Effects of Achieving SVR on Clinical Characteristics and Surgical Outcomes in Patients Who Developed Early-Stage HCV-Related Hepatocellular Carcinoma and Received Curative Resection: Preoperative versus Postoperative SVR
Abstract
1. Introduction
2. Materials and Methods
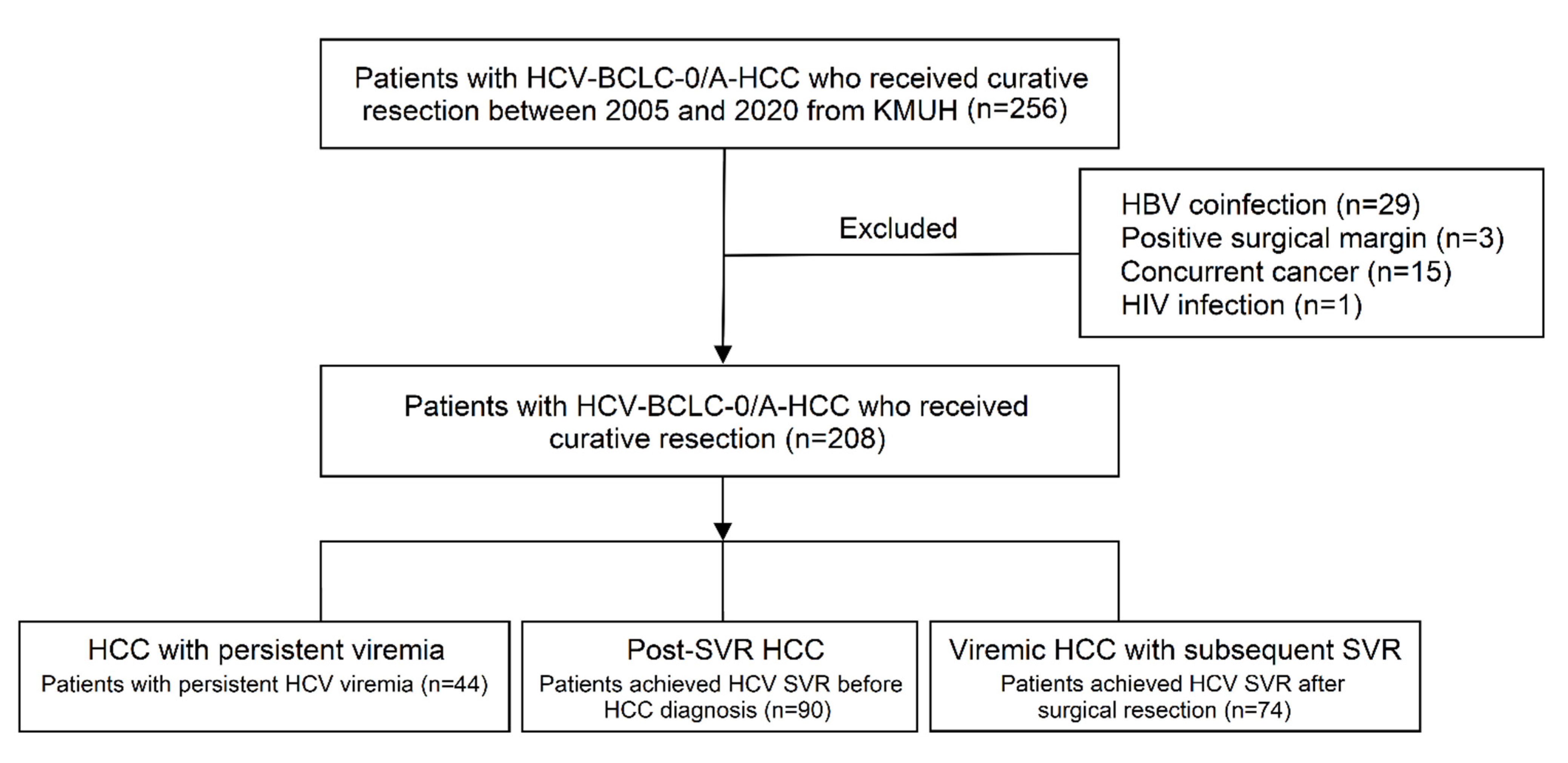
2.1. Study Population
2.2. Data Collection
2.3. Surgical Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
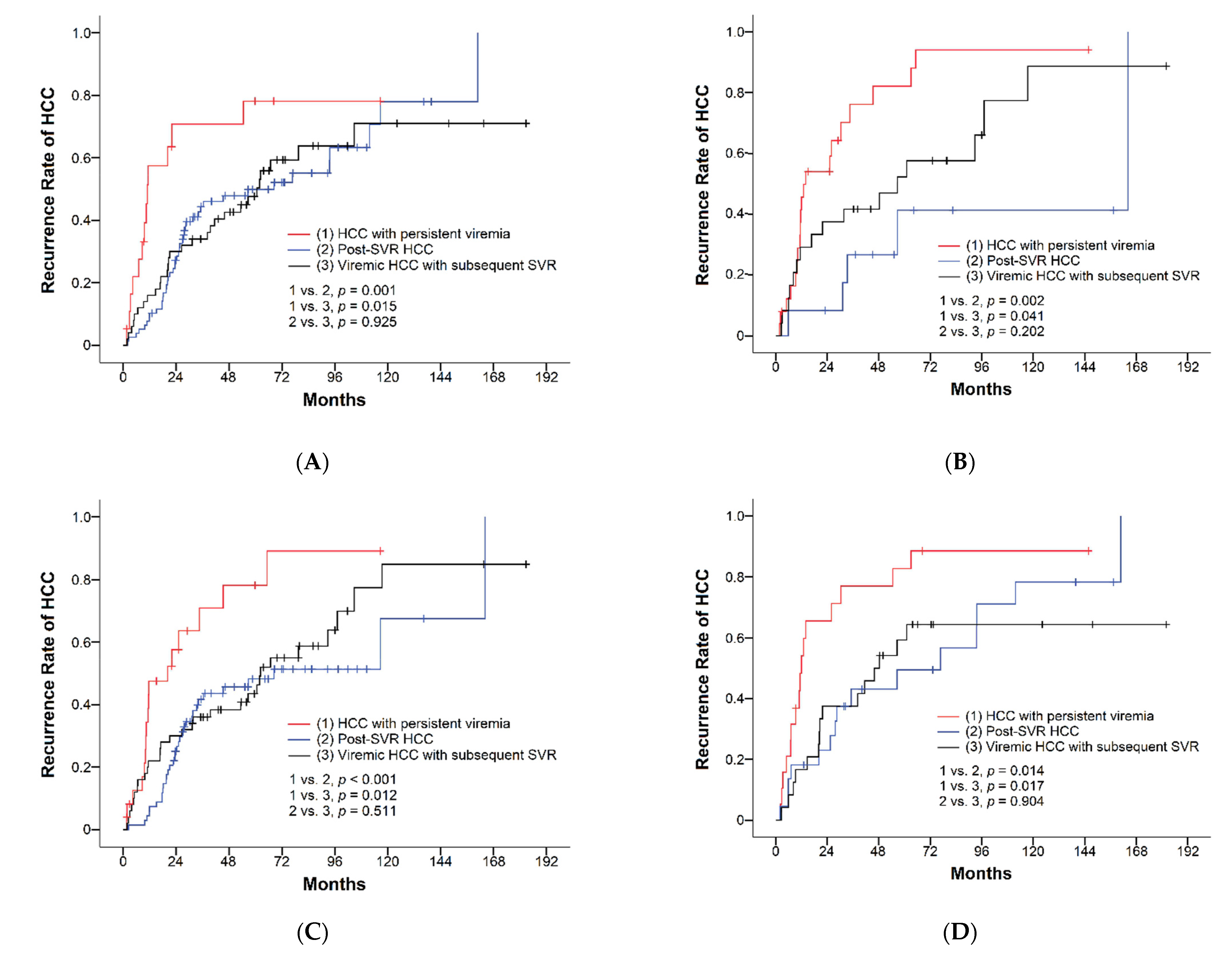
