Systematic Genomic Surveillance of SARS-CoV-2 Virus on Illumina Sequencing Platforms in the Slovak Republic—One Year Experience
Abstract
1. Introduction
2. Material and Methods
2.1. SARS-CoV-2 Samples
2.2. RNA Isolation
2.3. NGS Sequencing
2.4. Bioinformatic Analysis
2.5. SARS-CoV-2 Phylogeny
3. Results
3.1. SARS-CoV-2 Genome Surveillance Program
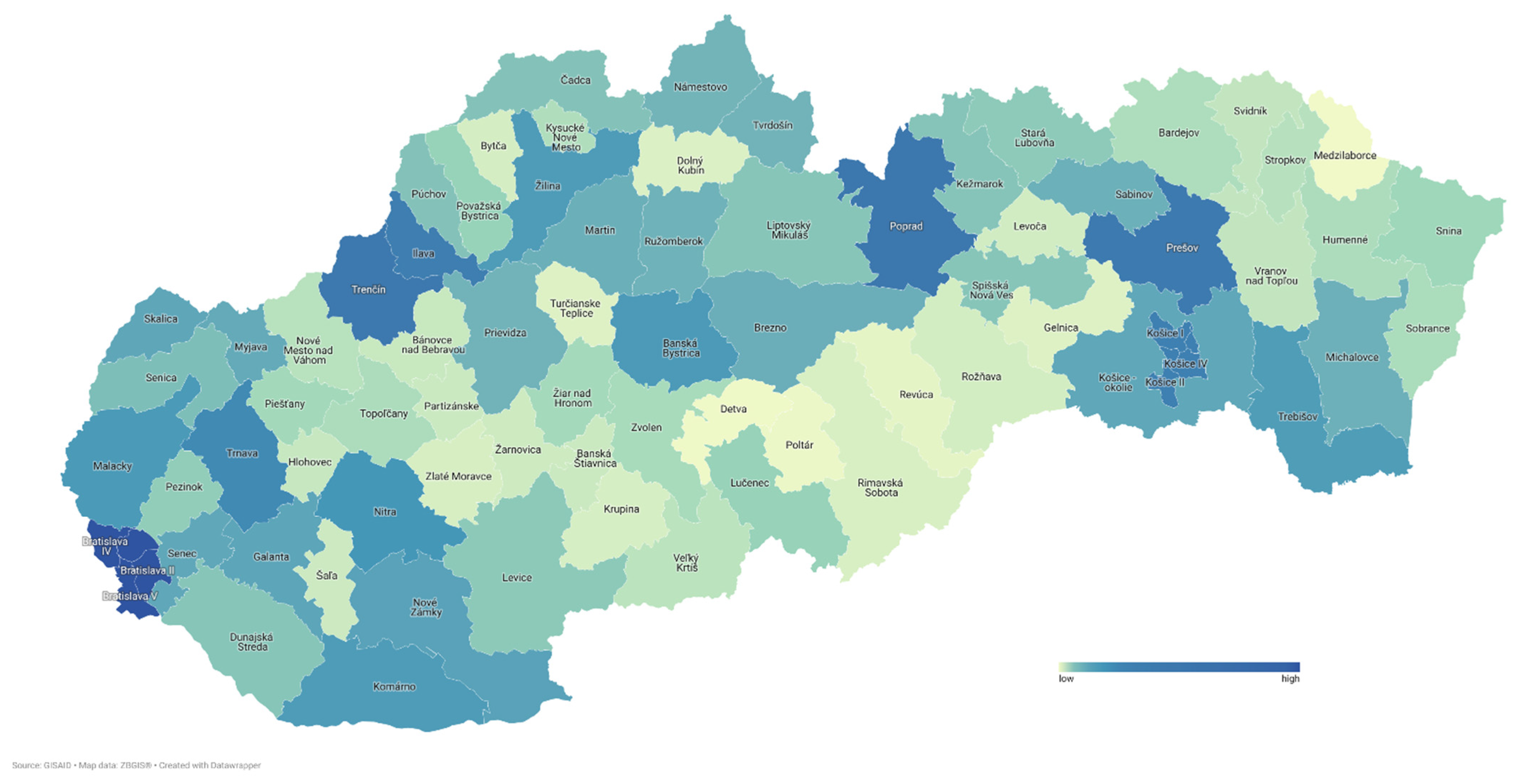
3.2. Sample Characteristics
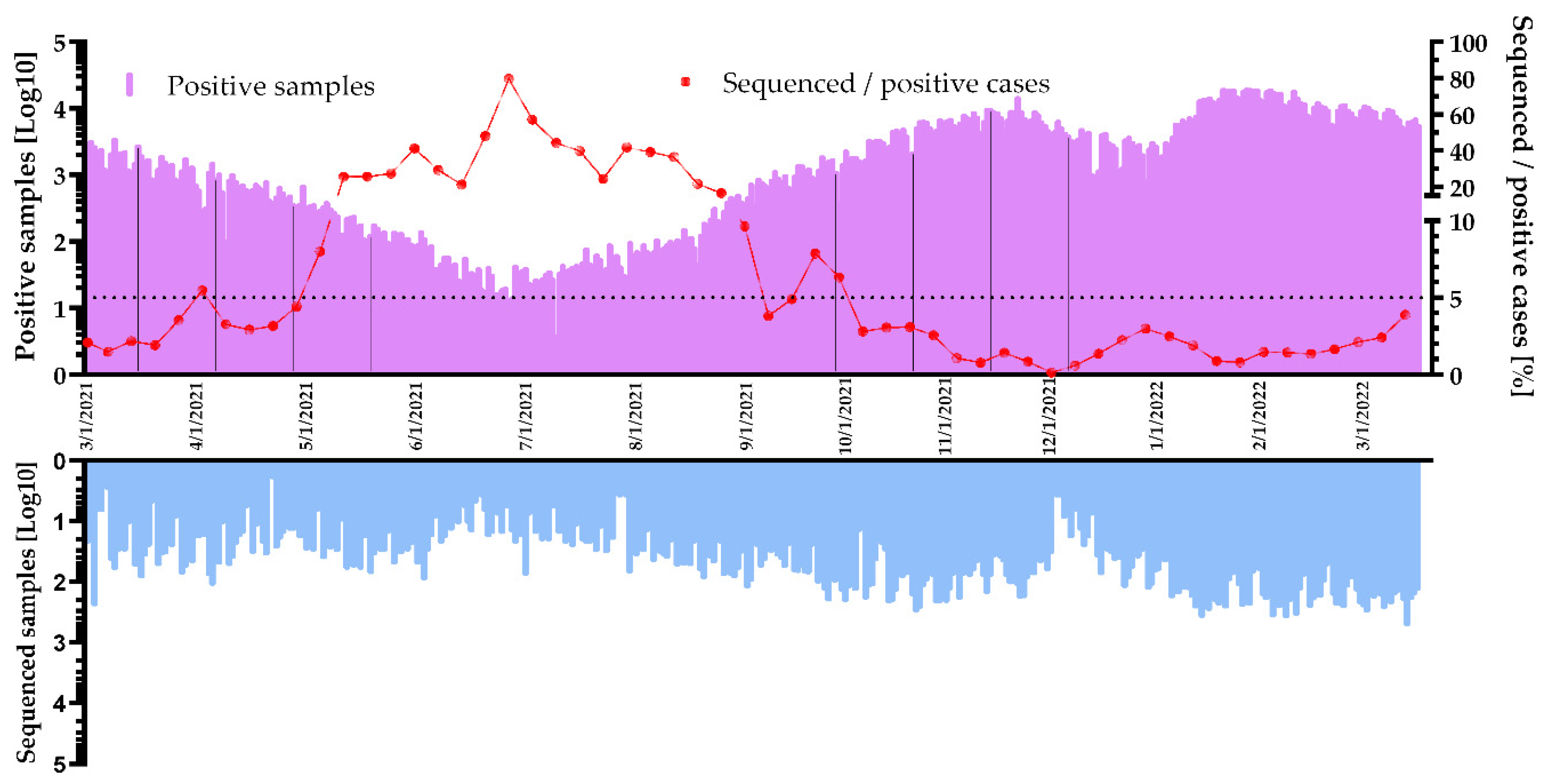
3.3. Sequencing Volume of the Surveillance Program
