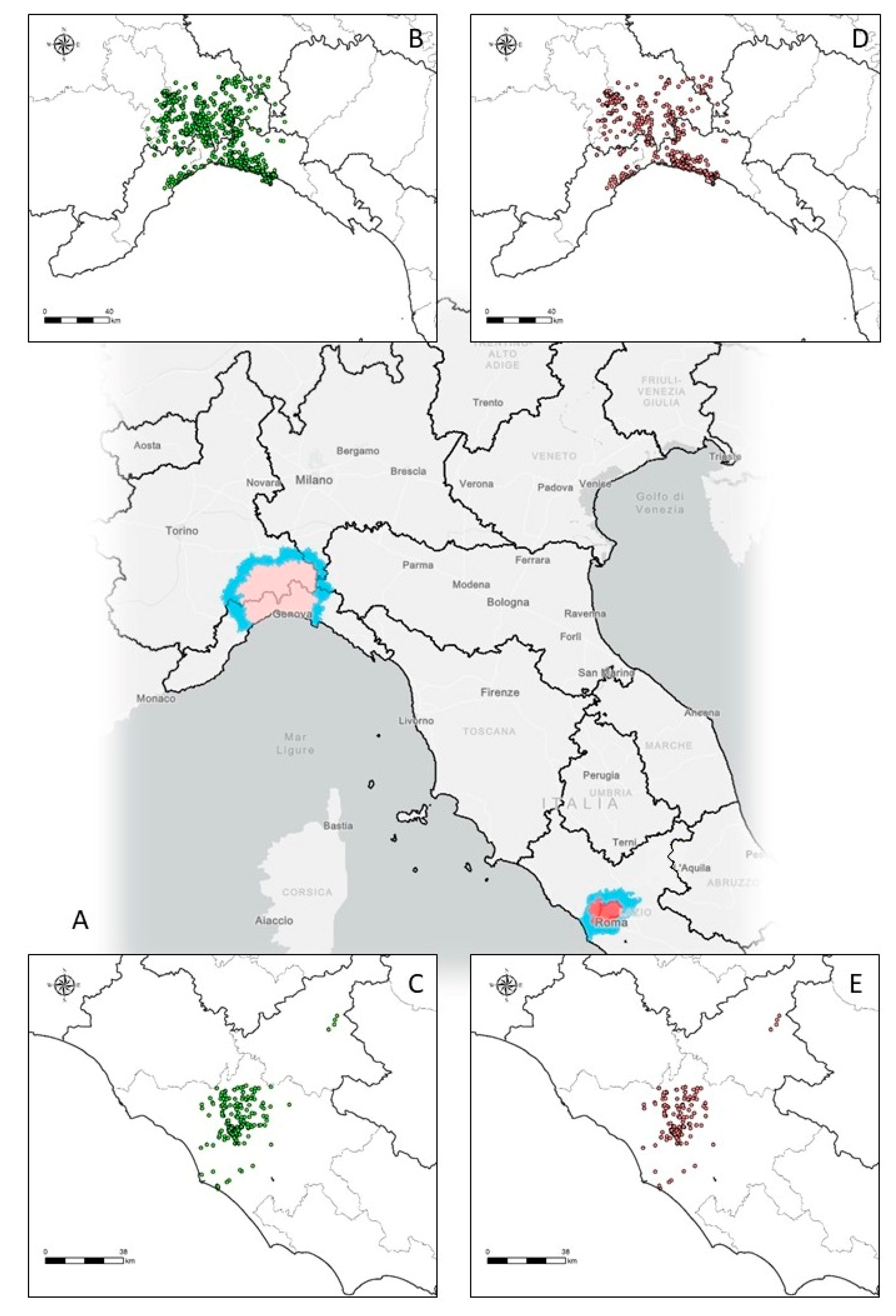
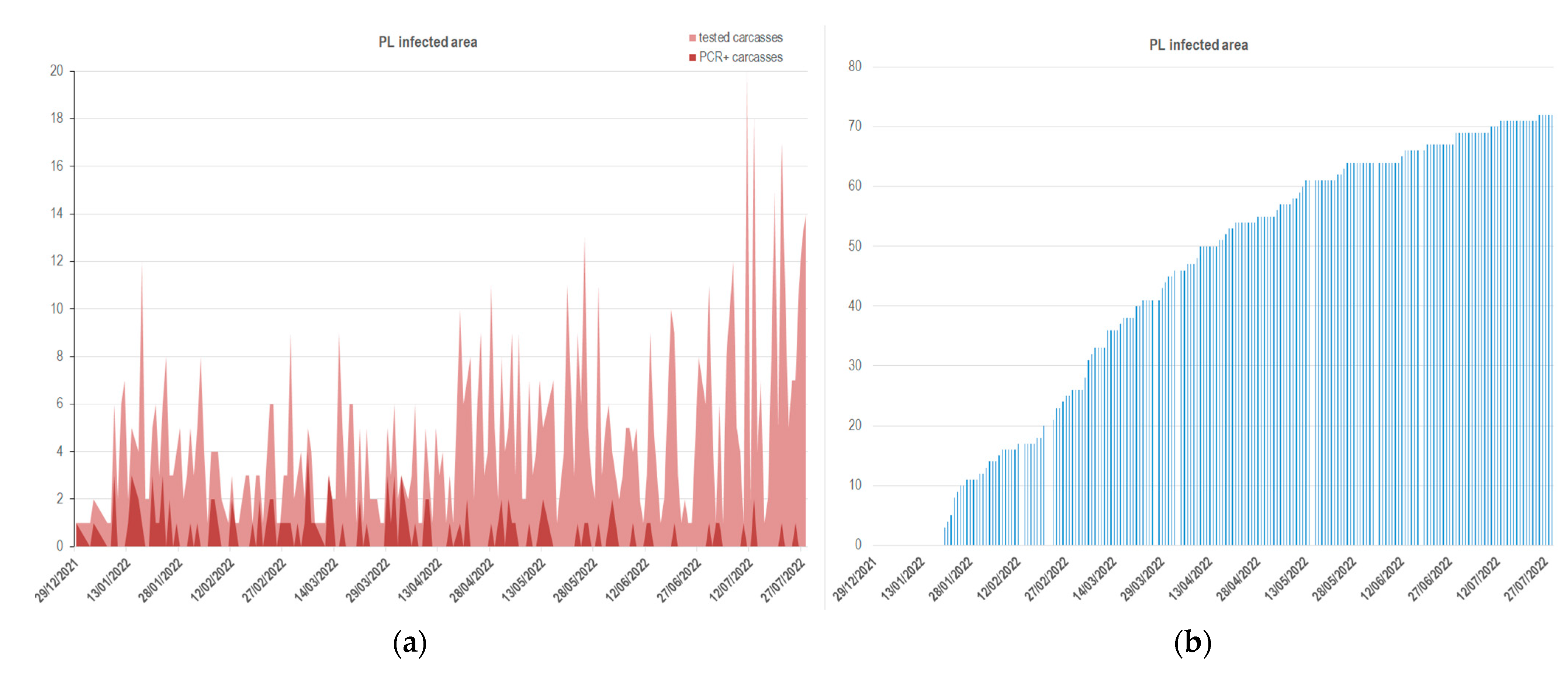

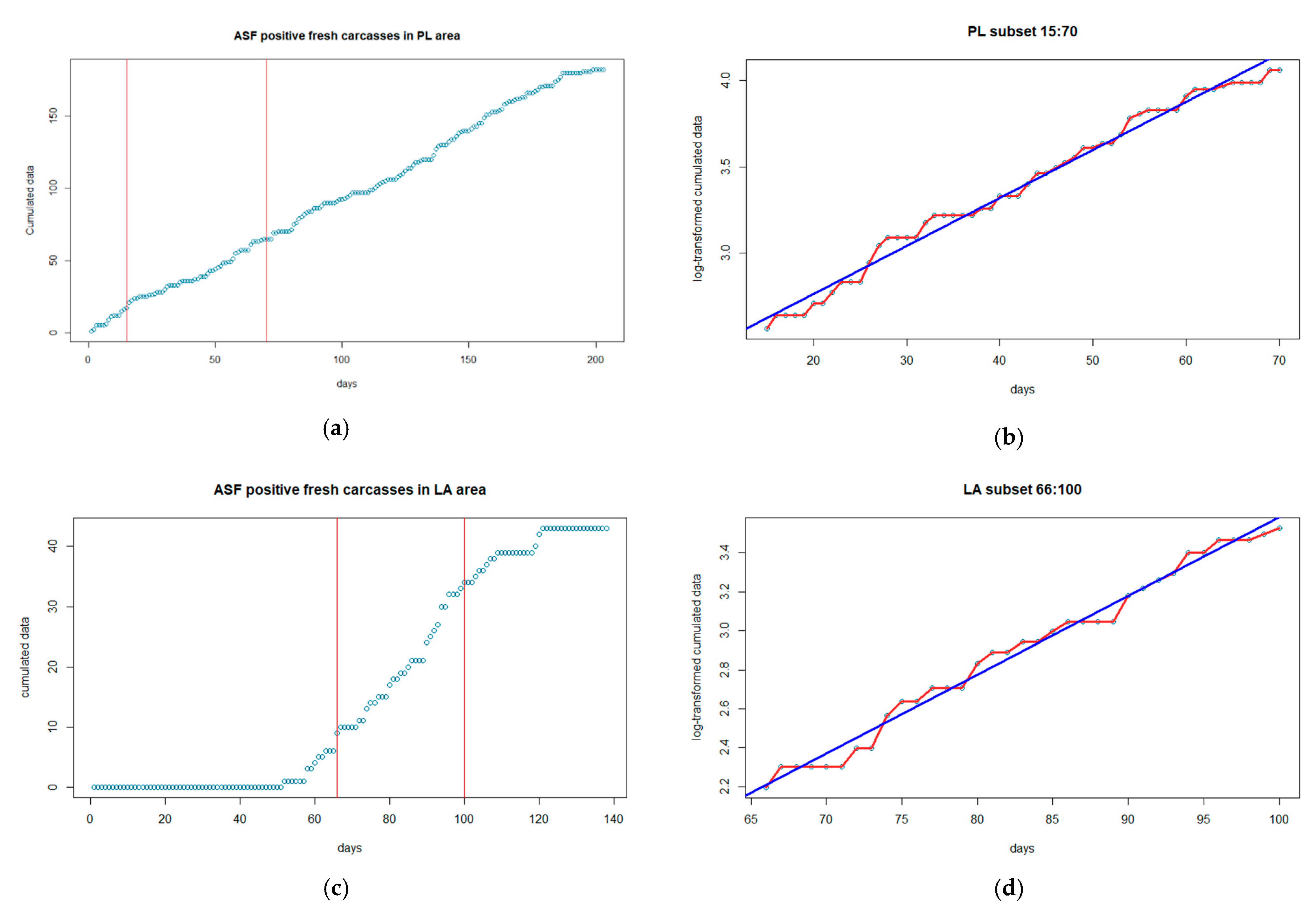
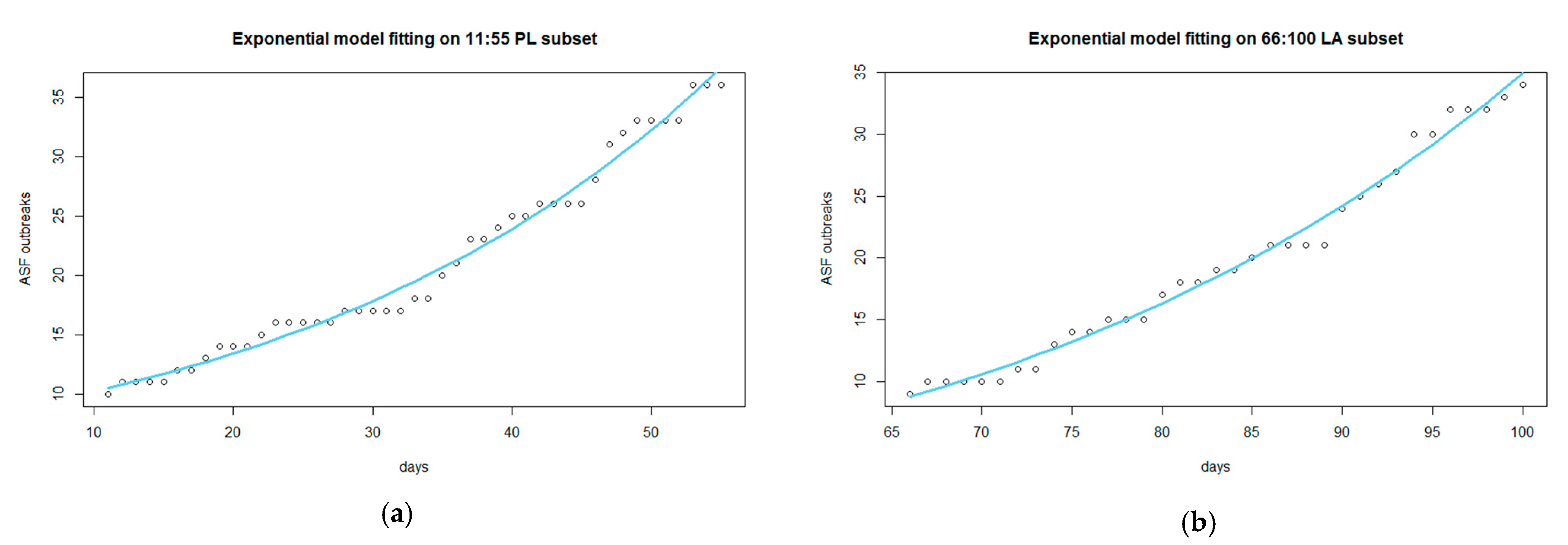
| Features of the Samples | Piedmont-Liguria (n = 1019) | Lazio (n = 287) |
|---|---|---|
| Female | 467 (51%) | 136 (58%) |
| Age | ||
| Young (0–6 months) | 272 (27%) | 148 (52%) |
| Subadult (6–18 months) | 277 (27%) | 56 (20%) |
| Adult (>18 months) | 379 (37%) | 81 (28%) |
| NA | 91 (17%) | 2 (9%) |
| Source of the sample | ||
| Active surveillance † | 411 (40%) | 191 (66%) |
| Passive surveillance * | 608 (59%) | 96 (33%) |
| PCR+ samples from active surveillance † | ||