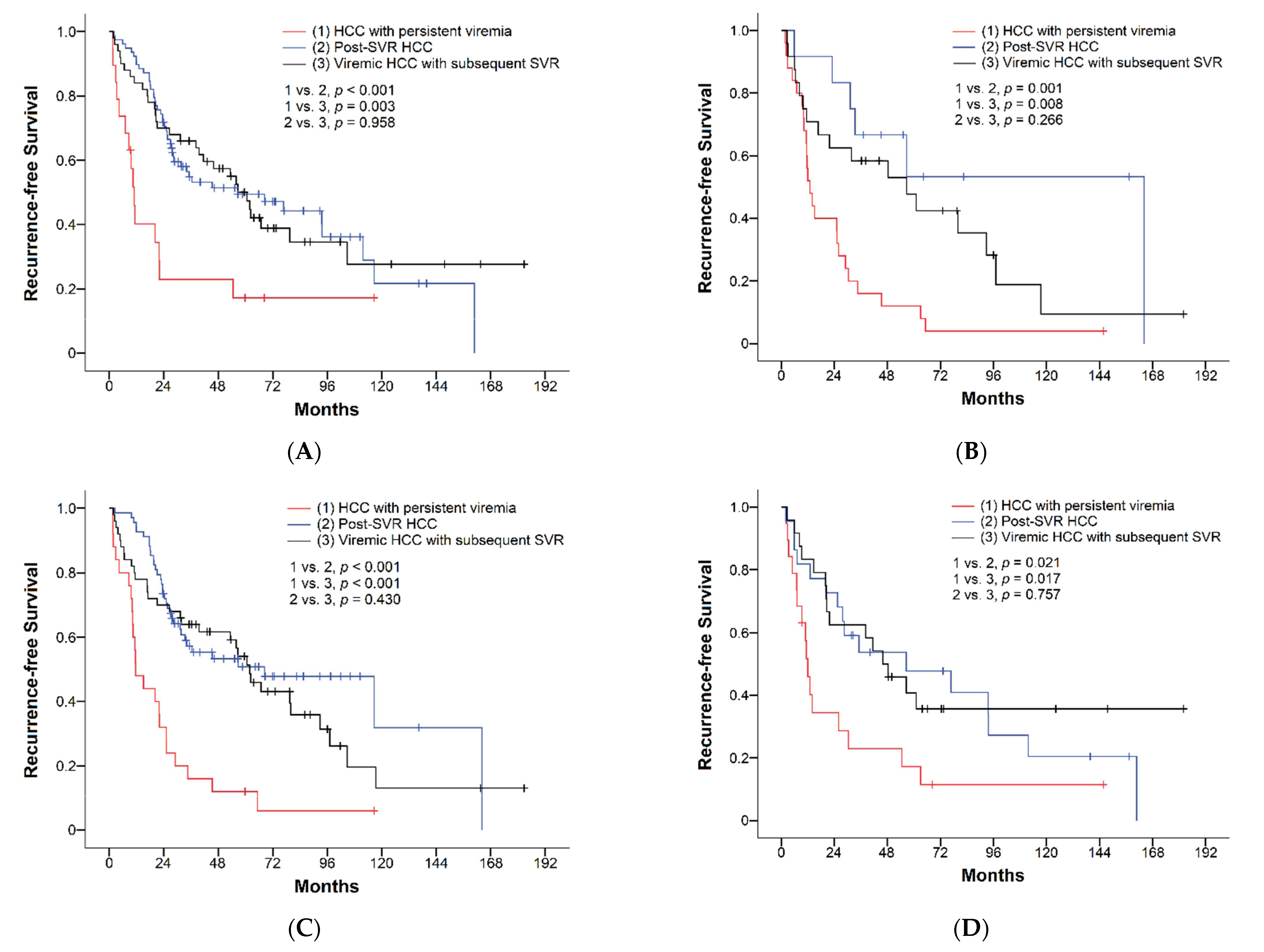
3.2. Surgical Outcomes of HCC with Different Viral Status
3.3. Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer, World Health Organization. Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 31 August 2022).