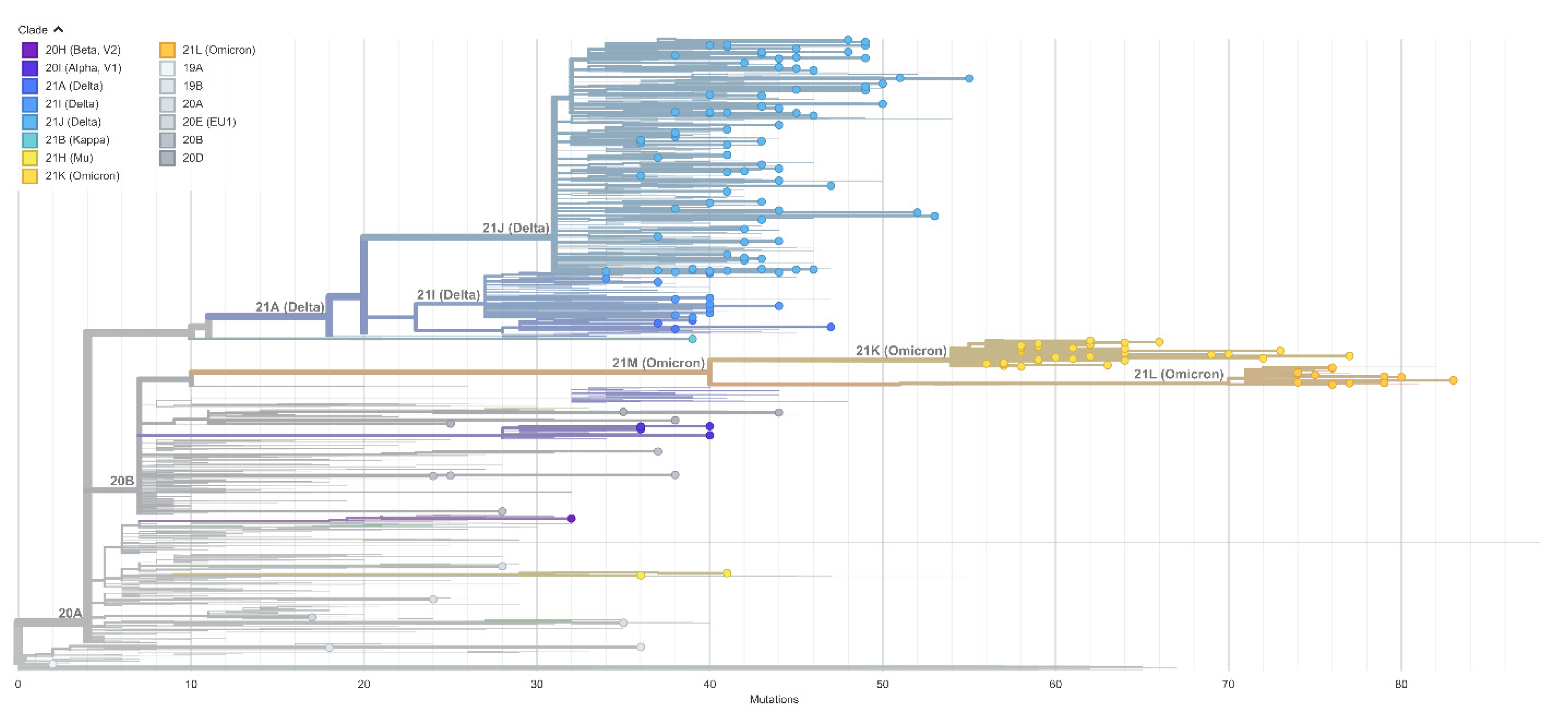
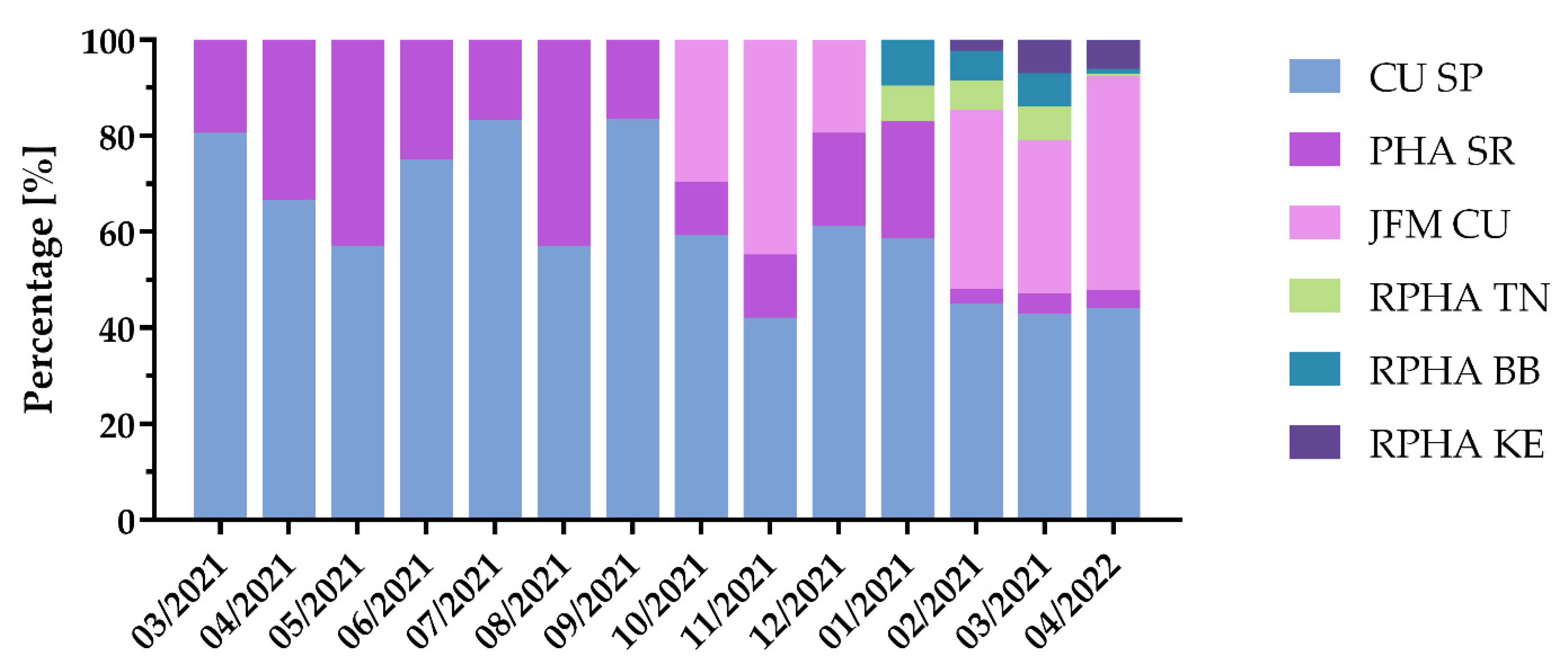
3.4. Virus Variant and Lineage Prevalence
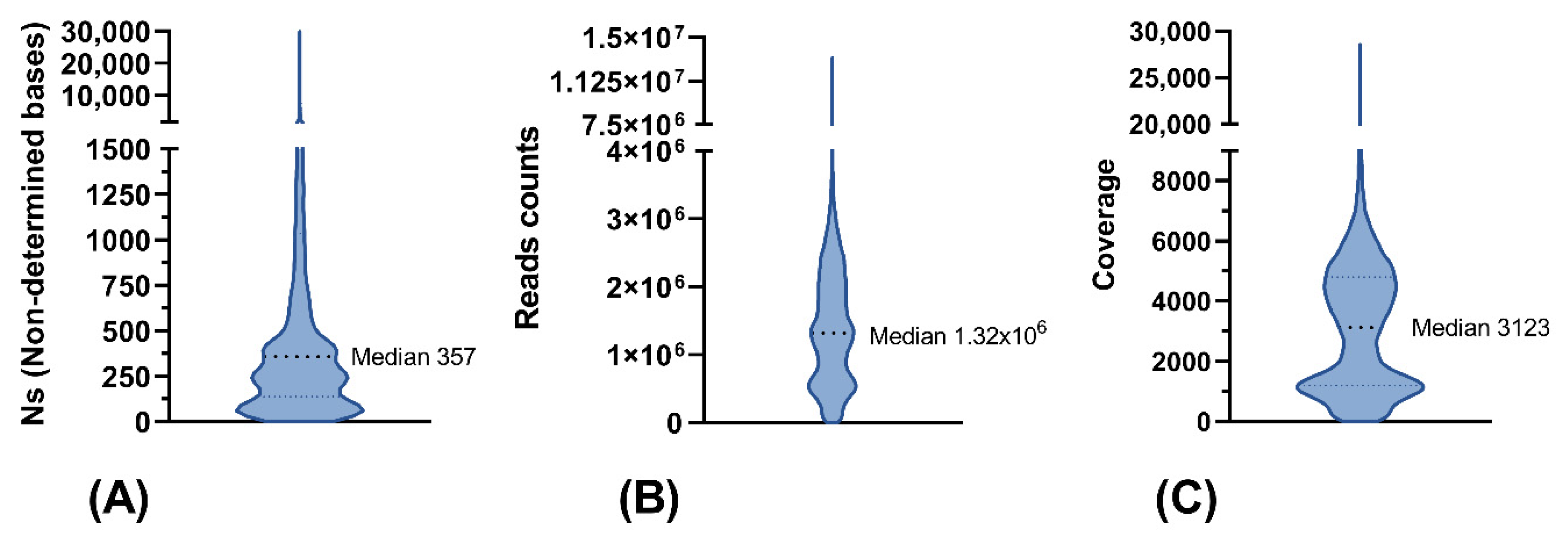
3.5. Sequence Quality of NGS Data
3.6. Genome Sequencing Centers Participating in the SARS-CoV-2 Genomic Surveillance Program
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 29 June 2022).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 29 June 2022).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Tan, W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