| Young (0–6 months) | 1 (33%) | 0 (0%) |
| Subadult (6–18 months) | 1 (33%) | 0 (0%) |
| Adult (>18 months) | 1 (33%) | 0 (0%) |
| PCR+ samples from passive surveillance * | ||
| Young (0–6 months) | 22 (13%) | 16 (35%) |
| Subadult (6–18 months) | 38 (22%) | 12 (27%) |
| Adult (>18 months) | 68 (40%) | 17 (38%) |
| NA | 42 (25%) | 0 (0%) |
| Carcass conservation stage | ||
| Fresh | 416 (90%) | 48 (50%) |
| In decomposition | 146 (24%) | 40 (42%) |
| Advanced decomposition | 25 (4%) | 6 (6%) |
| NA | 21 (3%) | 2 (2%) |
| PCR+ fresh carcasses | 72 (17%) | 19 (20%) |
| Infected Area | α | β | θ | Doubling Time | R0 (95% Bootstrap Confidence Interval) |
|---|---|---|---|---|---|
| Piedmont-Liguria | 26.14 | 0.016 | −20.90 | 11.89 days | 1.41 [1.37–1.45] |
| Lazio | 1.74 | 0.031 | −5.07 | 7.33 days | 1.66 [1.61–1.75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loi, F.; Di Sabatino, D.; Baldi, I.; Rolesu, S.; Gervasi, V.; Guberti, V.; Cappai, S. Estimation of R0 for the Spread of the First ASF Epidemic in Italy from Fresh Carcasses. Viruses 2022, 14, 2240. https://doi.org/10.3390/v14102240
Loi F, Di Sabatino D, Baldi I, Rolesu S, Gervasi V, Guberti V, Cappai S. Estimation of R0 for the Spread of the First ASF Epidemic in Italy from Fresh Carcasses. Viruses. 2022; 14(10):2240. https://doi.org/10.3390/v14102240
Chicago/Turabian StyleLoi, Federica, Daria Di Sabatino, Ileana Baldi, Sandro Rolesu, Vincenzo Gervasi, Vittorio Guberti, and Stefano Cappai. 2022. "Estimation of R0 for the Spread of the First ASF Epidemic in Italy from Fresh Carcasses" Viruses 14, no. 10: 2240. https://doi.org/10.3390/v14102240
APA StyleLoi, F., Di Sabatino, D., Baldi, I., Rolesu, S., Gervasi, V., Guberti, V., & Cappai, S. (2022). Estimation of R0 for the Spread of the First ASF Epidemic in Italy from Fresh Carcasses. Viruses, 14(10), 2240. https://doi.org/10.3390/v14102240





