Isolation and Genetic Characterization of a Bovine Coronavirus KBR-1 Strain from Calf Feces in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Diarrhea Samples and RNA Extraction
2.2. RT-PCR and Sequences Analysis of BCoV
2.3. Isolation of BCoV
2.4. Adjuvant Selection Using Mice and Goats
2.5. Immunization of Calves and Examination of Vaccine Efficacy
2.6. Serum Neutralizing Antibody and HI Tests
2.7. Real-Time RT-PCR
2.8. Statistical Analysis
3. Results
3.1. Prevalence of BCoV
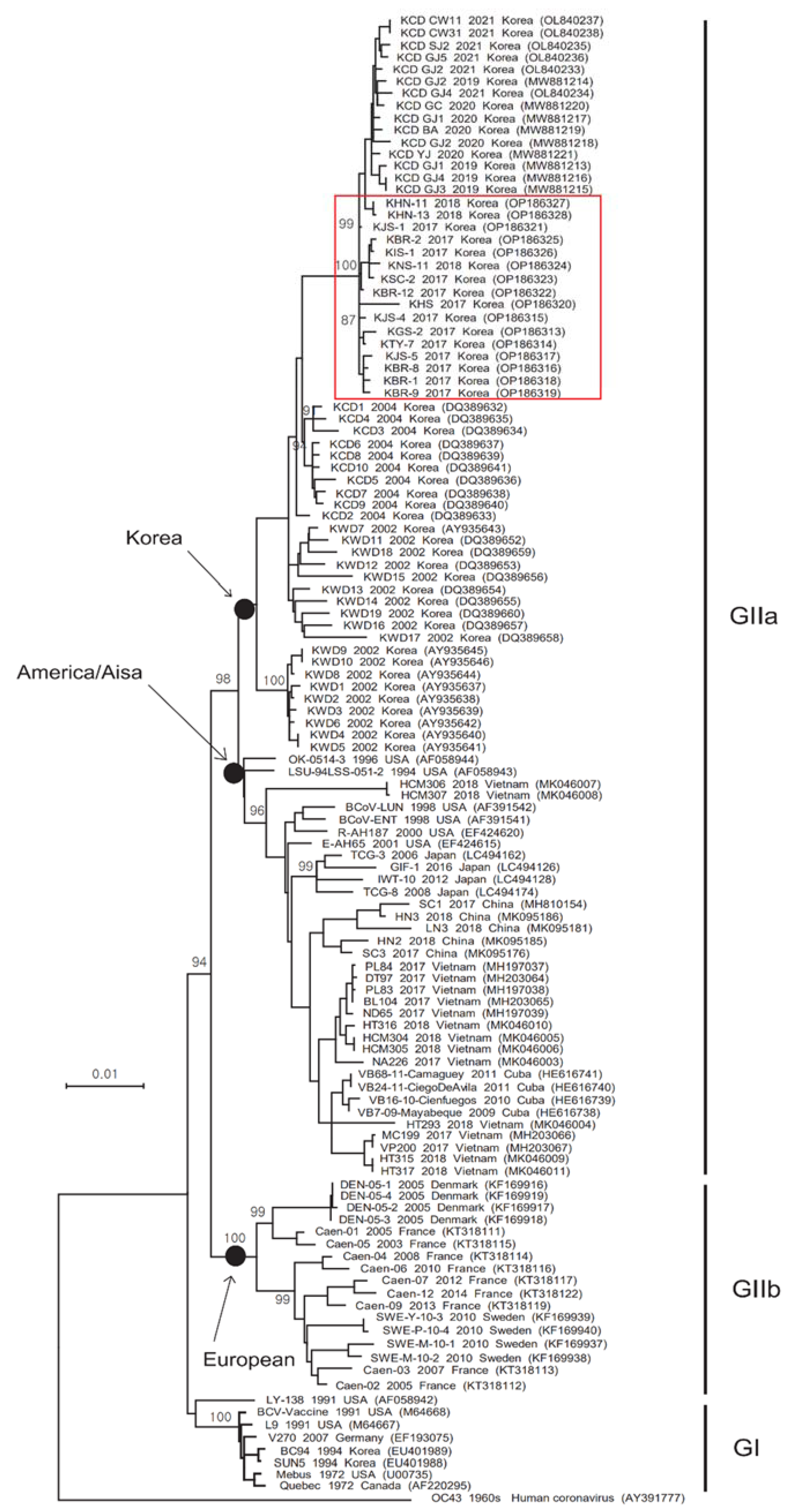
3.2. Phylogenetic Analysis of the S and HE Genes
3.3. Comparison of the S and HE Gene Sequences
3.4. Non-CPE of the BC94 and KBR-1 Strains
3.5. Antibody Titers and Protection of Mice and Goats Inoculated with the Inactivated KBR-1 Vaccine
3.6. Diarrhea Score, and Antigen and Antibody Levels, after Challenge of Calves with the Inactivated KBR-1 Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Groot, R.J.; Baker, S.C.; Baric, R.; Enjuanes, L.; Gorbalenya, A.E.; Holmes, K.V.; Perlman, S.; Poon, L.; Rottier, P.J.M.; Talbot, P.J.; et al. Family Coronaviridae. In Virus Taxonomy, Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses, 1st ed.; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2011; pp. 806–828. [Google Scholar]
- Lai, M.M.C.; Cavanagh, D. The molecular biology of coronaviruses. Adv. Virus Res. 1997, 48, 1–100. [Google Scholar]
- He, Q.; Guo, Z.; Zhang, B.; Yue, H.; Tang, C. First detection of bovine coronavirus in Yak (Bos grunniens) and a bovine coronavirus genome with a recombinant HE gene. J. Gen. Virol. 2019, 100, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Desjardins, J.; Zhang, C.; Talbot, P.J. The acetyl-esterase activity of the hemagglutinin-esterase protein of human coronavirus OC43 strongly enhances the production of infectious virus. J. Virol. 2013, 87, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J.; Jung, K. Comparative pathogenesis of bovine and porcine respiratory coronaviruses in the animal host species and SARS-CoV-2 in humans. J. Clin. Microbiol. 2020, 58, 3291–3298. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A. Bovine coronavirus. Br. Vet. J. 1993, 149, 51–70. [Google Scholar] [CrossRef]
- Tsunemitsu, H.; Saif, L.J. Antigenic and biological comparisons of bovine coronaviruses derived from neonatal calf diarrhea and winter dysentery of adult cattle. Arch. Virol. 1995, 140, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J. A review of evidence implicating bovine coronavirus in the etiology of winter dysentery in cows: An enigma resolved? Cornell Vet. 1990, 80, 303–311. [Google Scholar]