- Yu, M.L.; Yeh, M.L.; Tsai, P.C.; Huang, C.I.; Huang, J.F.; Huang, C.F.; Hsieh, M.H.; Liang, P.C.; Lin, Y.H.; Hsieh, M.Y.; et al. Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: A nationwide survey in Taiwan. Medicine 2015, 94, e690. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.; Waser, N.; Johnston, K.; Kao, J.H.; Lim, Y.S.; Duan, Z.P.; Lee, Y.J.; Wei, L.; Chen, C.J.; Sievert, W.; et al. A review of the burden of hepatitis C virus infection in China, Japan, South Korea and Taiwan. Hepatol. Int. 2015, 9, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S. Hepatocellular carcinoma in Taiwan. Hepatol. Res. 2007, 37 (Suppl. S2), S101–S105. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Hsu, C.; Chen, L.T.; Cheng, C.C.; Hu, F.C.; Cheng, A.L. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): A meta-regression approach. J. Hepatol. 2010, 52, 889–894. [Google Scholar] [CrossRef]
- Miyake, Y.; Takaki, A.; Iwasaki, Y.; Yamamoto, K. Meta-analysis: Interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J. Viral Hepat. 2010, 17, 287–292. [Google Scholar] [CrossRef]
- Breitenstein, S.; Dimitroulis, D.; Petrowsky, H.; Puhan, M.A.; Müllhaupt, B.; Clavien, P.-A. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br. J. Surg. 2009, 96, 975–981. [Google Scholar] [CrossRef]
- Ravi, S.; Axley, P.; Jones, D.; Kodali, S.; Simpson, H.; McGuire, B.M.; Singal, A.K. Unusually High Rates of Hepatocellular Carcinoma After Treatment With Direct-Acting Antiviral Therapy for Hepatitis C Related Cirrhosis. Gastroenterology 2017, 152, 911–912. [Google Scholar] [CrossRef]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Frazzoni, L.; Sikandar, U.; Metelli, F.; Sadalla, S.; Mazzella, G.; Bazzoli, F.; Fuccio, L.; Azzaroli, F. Hepatocellular Carcinoma Recurrence after Hepatitis C Virus Therapy with Direct-Acting Antivirals. A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1694. [Google Scholar] [CrossRef]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.P.; et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- The ANRS Collaborative Study Group on Hepatocellular Carcinoma. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J. Hepatol. 2016, 65, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Lee, C.M.; Wang, J.H.; Hu, T.H.; Chen, C.H.; Lin, C.Y.; Lu, S.N. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int. J. Cancer 2011, 128, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Ooka, Y.; Miho, K.; Shuntaro, O.; Nakamura, M.; Ogasawara, S.; Suzuki, E.; Yasui, S.; Chiba, T.; Arai, M.; Kanda, T.; et al. Prediction of the very early occurrence of HCC right after DAA therapy for HCV infection. Hepatol. Int. 2018, 12, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Tsuchiya, K.; Nishimura, T.; Muraoka, M.; Suzuki, Y.; Tamaki, N.; Yasui, Y.; Hosokawa, T.; Ueda, K.; Nakanishi, H.; et al. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology 2013, 58, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.Y.; Lin, C.Y.; Tsai, P.C.; Lin, P.Y.; Yeh, M.L.; Huang, C.F.; Chang, W.T.; Huang, J.F.; Yu, M.L.; Chen, Y.L. Impact of tumor size on the prognosis of hepatocellular carcinoma in patients who underwent liver resection. J. Chin. Med. Assoc. 2018, 81, 155–163. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of hepatocellular cancer after resection: Patterns, treatments, and prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar] [CrossRef]