- John, G.; Sahajpal, N.S.; Mondal, A.K.; Ananth, S.; Williams, C.; Chaubey, A.; Kolhe, R. Next-Generation Sequencing (NGS) in COVID-19: A Tool for SARS-CoV-2 Diagnosis, Monitoring New Strains and Phylodynamic Modeling in Molecular Epidemiology. Curr. Issues Mol. Biol. 2021, 43, 845–867. [Google Scholar] [CrossRef] [PubMed]
- Chiara, M.; D’Erchia, A.M.; Gissi, C.; Manzari, C.; Parisi, A.; Resta, N.; Zambelli, F.; Picardi, E.; Pavesi, G.; Horner, D.S. Next generation sequencing of SARS-CoV-2 genomes: Challenges, applications and opportunities. Brief. Bioinform. 2021, 22, 616–630. [Google Scholar] [CrossRef]
- Novel Coronavirus—China. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON233 (accessed on 29 June 2022).
- Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on the risk related to the spread of new SARS-CoV-2 variants of concern in the EU/EEA—First update. Eurosurveillance 2021, 26, 2101211. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Tan, W. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 29 June 2022).
- GISAID—hCov19 Variants. Available online: https://www.gisaid.org/hcov19-variants (accessed on 29 June 2022).
- CoVariants. Available online: https://covariants.org/ (accessed on 29 June 2022).
- Cov-Lineages. Available online: https://cov-lineages.org/lineage_list.html (accessed on 29 June 2022).
- European Centre for Disease Prevention and Control. SARS-CoV-2 Variants of Concern as of 9 June 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 29 June 2022).