- Cho, K.O.; Halbur, P.G.; Bruna, J.D.; Sorden, S.D.; Yoon, K.J.; Janke, B.H.; Chang, K.O.; Saif, L.J. Detection and isolation of coronavirus from feces of three herds of feedlot cattle during outbreaks of winter dysentery-like disease. J. Am. Vet. Med. Assoc. 2000, 217, 1191–1194. [Google Scholar] [CrossRef]
- Tråvén, M.; Näslund, K.; Linde, N.; Linde, B.; Silván, A.; Fossum, C.; Hedlund, K.O.; Larsson, B. Experimental reproduction of winter dysentery in lactating cows using BCV—Comparison with BCV infection in milk-fed calves. Vet. Microbiol. 2001, 81, 127–151. [Google Scholar] [CrossRef]
- Lathrop, S.L.; Wittum, T.E.; Brock, K.V.; Loerch, S.C.; Perino, L.J.; Bingham, H.R.; McCollum, F.T.; Saif, L.J. Association between infection of the respiratory tract attributable to bovine coronavirus and health and growth performance of cattle in feedlots. Am. J. Vet. Res. 2000, 61, 1062–1066. [Google Scholar] [CrossRef]
- Ellis, J. What is the evidence that bovine coronavirus is a biologically significant respiratory pathogen in cattle? Can. Vet. J. 2019, 60, 147–152. [Google Scholar] [PubMed]
- Jeong, J.H.; Kim, G.Y.; Yoon, S.S.; Park, S.J.; Kim, Y.J.; Sung, C.M.; Jang, O.J.; Shin, S.S.; Koh, H.B.; Lee, B.J.; et al. Detection and isolation of winter dysentery bovine coronavirus circulated in Korea during 2002–2004. J. Vet. Med. Sci. 2005, 67, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, G.Y.; Choy, H.E.; Hong, Y.J.; Saif, L.J.; Jeong, J.H.; Park, S.I.; Kim, H.H.; Kim, S.K.; Shin, S.S.; et al. Dual enteric and respiratory tropisms of winter dysentery bovine coronavirus in calves. Arch. Virol. 2007, 152, 1885–1900. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Hwang, I.J.; Kim, B.H.; Kweon, C.H.; Lee, K.W.; Kang, M.I.; Lee, C.S.; Cho, K.O. Serosurveillance of viral diseases in Korean native goats (Capra hircus). J. Vet. Med. Sci. 2008, 70, 977–979. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Jang, J.H.; Yoon, S.W.; Noh, J.Y.; Ahn, M.J.; Kim, Y.; Jeong, D.G.; Kim, H.K. Detection of bovine coronavirus in nasal swab of non-captive wild water deer, Korea. Transbound. Emerg. Dis. 2018, 65, 627–631. [Google Scholar] [CrossRef]
- Kim, E.M.; Cho, H.C.; Shin, S.U.; Park, J.; Choi, K.S. Prevalence and genetic characterization of bovine coronavirus identified from diarrheic pre-weaned native Korean calves from 2019 to 2021. Infect. Genet. Evol. 2022, 100, 105263. [Google Scholar] [CrossRef]