- Chan, A.W.H.; Zhong, J.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.; Tada, T.; Chong, C.C.N.; Xiang, B.D.; Li, L.Q.; et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef]
- Andreana, L.; Burroughs, A.K. Treatment of early hepatocellular carcinoma: How to predict and prevent recurrence. Dig. Liver Dis. 2010, 42 (Suppl. S3), S249–S257. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Scheuer, P.J. Classification of chronic viral hepatitis: A need for reassessment. J. Hepatol. 1991, 13, 372–374. [Google Scholar] [CrossRef]
- Sou, F.M.; Wu, C.K.; Chang, K.C.; Lu, S.N.; Wang, J.H.; Hung, C.H.; Chen, C.H.; Kee, K.M.; Yen, Y.H.; Lin, M.T.; et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J. Formos. Med. Assoc. 2019, 118, 504–513. [Google Scholar] [CrossRef]
- Yeh, M.L.; Liang, P.C.; Tsai, P.C.; Wang, S.C.; Leong, J.; Ogawa, E.; Jun, D.W.; Tseng, C.H.; Landis, C.; Tanaka, Y.; et al. Characteristics and Survival Outcomes of Hepatocellular Carcinoma Developed after HCV SVR. Cancers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Calvaruso, V.; Craxì, A. Hepatic benefits of HCV cure. J. Hepatol. 2020, 73, 1548–1556. [Google Scholar] [CrossRef]
- Nakajima, M.; Kobayashi, S.; Wada, H.; Tomokuni, A.; Takahashi, H.; Noda, T.; Matsui, H.; Matsukuma, S.; Kanekiyo, S.; Shindo, Y.; et al. Viral elimination is essential for improving surgical outcomes of hepatitis C virus-related hepatocellular carcinoma: Multicenter retrospective analysis. Ann. Gastroenterol. Surg. 2020, 4, 710–720. [Google Scholar] [CrossRef]
- Perez, S.; Kaspi, A.; Domovitz, T.; Davidovich, A.; Lavi-Itzkovitz, A.; Meirson, T.; Alison Holmes, J.; Dai, C.Y.; Huang, C.F.; Chung, R.T.; et al. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019, 15, e1008181. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.; et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist after Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e7. [Google Scholar] [CrossRef]
- Singal, A.G.; Rich, N.E.; Mehta, N.; Branch, A.; Pillai, A.; Hoteit, M.; Volk, M.; Odewole, M.; Scaglione, S.; Guy, J.; et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019, 156, 1683–1692.e1. [Google Scholar] [CrossRef]
- Dang, H.; Yeo, Y.H.; Yasuda, S.; Huang, C.F.; Iio, E.; Landis, C.; Jun, D.W.; Enomoto, M.; Ogawa, E.; Tsai, P.C.; et al. Cure With Interferon-Free Direct-Acting Antiviral Is Associated With Increased Survival in Patients With Hepatitis C Virus-Related Hepatocellular Carcinoma From Both East and West. Hepatology 2020, 71, 1910–1922. [Google Scholar] [CrossRef]
- Koda, M.; Tanaka, S.; Takemura, S.; Shinkawa, H.; Kinoshita, M.; Hamano, G.; Ito, T.; Kawada, N.; Shibata, T.; Kubo, S. Long-Term Prognostic Factors after Hepatic Resection for Hepatitis C Virus-Related Hepatocellular Carcinoma, with a Special Reference to Viral Status. Liver Cancer 2018, 7, 261–276. [Google Scholar] [CrossRef]
- Yu, M.L.; Huang, C.F.; Wei, Y.J.; Lin, W.Y.; Lin, Y.H.; Hsu, P.Y.; Hsu, C.T.; Liu, T.W.; Lee, J.J.; Niu, S.W.; et al. Establishment of an outreach, grouping healthcare system to achieve microelimination of HCV for uremic patients in haemodialysis centres (ERASE-C). Gut 2021, 70, 2349–2358. [Google Scholar] [CrossRef]
- Yang, T.H.; Fang, Y.J.; Hsu, S.J.; Lee, J.Y.; Chiu, M.C.; Yu, J.J.; Kuo, C.C.; Chen, C.H. Microelimination of Chronic Hepatitis C by Universal Screening Plus Direct-Acting Antivirals for Incarcerated Persons in Taiwan. Open Forum Infect. Dis. 2020, 7, ofaa301. [Google Scholar] [CrossRef]
