Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunoprophylaxis Procedures
2.3. Data Collection and Outcomes
2.4. Statistical Analyses
3. Results
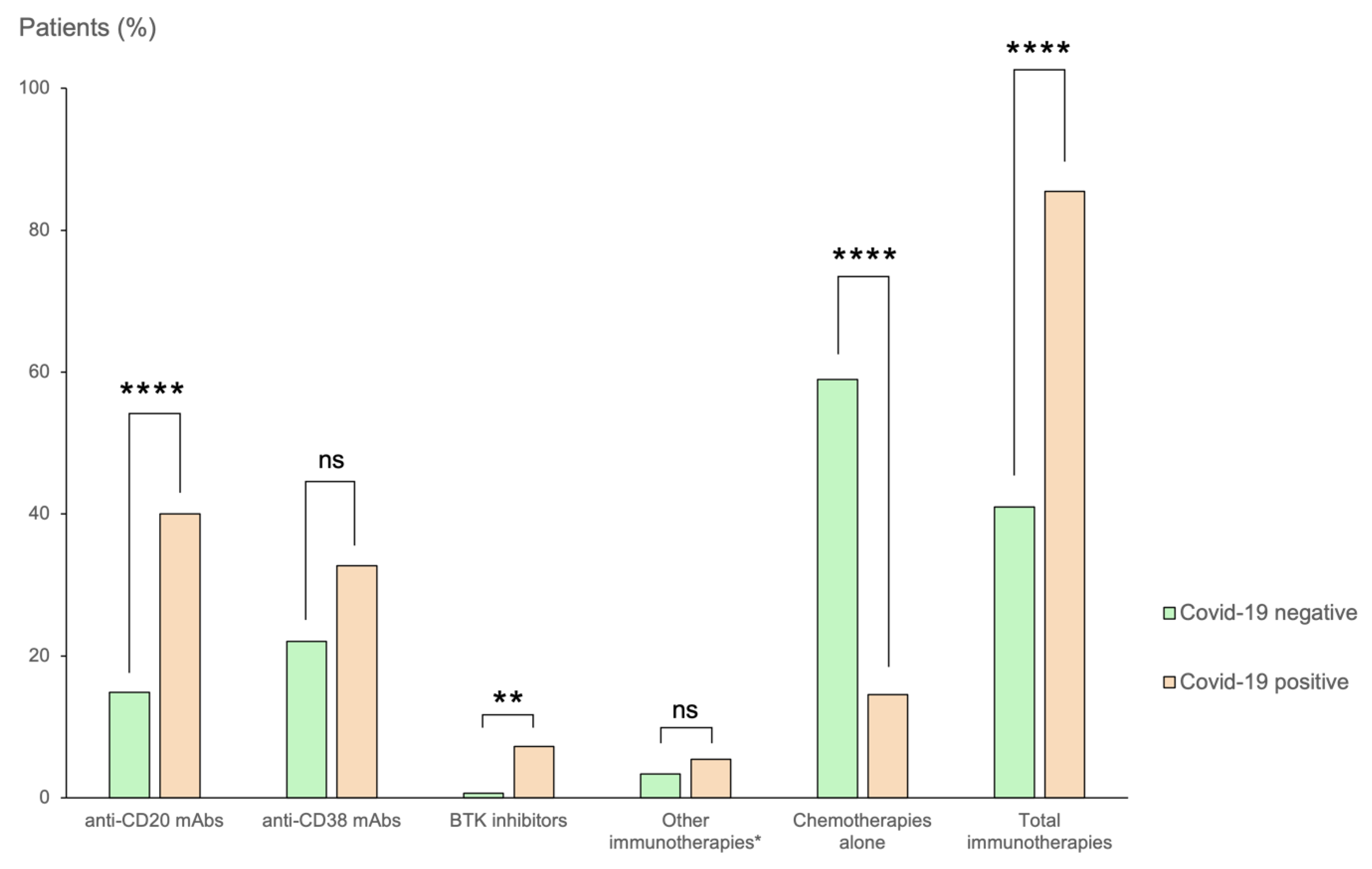
3.1. Patient Population and COVID-19-Positive Cases
3.2. Hospitalization and Mortality Due to COVID-19
3.3. Effects of Anti-SARS-CoV-2 Serology and mAb Prophylaxis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avouac, J.; Drumez, E.; Hachulla, E.; Seror, R.; Georgin-Lavialle, S.; El Mahou, S.; Pertuiset, E.; Pham, T.; Marotte, H.; Servettaz, A.; et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: A cohort study. Lancet Rheumatol. 2021, 3, e419–e426. [Google Scholar] [CrossRef]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Rinaldi, M.; Bussini, L.; Bonazzetti, C.; Pascale, R.; Pasquini, Z.; Faní, F.; Guedes, M.N.P.; Azzini, A.M.; Carrara, E.; et al. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Duléry, R.; Lamure, S.; Delord, M.; Di Blasi, R.; Chauchet, A.; Hueso, T.; Rossi, C.; Drenou, B.; Fischer, B.D.; Soussain, C.; et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am. J. Hematol. 2021, 96, 934–944. [Google Scholar] [CrossRef]
- Lamure, S.; Duléry, R.; Di Blasi, R.; Chauchet, A.; Laureana, C.; Deau-Fischer, B.; Drenou, B.; Soussain, C.; Rossi, C.; Noël, N.; et al. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: A retro-spective multicentric cohort study. eClinicalMedicine 2020, 27, 100549. [Google Scholar] [CrossRef]
- Xhaard, A.; Xhaard, C.; D’Aveni, M.; Salvator, H.; Chabi, M.L.; Berceanu, A.; Coman, T.; Beguin, Y.; Chalandon, Y.; Poiré, X.; et al. Risk factors for a severe form of COVID-19 after allogeneic haematopoietic stem cell transplantation: A Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM-TC) multicentre cohort study. Br. J. Haematol. 2021, 192, e121–e124. [Google Scholar] [CrossRef]
- Re, D.; Seitz-Polski, B.; Brglez, V.; Carles, M.; Graça, D.; Benzaken, S.; Liguori, S.; Zahreddine, K.; Delforge, M.; Bailly-Maitre, B.; et al. Humoral and cellular responses after a third dose of SARS-CoV-2 BNT162b2 vaccine in patients with lymphoid malignancies. Nat. Commun. 2022, 13, 864. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lym-phocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Henriquez, S.; Zerbit, J.; Bruel, T.; Ouedrani, S.; Planas, D.; Deschamps, P.; Staropoli, I.; Hadjadj, J.; Varet, B.; Ermak, N.; et al. Anti-CD38 therapy impairs SARS-CoV-2 vaccine response against alpha and delta variants in patients with multiple myeloma. Blood 2022, 139, 942–946. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Desai, S.; Marti, M.; Nohynek, H.; Kaslow, D.C.; Kochhar, S.; O’Brien, K.L.; Hombach, J.; Wilder-Smith, A. Response to additional COVID-19 vaccine doses in people who are immunocompromised: A rapid review. Lancet Glob. Health 2022, 10, e326–e328. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Planas, D.; Ouedrani, A.; Buffier, S.; Delage, L.; Nguyen, Y.; Bruel, T.; Stolzenberg, M.; Staropoli, I.; Ermak, N.; et al. Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in im-munocompromised patients with systemic inflammatory diseases. Ann. Rheum. Dis. 2022, 81, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Gressens, S.B.; Fourati, S.; Le Bouter, A.; Le Bras, F.; Dupuis, J.; Hammoud, M.; El Gnaoui, T.; Gounot, R.; Roulin, L.; Belhadj, K.; et al. Anti-SARS-CoV-2 antibody response after 2 and 3 doses of BNT162b2 mRNA vaccine in patients with lymphoid malignancies. Clin. Microbiol. Infect. 2022, 28, 885.e7–885.e11. [Google Scholar] [CrossRef] [PubMed]
- Asderakis, A.; Khalid, U.; Koimtzis, G.; Ponsford, M.J.; Szabo, L.; Chalklin, C.; Bramhall, K.; Grant, L.; Moat, S.J.; Humphreys, I.R.; et al. An Analysis of Serological Response and Infection Outcomes Following Ox-ford-AstraZeneca (AZD1222) and Pfizer-BioNTech (mRNA BNT162b2) SARS-CoV-2 Vaccines in Kidney and Kidney Pancreas Transplants. Transplantation 2022, 106, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Bruel, T.; Hadjadj, J.; Maes, P.; Planas, D.; Seve, A.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Bolland, W.; Nguyen, Y.; et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat. Med. 2022, 28, 1297–1302. [Google Scholar] [CrossRef]
- WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 1 September 2022).
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; De Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef]
- Public Health France. Indicators: Maps, Data and Graphs. Available online: https://geodes.santepubliquefrance.fr/#c=indicator&view=map2 (accessed on 1 September 2022).
- French Government. Governement COVID-19 Dashboard. Available online: https://www.gouvernement.fr/info-coronavirus/carte-et-donnees# (accessed on 1 September 2022).
- O’Brien, M.P.; Forleo-Neto, E.; Musser, B.J.; Isa, F.; Chan, K.-C.; Sarkar, N.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; Cohen, M.S.; et al. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N. Engl. J. Med. 2021, 385, 1184–1195. [Google Scholar] [CrossRef]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Releases Important Information about Risk of COVID-19 due to Certain Variants Not Neutralized by Evusheld. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-releases-important-information-about-risk-covid-19-due-certain-variants-not-neutralized-evusheld (accessed on 13 October 2022).