- Park, S.J.; Jeong, C.; Yoon, S.S.; Choy, H.E.; Saif, L.J.; Park, S.H.; Kim, Y.J.; Jeong, J.H.; Park, S.I.; Kim, H.H.; et al. Detection and Characterization of Bovine Coronaviruses in Fecal Specimens of Adult Cattle with Diarrhea during the Warmer Seasons. J. Clin. Microbiol. 2006, 44, 3178–3188. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Dey, A.K.; Burke, B.; Sun, Y.; Hartog, K.; Heeney, J.L.; Montefiori, D.; Srivastava, I.K.; Barnett, S.W. Use of a polyanionic carbomer, Carbopol971P, in combination with MF59, improves antibody responses to HIV-1 envelope glycoprotein. Vaccine 2012, 30, 2749–2759. [Google Scholar] [CrossRef]
- Cho, Y.I.; Kim, W.I.; Liu, S.; Kinyon, J.M.; Yoon, K.J. Development of a panel of multiplex real-time polymerase chain reaction assays for simultaneous detection of major agents causing calf diarrhea in feces. J. Vet. Diagn. Investig. 2010, 22, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.B.; Kim, H.C.; Kang, J.G.; Choi, K.S.; Chae, J.S.; Yu, D.H.; Park, B.K.; Oh, Y.S.; Choi, H.J.; Park, J. The prevalence of causative agents of calf diarrhea in Korean native calves. J. Anim. Sci. Technol. 2021, 63, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, B.; Sun, D. Advances in Bovine Coronavirus Epidemiology. Viruses 2022, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, G.Y.; Yoon, S.S.; Park, S.J.; Kim, Y.J.; Sung, C.M.; Shin, S.S.; Lee, B.J.; Kang, M.I.; Park, N.Y.; et al. Molecular analysis of S gene of spike glycoprotein of winter dysentery bovine coronavirus circulated in Korea during 2002–2003. Virus Res. 2005, 108, 207–212. [Google Scholar] [CrossRef]
- Takamura, K.; Matsumoto, Y.; Shimizu, Y. Field study of bovine coronavirus vaccine enriched with hemagglutinating antigen for winter dysentery in dairy cows. Can. J. Vet. Res. 2002, 66, 278–281. [Google Scholar]



| Strain | Gene | Korean BCoV Strains a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1994 | 2002 | 2004 | 2015 | 2017 | 2018 | 2019 | 2020 | 2021 | ||
| Mebus | Spike | 99.5–99.6 b | 97.5–98.2 | 97.9–98.1 | - | 97.3–97.8 | 97.5–97.6 | 97.6–97.7 | 97.5–97.6 | 97.5–97.6 |
| (98.8–98.9) c | (96.1–97.6) | (97.1–97.5) | - | (96.4–97.6) | (97.1–97.4) | (97.0) | (97.2–97.4) | (97.1–97.4) | ||
| HE | 99.8 | 97.5–98.0 | 97.7–98.0 | 97.9–98.0 | 97.6–98.0 | 97.6–97.8 | 96.9–97.6 | 97.3–98.0 | 97.5–97.8 | |
| (99.3–99.5) | (97.4–98.6) | (98.6–99.1) | (98.8) | (98.1–98.8) | (98.4–98.6) | (97.1–97.9) | (97.4–98.8) | (98.1–98.6) | ||
| Ref. Strain | Korean BCoV Strains a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1971 | 1994 | 2002 | 2004 | 2005 | 2017 | 2018 | 2019 | 2020 | 2021 |