- Tsai, P.C.; Huang, C.I.; Yeh, M.L.; Huang, C.F.; Hsieh, M.H.; Yang, J.F.; Hsu, P.Y.; Liang, P.C.; Lin, Y.H.; Jang, T.Y.; et al. Significant amelioration of hepatitis C virus infection in a hyperendemic area: Longitudinal evidence from the COMPACT Study in Taiwan. BMJ Open 2021, 11, e042861. [Google Scholar] [CrossRef]
- Ryu, T.; Takami, Y.; Wada, Y.; Tateishi, M.; Matsushima, H.; Yoshitomi, M.; Mikagi, K.; Saitsu, H. Effect of achieving sustained virological response before hepatitis C virus-related hepatocellular carcinoma occurrence on survival and recurrence after curative surgical microwave ablation. Hepatol. Int. 2018, 12, 149–157. [Google Scholar] [CrossRef]
- Krassenburg, L.A.P.; Maan, R.; Ramji, A.; Manns, M.P.; Cornberg, M.; Wedemeyer, H.; de Knegt, R.J.; Hansen, B.E.; Janssen, H.L.A.; de Man, R.A.; et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J. Hepatol. 2021, 74, 1053–1063. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology 2018, 155, 411–421.e414. [Google Scholar] [CrossRef]
- Mu, S.C.; Lin, Y.M.; Jow, G.M.; Chen, B.F. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J. Hepatol. 2009, 50, 264–272. [Google Scholar] [CrossRef]
- Mak, L.Y.; Wong, D.K.; Pollicino, T.; Raimondo, G.; Hollinger, F.B.; Yuen, M.F. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J. Hepatol. 2020, 73, 952–964. [Google Scholar] [CrossRef]
- Okimoto, S.; Kobayashi, T.; Kuroda, S.; Ishiyama, K.; Ide, K.; Ohira, M.; Tahara, H.; Shimizu, S.; Iwako, H.; Hamaoka, M.; et al. Prediction of recurrence following hepatectomy in patients with hepatitis C virus infection-related hepatocellular carcinoma who achieved a sustained virological response. Hepatol. Res. 2017, 47, 1186–1195. [Google Scholar] [CrossRef]
- Yu, M.L.; Chen, P.J.; Dai, C.Y.; Hu, T.H.; Huang, C.F.; Huang, Y.H.; Hung, C.H.; Lin, C.Y.; Liu, C.H.; Liu, C.J.; et al. 2020 Taiwan consensus statement on the management of hepatitis C: Part (I) general population. J. Formos. Med. Assoc. 2020, 119, 1019–1040. [Google Scholar] [CrossRef] [PubMed]

| Variable | All Patients (n = 208) | HCC with Persistent Viremia (n = 44) | Post-SVR HCC (n = 90) | Viremic HCC with Subsequent SVR (n = 74) | p Value |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 64.6 ± 8.6 | 67.5 ± 8.2 | 64.7 ± 8.1 | 62.8 ± 8.9 † | 0.016 |
| Male gender, n (%) | 136 (65.4) | 25 (56.8) | 65 (72.2) | 46 (62.2) | 0.163 |
| BMI (kg/m2), mean ± SD | 24.6 ± 3.8 | 24.2 ± 3.5 | 25.4 ± 4.1 | 23.9 ± 3.3 ‡ | 0.032 |
| Diabetes mellitus, n (%) | 68 (32.7) | 17 (38.6) | 32 (35.6) | 19 (25.7) | 0.260 |
| Hypertension, n (%) | 104 (50.0) | 25 (56.8) | 54 (60.0) | 25 (33.8) †‡ | 0.002 |
| Alcohol drinking, n (%) | 44 (21.2) | 8 (18.2) | 22 (24.4) | 14 (18.9) | 0.595 |
| Smoking, n (%) | 61 (29.3) | 9 (20.5) | 29 (32.2) | 23 (31.1) | 0.342 |
| AST (IU/L), mean ± SD | 53.6 ± 44.2 | 68.3 ± 28.8 | 37.9 ± 52.1 † | 64.0 ± 34.6 ‡ | <0.001 |
| ALT (IU/L), mean ± SD | 55.2 ± 42.5 | 68.7 ± 39.3 | 34.7 ± 32.2 † | 72.1 ± 44.9 ‡ | <0.001 |
| Platelet count (103/μL), mean ± SD | 166.9 ± 71.1 | 148.0 ± 54.5 | 181.2 ± 62.9 † | 160.8 ± 85.3 | 0.025 |
| ALBI grade I/II/III | <0.001 | ||||
| Grade I, n (%) | 147 (70.7) | 19 (43.2) | 78 (86.7) † | 50 (67.6) †‡ | |
| Grade II, n (%) | 59 (28.4) | 24 (54.5) | 11 (12.2) † | 24 (32.4) ‡ | |
| Grade III, n (%) | 2 (1.0) | 1 (2.3) | 1 (1.1) | 0 (0) | |
| AFP ≥ 20 ng/mL, n (%) | 80 (38.5) | 25 (56.8) | 26 (28.9) † | 29 (39.2) | 0.008 |
| AFP ≥ 200 ng/mL, n (%) | 32 (15.4) | 11 (25.0) | 12 (13.3) | 9 (12.2) | 0.135 |