- European Centre for Disease Prevention and Control. Guidance for Representative and Targeted Genomic SARS-CoV-2 Monitoring. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/guidance-representative-and-targeted-genomic-sars-cov-2-monitoring (accessed on 29 June 2022).
- COVID-19 Genomics UK (COG-UK) Consortium. An integrated national scale SARS-CoV-2 genomic surveillance network. Lancet Microbe 2020, 1, e99–e100. [Google Scholar] [CrossRef]
- Wilkinson, E.; Giovanetti, M.; Tegally, H.; San, J.E.; Lessells, R.; Cuadros, D.; Tumedi, K.A. A year of genomic surveillance reveals how the SARS-CoV-2 pandemic unfolded in Africa. Science 2021, 374, 423–431. [Google Scholar] [CrossRef]
- Lamarca, A.P.; de Almeida, L.G.P.; da Silva Francisco, R.; Cavalcante, L.; Machado, D.T.; Brustolini, O.; Vasconcelos, A.T.R. Genomic Surveillance Tracks the First Community Outbreak of the SARS-CoV-2 Delta (B.1.617.2) Variant in Brazil. J. Virol. 2022, 96, e01228-21. [Google Scholar] [CrossRef]
- Dzinamarira, T.; Mukwenha, S.; Mukandavire, Z.; Cuadros, D.F.; Murewanhema, G.; Madziva, R.; Musuka, G. Insights from Zimbabwe’s SARS-CoV-2 genomic surveillance. Lancet Glob. Health 2021, 9, e1624–e1625. [Google Scholar] [CrossRef]
- Lancet, T. Genomic sequencing in pandemics. Lancet 2021, 397, 445. [Google Scholar] [CrossRef]
- Brito, A.F.; Semenova, E.; Dudas, G.; Hassler, G.W.; Kalinich, C.C.; Kraemer, M.U.G.; Ho, J.; Tegally, H.; Githinji, G.; Agoti, C.N.; et al. Global disparities in SARS-CoV-2 genomic surveillance. medRxiv 2021, medRxiv:21262393. [Google Scholar] [CrossRef]
- Goga, A.; Böhmer, M.; Hekel, R.; Krampl, W. SnakeLines Workflow for SARS-CoV-2 Variant Detection from Next-Generation Sequencing Reads And Theory, ITAT 2021. Available online: http://ceur-ws.org/Vol-2962/paper15.pdf (accessed on 29 June 2022).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. Available online: http://journal.embnet.org/index.php/embnetjournal/article/view/200 (accessed on 8 June 2022). [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Li, H. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Aksamentov, I.; Roemer, C.; Hodcroft, E.B.; Neher, R.A. Nextclade: Clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 2021, 6, 3773. [Google Scholar] [CrossRef]
- EMBL-EBI. ENA Browser. Available online: http://www.ebi.ac.uk/ena (accessed on 29 June 2022).
- GISAID—Initiative. Available online: https://www.gisaid.org (accessed on 29 June 2022).
- Budiš, J.; Krampl, W.; Kucharík, M.; Hekel, R.; Lichvár, M.; Smol’ak, D.; Szemes, T. SnakeLines: Integrated set of computational pipelines for paired-end sequencing reads. arXiv 2020, arXiv:2106.13649. [Google Scholar]
- Nextclade. Available online: https://clades.nextstrain.org (accessed on 29 June 2022).
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef]
- Národné Centrum Zdravotníckych Informácií. Available online: http://www.nczisk.sk/ (accessed on 29 June 2022).
- Statistical Office of the Slovak Republic. Available online: https://www.scitanie.sk/en (accessed on 24 October 2022).