| Spike aa | Mebus | BCoV (n = 2) | BCoV (n = 19) | BCoV (n = 10) | BCoV (n = 0) | BCoV (n = 13) | BCoV (n = 3) | BCoV (n = 4) | BCoV (n = 5) | BCoV (n = 6) |
| 11 | M | * | */T | */T | - | T | T | T | T | T |
| 16 | I | * | * | * | - | * | * | */V | */V | */V |
| 33 | A | * | V | V | - | V | V | V | V | V |
| 40 | I | */T | T | T | - | T | T | T | T | T |
| 88 | R | * | T | */T | - | T | T | T | T | T |
| 100 | I | * | T | T | - | T | T | T | T | T |
| 115 | K | * | D | D | - | D | D | D | D | D |
| 147 | L | * | F | F | - | F | F | F | F | F |
| 149 | N | * | S | S | - | S | S | S | S | S |
| 169 | H | * | N | N | - | N | N | N | N | N |
| 173 | H | * | N | */N | - | N | N | N | N | N |
| 179 | K | R/Q | R/Q | Q | - | Q | Q | Q | Q | Q |
| 248 | L | * | M | M | - | M | M | M | M | M |
| 253 | S | */Y | N | N | - | N | N | N | N | N |
| 256 | M | * | *L | L | - | L | L | L | L | L |
| 458 | F | S | S | S | - | S | S | S | S | S |
| 465 | V | * | A | */A | - | A | A | A | A | A |
| 470 | H | * | D | D | - | */D | D | D | D | D |
| 484 | S | T | T | T | - | T | T | T | T | T |
| 499 | N | * | */S/T/K | S/T | - | T | T | T | T | T |
| 501 | P | * | */S | S | - | S/Y | S | S | F/S | */S |
| 510 | S | * | T | T | - | T | T | T | T | T |
| 531 | N | D | */D | */D | - | D | D | D | D | D |
| 543 | S | * | A | A | - | A | A | A | A | A |
| 578 | T | * | S | S | - | S | S | S | S | S |
| 769 | A | * | S | S | - | S | S | S | S | S |
| 779 | A | N | N | N | - | N | N | N | N | N |
| 965 | V | * | E | E | - | E | E | E | E | E |
| 984 | L | W | W | W | - | W | W | W | W | W |
| 988 | V | A | A | A | - | A | A | A | A | A |
| 1026 | D | * | G | G | - | G | G | G | G | G |
| 1030 | E | * | */D | D | - | D | D | D | D | D |
| 1100 | V | A | A | A | - | A | A | A | A | A |
| 1241 | H | * | P | */P | - | P | P | P | P | P |
| 1341 | I | * | */K | K | - | K | K | K | K | K |
| Ref. Strain | Korean BCoV Strains a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1971 | 1994 | 2002 | 2004 | 2005 | 2017 | 2018 | 2019 | 2020 | 2021 |
| HE aa | Mebus | BCoV (n = 3) | BCoV (n = 19) | BCoV (n = 10) | BCoV (n = 2) | BCoV (n = 13) | BCoV (n = 3) | BCoV (n = 4) | BCoV (n = 5) | BCoV (n = 6) |
| 5 | L | * | P | */P | P | P | P | */P | P/H | P/H |
| 29 | N | * | T | T | T | T | T | T | T | T |
| 46 | D | * | G | G | G | G | G | G | */G | G |
| 339 | S | * | N | N | S | N | N | */N | */N | */N |
| 367 | S | * | P | P | D | P | P | P | P | P |
| 392 | L | * | I | I | I | I | I | I | I | I |