| BCLC stage 0/A, n (%) | 71/137 (34.1/65.9) | 13/31 (29.5/70.5) | 31/59 (34.4/65.6) | 27/47 (36.5/63.5) | 0.742 |
| Histological grade | 0.168 | ||||
| Well-differentiated, n (%) | 27 (13.0) | 7 (15.9) | 7 (7.8) | 13 (17.6) | |
| Moderately differentiated, n (%) | 144 (69.2) | 33 (75.0) | 64 (71.1) | 47 (63.5) | |
| Poorly differentiated, n (%) | 37 (17.8) | 4 (9.1) | 19 (21.1) | 14 (18.9) | |
| Multiple tumors, n (%) | 19 (9.1) | 3 (6.8) | 8 (8.9) | 8 (10.8) | 0.822 |
| Largest tumor size (cm), mean ± SD | 2.6 ± 1.1 | 2.5 ± 1.1 | 2.5 ± 1.0 | 2.6 ± 1.2 | 0.832 |
| Microvascular invasion, n (%) | 43 (20.7) | 9 (20.5) | 20 (22.2) | 14 (18.9) | 0.873 |
| Capsule invasion, n (%) | 75 (36.1) | 13 (29.5) | 32 (35.6) | 30 (40.5) | 0.481 |
| Satellite nodules, n (%) | 52 (25.0) | 14 (31.8) | 19 (21.1) | 19 (25.7) | 0.400 |
| Hepatic steatosis, n (%) | 100 (48.1) | 26 (59.1) | 46 (51.1) | 28 (37.8) | 0.061 |
| Liver cirrhosis, n (%) ¶ | 65 (31.3) | 19 (43.2) | 22 (24.4) | 24 (32.4) | 0.086 |
| Follow-up (months), mean ± SD | 70.7 ± 43.3 | 50.5 ± 37.6 | 67.0 ± 40.4 | 87.3 ± 44.2 †‡ | <0.001 |
| All Patients (n = 208) | HCC with Persistent Viremia (n = 44) | Post-SVR HCC (n = 90) | Viremic HCC with Subsequent SVR (n = 74) | HCC with Persistent Viremia vs. Post-SVR HCC p Value | HCC with Persistent Viremia vs. Viremic HCC with Subsequent SVR p Value | Post-SVR HCC vs. Viremic HCC with Subsequent SVR p Value | |
|---|---|---|---|---|---|---|---|
| Cumulative recurrence rates | <0.001 | <0.001 | 0.638 | ||||
| 1-year recurrence rate | 35% | 60% | 25% | 32% | |||
| 3-year recurrence rate | 50% | 76% | 45% | 42% | |||
| 5-year recurrence rate | 62% | 89% | 51% | 59% | |||
| Recurrence-free survival, median (RFS), months (95% CI) | 39.5 (23.1–55.9) | 11.6 (9.5–13.7) | 56.7 (11.8–101.6) | 56.7 (43.3–70.0) | <0.001 | <0.001 | 0.616 |
| 1-year RFS | 63% | 33% | 73% | 68% | |||
| 3-year RFS | 47% | 16% | 53% | 58% | |||
| 5-year RFS | 36% | 8% | 48% | 40% |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age ≥ 60 (years) | 1.38 (0.92–2.07) | 0.126 | ||
| Male | 1.11 (0.77–1.61) | 0.569 | ||
| Diabetes mellitus | 1.25 (0.86–1.80) | 0.243 | ||
| Hypertension | 0.84 (0.59–1.19) | 0.332 | ||
| Alcohol drinking | 0.90 (0.58–1.39) | 0.619 | ||
| Smoking | 0.79 (0.53–1.18) | 0.244 | ||
| AST ≥ 40 (IU/L) | 1.31 (0.92–1.86) | 0.138 | ||
| ALT ≥ 40 (IU/L) | 1.36 (0.96–1.94) | 0.085 | 1.21 (0.81–1.80) | 0.358 |
| Platelet ≥ 150 (103/μL) | 0.72 (0.50–1.02) | 0.064 | 0.72 (0.50–1.05) | 0.089 |
| ALBI grade II/III (vs. grade I) | 1.26 (0.86–1.83) | 0.233 | ||
| AFP ≥ 200 ng/mL | 1.44 (0.90–2.31) | 0.127 | ||
| HCV status | ||||
| HCC with persistent viremia | Reference | Reference | ||
| Post-SVR HCC | 0.37 (0.24–0.58) | <0.001 | 0.42 (0.26–0.68) | <0.001 |
| Viremic HCC with subsequent SVR | 0.41 (0.26–0.65) | <0.001 | 0.41 (0.26–0.66) | <0.001 |
| BCLC stage A (vs. stage 0) | 1.41 (0.96–2.06) | 0.079 | 1.25 (0.74–2.11) | 0.413 |
| Multiple tumors | 1.04 (0.59–1.85) | 0.886 | ||
| Largest tumor size (cm) | 1.16 (0.99–1.37) | 0.065 | 1.11 (0.89–1.38) | 0.377 |
| Histological grade: poor/moderate (vs. well) | 1.40 (0.81–2.45) | 0.232 | ||
| Microvascular invasion | 1.14 (0.74–1.77) | 0.548 | ||
| Capsule invasion | 1.12 (0.78–1.61) | 0.538 | ||
| Satellite nodules | 1.53 (1.04–2.26) | 0.031 | 1.59 (1.06–2.38) | 0.025 |
| Hepatic steatosis | 1.38 (0.97–1.96) | 0.072 | 1.41 (0.97–2.04) | 0.070 |