- El-Shabasy, R.M.; Nayel, M.A.; Taher, M.M.; Abdelmonem, R.; Shoueir, K.R.; Kenawy, E.R. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int. J. Biol. Macromol. 2022, 204, 161–168. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Zhang, Y.Z. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Bernard Stoecklin, S.; Rolland, P.; Silue, Y.; Mailles, A.; Campese, C.; Simondon, A.; Levy-Bruhl, D. First cases of coronavirus disease 2019 (COVID-19) in France: Surveillance, investigations and control measures, January 2020. Eurosurveillance 2020, 25, 2000094. [Google Scholar] [CrossRef]
- API. Available online: https://data.korona.gov.sk (accessed on 29 June 2022).
- Preliminary Genomic Characterisation of an Emergent SARS-CoV-2 Lineage in the UK Defined by a Novel Set of Spike Mutations. 2020. Available online: https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 (accessed on 22 February 2022).
- Alm, E.; Broberg, E.K.; Connor, T.; Hodcroft, E.B.; Komissarov, A.B.; Maurer-Stroh, S.; Pereyaslov, D. Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European Region, January to June 2020. Eurosurveillance 2020, 25, 2001410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Azman, A.S.; Chen, X.; Zou, J.; Tian, Y.; Sun, R.; Yu, H. Global landscape of SARS-CoV-2 genomic surveillance and data sharing. Nat. Genet. 2022, 54, 499–507. [Google Scholar] [CrossRef] [PubMed]
- National Health Information Center. Available online: https://covid-19.nczisk.sk/en (accessed on 24 October 2022).
- Duong, B.V.; Larpruenrudee, P.; Fang, T.; Hossain, S.I.; Saha, S.C.; Gu, Y.; Islam, M.S. Is the SARS-CoV-2 Omicron Variant Deadlier and More Transmissible Than Delta Variant? Int. J. Environ. Res. Public Health 2022, 19, 4586. [Google Scholar] [CrossRef] [PubMed]
- Severity of Disease Associated with Omicron Variant as Compared with Delta Variant in Hospitalized Patients with Suspected or Confirmed SARS-CoV-2 Infection. Available online: https://www.who.int/publications/i/item/9789240051829 (accessed on 24 October 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusňáková, D.; Sedláčková, T.; Radvák, P.; Böhmer, M.; Mišenko, P.; Budiš, J.; Bokorová, S.; Lipková, N.; Forgáčová-Jakúbková, M.; Sládeček, T.; et al. Systematic Genomic Surveillance of SARS-CoV-2 Virus on Illumina Sequencing Platforms in the Slovak Republic—One Year Experience. Viruses 2022, 14, 2432. https://doi.org/10.3390/v14112432
Rusňáková D, Sedláčková T, Radvák P, Böhmer M, Mišenko P, Budiš J, Bokorová S, Lipková N, Forgáčová-Jakúbková M, Sládeček T, et al. Systematic Genomic Surveillance of SARS-CoV-2 Virus on Illumina Sequencing Platforms in the Slovak Republic—One Year Experience. Viruses. 2022; 14(11):2432. https://doi.org/10.3390/v14112432
Chicago/Turabian StyleRusňáková, Diana, Tatiana Sedláčková, Peter Radvák, Miroslav Böhmer, Pavol Mišenko, Jaroslav Budiš, Silvia Bokorová, Nikola Lipková, Michaela Forgáčová-Jakúbková, Tomáš Sládeček, and et al. 2022. "Systematic Genomic Surveillance of SARS-CoV-2 Virus on Illumina Sequencing Platforms in the Slovak Republic—One Year Experience" Viruses 14, no. 11: 2432. https://doi.org/10.3390/v14112432
APA StyleRusňáková, D., Sedláčková, T., Radvák, P., Böhmer, M., Mišenko, P., Budiš, J., Bokorová, S., Lipková, N., Forgáčová-Jakúbková, M., Sládeček, T., Sitarčík, J., Krampl, W., Gažiová, M., Kaliňáková, A., Staroňová, E., Tichá, E., Vrábľová, T., Ševčíková, L., Kotvasová, B., ... Szemes, T. (2022). Systematic Genomic Surveillance of SARS-CoV-2 Virus on Illumina Sequencing Platforms in the Slovak Republic—One Year Experience. Viruses, 14(11), 2432. https://doi.org/10.3390/v14112432