| Group | No. Cow | Vaccination | Challenge (after Vaccination) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPV 0 | DPV 21 | DPV 35 | DPC 1 | DPC 2 | DPC 3 | DPC 6 | DPC 9 | DPC 12 | DPC 14 | ||
| KBR-1 (106.0TCID50/dose) | A1 | b/<10 c/0 d | /40/0 | /80/0 | -/NT/0 | -/NT/0 | -/80/0 | -/160/0 | -/160/0 | -/160/0 | -/160/0 |
| A2 | -/<10/0 | /20/0 | /40/0 | -/NT/0 | -/NT/0 | -/160/0 | -/80/0 | -/160/0 | -/160/0 | -/160/0 | |
| KBR-1 (104.0TCID50/dose) | B1 | -/<20/0 | /20/0 | /20/0 | 30.3/NT/0 | 28.7/NT/0 | 33.1/40/2 | -/40/1 | -/80/0 | -/40/0 | -/80/0 |
| B2 | -/<10/0 | /10/0 | /20/0 | -/NT/0 | 29.1/NT/0 | 32.9/40/2 | 30.8/20/2 | -/40/0 | -/40/0 | -/40/0 | |
| Mock | C1 | -/<20/0 | /10/0 | /10/0 | -/NT/0 | -/NT/1 | 23.4/10/1 | 20.7/10/2 | 26.8/20/2 | 31.1/10/2 | -/20/1 |
| C2 | -/<10/0 | /10/0 | /10/0 | -/NT/0 | 28.1/NT/1 | 22.5/20/2 | 23.1/10/2 | 29.2/20/1 | -/20/2 | -/40/0 | |
| Group | No. Cow | Organs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Br * | Lv | Sp | Ln | Ht | Kd | SI | Ts | Li | Dd | Ji | Il | Ml | ||
| KBR-1 (106.0TCID50/mL) | A1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| A2 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| KBR-1 (104.0TCID50/mL) | B1 | - | - | - | - | - | - | - | - | 32.6 | - | 28.9 | 26.1 | - |
| B2 | - | - | - | - | - | - | - | - | 33.2 | - | 30.5 | 29.8 | - | |
| Mock | C1 | - | - | - | - | - | - | - | - | - | - | 23.6 | 26.2 | - |
| C2 | - | - | - | - | - | - | - | - | - | 31.8 | 24.7 | 25.5 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Choe, S.; Park, G.-N.; Song, S.; Kim, K.-S.; An, B.-H.; Hyun, B.-H.; An, D.-J. Isolation and Genetic Characterization of a Bovine Coronavirus KBR-1 Strain from Calf Feces in South Korea. Viruses 2022, 14, 2376. https://doi.org/10.3390/v14112376
Shin J, Choe S, Park G-N, Song S, Kim K-S, An B-H, Hyun B-H, An D-J. Isolation and Genetic Characterization of a Bovine Coronavirus KBR-1 Strain from Calf Feces in South Korea. Viruses. 2022; 14(11):2376. https://doi.org/10.3390/v14112376
Chicago/Turabian StyleShin, Jihye, SeEun Choe, Gyu-Nam Park, Sok Song, Ki-Sun Kim, Byung-Hyun An, Bang-Hun Hyun, and Dong-Jun An. 2022. "Isolation and Genetic Characterization of a Bovine Coronavirus KBR-1 Strain from Calf Feces in South Korea" Viruses 14, no. 11: 2376. https://doi.org/10.3390/v14112376
APA StyleShin, J., Choe, S., Park, G.-N., Song, S., Kim, K.-S., An, B.-H., Hyun, B.-H., & An, D.-J. (2022). Isolation and Genetic Characterization of a Bovine Coronavirus KBR-1 Strain from Calf Feces in South Korea. Viruses, 14(11), 2376. https://doi.org/10.3390/v14112376