- Niemann, C.U.; Da Cunha-Bang, C.; Helleberg, M.; Ostrowski, S.R.; Brieghel, C. Patients with CLL have lower risk of death from COVID-19 in the omicron era. Blood 2022, 140, 445–450. [Google Scholar] [CrossRef]
- Madhi, S.A.; Kwatra, G.; Myers, J.E.; Jassat, W.; Dhar, N.; Mukendi, C.K.; Nana, A.J.; Blumberg, L.; Welch, R.; Ngorima-Mabhena, N.; et al. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; López-García, A.; Lamure, S.; Itri, F.; da Silva, M.G.; Dragonetti, G.; Falces-Romero, I.; van Doesum, J.; et al. COVID-19 in vaccinated adult patients with hematological malignancies: Preliminary results from EPICOVIDEHA. Blood 2022, 139, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wu, M.; Harvey, R.; Schmitt, A.M.; Tippu, Z.; Shum, B.; Farag, S.; Rogiers, A.; et al. Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet 2022, 399, 905–907. [Google Scholar] [CrossRef]
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef]

| Outpatients | Inpatients | |
|---|---|---|
| n = 44 | n = 13 | |
| Median age (IQR)—yr | 69 (60.5–78) | 75 (69–77) |
| Age category—no. (%) | ||
| <75 ans | 30 (68) | 6 (46.2) |
| > or = 75 ans | 14 (31.8) | 7 (53.8) |
| Male sex—no. (%) | 24 (54.5) | 12 (92.3) |
| Median BMI (IQR) | 24.1 (22.5–26.3) | 23.5 (22.5–26.3) |
| Hematologic malignancy—no. (%) | ||
| Multiple myeloma | 20 (45.5) | 5 (38.5) |
| Low-grade lymphoma | 7 (15.9) | 3 (23.1) |
| High-grade lymphoma | 8 (18.2) | 1 (7.7) |
| Chronic lymphocytic leukemia | 3 (6.8) | 2 (15.4) |
| Others * | 6 (13.6) | 2 (15.4) |
| On treatment—no. (%) | ||
| Anti-CD20 | 16 (36.4) | 6 (46.2) |
| Anti-CD38 | 15 (34.1) | 3 (23.1) |
| IBTK | 2 (4.5) | 2 (15.4) |
| Other immunotherapies ** | 1 (2.3) | 2 (15.4) |
| Others *** | 8 (18.2) | - |
| Median lymphocyte count (IQR)—G/L | 0.79 (0.47–1.03) | 0.5 (0.4–1.1) |
| Missing information—no (%) | 11 (25) | 1 (7.7) |
| Hypogammaglobulinemia—no. (%) | ||
| Present | 19 (43.2) | 8 (61.5) |
| Absent | 7 (15.9) | 1 (7.7) |
| Missing information | 18 (40.9) | 4 (30.1) |
| Vaccination—no. (%) | ||
| Initial scheme | 41 | 13 |
| Full scheme | 34 | 13 |
| Serology—no. (%) | ||
| Negative | 22 (50) | 9 (69) |
| <264 BAU | 4 (9.1) | 2 (15.4) |
| 264—1000 BAU | 1 (2.3) | 1(7.7) |
| >1000 BAU | 6 (13.6) | 1(7.7) |
| Missing information | 11 (25) | - |
| Casirivimab/imdevimab prophylaxis—no. (%) | 5 (11.4) | 2 (15.4) |
| Tixagevimab/cilgavimab prophylaxis—no. (%) | 3 (6.8) | 2 (15.4) |
| Median duration between last vaccination and COVID-19 diagnosis (IQR)—days | 117 (66–151) | 84 (49–107) |
| Anti-SARS-CoV-2 curative specific treatment—no. (%) | 9 (20.5) | 3 (23.1) |
| Tixagevimab/cilgavimab | 3 (6.8) | 2 (15.4) |
| Sotrovimab | 3 (6.8) | 1 (7.7) |
| Nirmatrelvir/ritonavir | 3 (6.8) | - |
| Outcomes | ||
| Invasive mechanical ventilation | - | 11 (84.6) |
| Deaths | - | 3 (23) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerbit, J.; Detroit, M.; Meyer, A.; Decroocq, J.; Deau-Fischer, B.; Deschamps, P.; Birsen, R.; Mondesir, J.; Franchi, P.; Miekoutima, E.; et al. Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era. Viruses 2022, 14, 2377. https://doi.org/10.3390/v14112377
Zerbit J, Detroit M, Meyer A, Decroocq J, Deau-Fischer B, Deschamps P, Birsen R, Mondesir J, Franchi P, Miekoutima E, et al. Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era. Viruses. 2022; 14(11):2377. https://doi.org/10.3390/v14112377
Chicago/Turabian StyleZerbit, Jeremie, Marion Detroit, Antoine Meyer, Justine Decroocq, Benedicte Deau-Fischer, Paul Deschamps, Rudy Birsen, Johanna Mondesir, Patricia Franchi, Elsa Miekoutima, and et al. 2022. "Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era" Viruses 14, no. 11: 2377. https://doi.org/10.3390/v14112377
APA StyleZerbit, J., Detroit, M., Meyer, A., Decroocq, J., Deau-Fischer, B., Deschamps, P., Birsen, R., Mondesir, J., Franchi, P., Miekoutima, E., Guerin, C., Batista, R., Bouscary, D., Willems, L., & Vignon, M. (2022). Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era. Viruses, 14(11), 2377. https://doi.org/10.3390/v14112377