| Liver cirrhosis † | 1.30 (0.90-1.87) | 0.159 | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Predictor | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age ≥ 60 (years) | 1.40 (0.95–2.08) | 0.093 | 1.31 (0.86–1.99) | 0.214 |
| Male | 1.06 (0.74–1.50) | 0.756 | ||
| Diabetes mellitus | 1.23 (0.86–1.76) | 0.257 | ||
| Hypertension | 0.83 (0.59–1.16) | 0.263 | ||
| Alcohol drinking | 0.89 (0.59–1.36) | 0.601 | ||
| Smoking | 0.77 (0.52–1.13) | 0.184 | ||
| AST ≥ 40 (IU/L) | 1.33 (0.94–1.86) | 0.104 | ||
| ALT ≥ 40 (IU/L) | 1.35 (0.96–1.89) | 0.085 | 1.25 (0.84–1.85) | 0.275 |
| Platelet ≥ 150 (103/μL) | 0.71 (0.51–0.99) | 0.049 | 0.71 (0.49–1.04) | 0.077 |
| ALBI grade II/III (vs. grade I) | 1.38 (0.97–1.96) | 0.077 | 0.88 (0.58–1.34) | 0.560 |
| AFP ≥ 200 ng/mL | 1.48 (0.94–2.32) | 0.091 | 1.59 (1.00–2.54) | 0.051 |
| HCV status | ||||
| HCC with persistent viremia | Reference | Reference | ||
| Post-SVR HCC | 0.32 (0.21–0.49) | <0.001 | 0.35 (0.21–0.57) | <0.001 |
| Viremic HCC with subsequent SVR | 0.36 (0.23–0.56) | <0.001 | 0.34 (0.21–0.54) | <0.001 |
| BCLC stage A (vs. stage 0) | 1.46 (1.01–2.11) | 0.046 | 1.25 (0.75–2.08) | 0.392 |
| Multiple tumors | 1.05 (0.60–1.82) | 0.877 | ||
| Largest tumor size (cm) | 1.17 (1.00–1.37) | 0.046 | 1.10 (0.89–1.37) | 0.370 |
| Histological grade: poor/moderate (vs. well) | 1.42 (0.83–2.42) | 0.204 | ||
| Microvascular invasion | 1.25 (0.83–1.89) | 0.286 | ||
| Capsule invasion | 1.09 (0.77–1.55) | 0.616 | ||
| Satellite nodules | 1.48 (1.02–2.16) | 0.040 | 1.43 (0.97–2.11) | 0.069 |
| Hepatic steatosis | 1.29 (0.92–1.81) | 0.141 | ||
| Liver cirrhosis † | 1.20 (0.84–1.71) | 0.318 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-Y.; Liang, P.-C.; Huang, C.-I.; Hsieh, M.-H.; Tsai, Y.-S.; Lin, T.-C.; Yeh, M.-L.; Huang, C.-F.; Wang, C.-W.; Jang, T.-Y.; et al. Effects of Achieving SVR on Clinical Characteristics and Surgical Outcomes in Patients Who Developed Early-Stage HCV-Related Hepatocellular Carcinoma and Received Curative Resection: Preoperative versus Postoperative SVR. Viruses 2022, 14, 2412. https://doi.org/10.3390/v14112412
Hsu P-Y, Liang P-C, Huang C-I, Hsieh M-H, Tsai Y-S, Lin T-C, Yeh M-L, Huang C-F, Wang C-W, Jang T-Y, et al. Effects of Achieving SVR on Clinical Characteristics and Surgical Outcomes in Patients Who Developed Early-Stage HCV-Related Hepatocellular Carcinoma and Received Curative Resection: Preoperative versus Postoperative SVR. Viruses. 2022; 14(11):2412. https://doi.org/10.3390/v14112412
Chicago/Turabian StyleHsu, Po-Yao, Po-Cheng Liang, Ching-I Huang, Meng-Hsuan Hsieh, Yi-Shan Tsai, Tzu-Chun Lin, Ming-Lun Yeh, Chung-Feng Huang, Chih-Wen Wang, Tyng-Yuan Jang, and et al. 2022. "Effects of Achieving SVR on Clinical Characteristics and Surgical Outcomes in Patients Who Developed Early-Stage HCV-Related Hepatocellular Carcinoma and Received Curative Resection: Preoperative versus Postoperative SVR" Viruses 14, no. 11: 2412. https://doi.org/10.3390/v14112412
APA StyleHsu, P.-Y., Liang, P.-C., Huang, C.-I., Hsieh, M.-H., Tsai, Y.-S., Lin, T.-C., Yeh, M.-L., Huang, C.-F., Wang, C.-W., Jang, T.-Y., Lin, Y.-H., Lin, Z.-Y., Chuang, W.-L., & Dai, C.-Y. (2022). Effects of Achieving SVR on Clinical Characteristics and Surgical Outcomes in Patients Who Developed Early-Stage HCV-Related Hepatocellular Carcinoma and Received Curative Resection: Preoperative versus Postoperative SVR. Viruses, 14(11), 2412. https://doi.org/10.3390/v14112412







